Assessment and Improvement of Masticatory Performance in Frail Older People: A Narrative Review
Abstract
:1. Introduction
2. A Conceptual Model of Oro-Facial Health with an Emphasis on Function
3. Factors Influencing Mastication
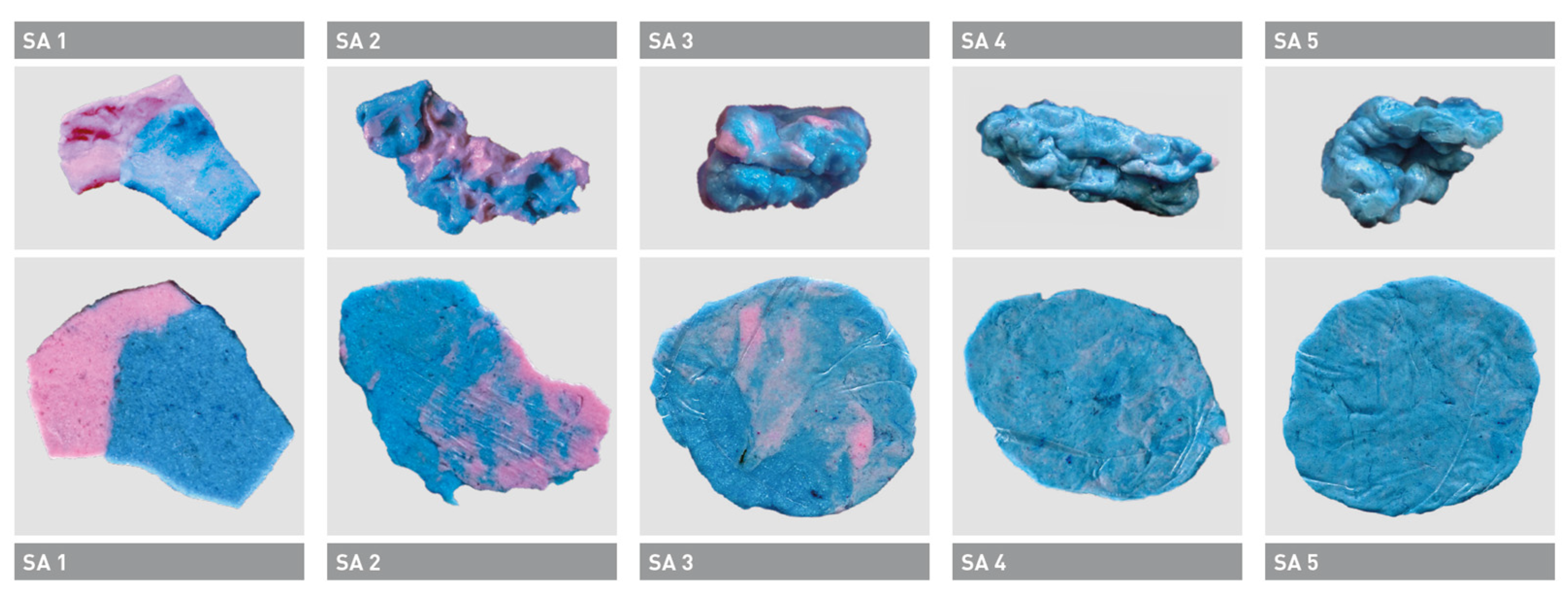
4. Assessment of the Masticatory Performance in Frail Older People
5. Chewing Efficiency
| Test | Methodology | Functioning |
|---|---|---|
| Color mixing-ability test | Two-colored chewing gum | Might contain sugar, older adults might not be familiar with chewing gum, easy to control bolus, easy to evaluate, easy to evaluate (scale) [14,81]. |
| Color mixing-ability test | Two-colored wax | Older adults might not be comfortable with chewing on wax, easy to control bolus, easy to evaluate, easy to evaluate (scale) [82]. |
| Glucosensor © (GC) | Glucose extraction from gum jelly | Needs specialized equipment and specimens [83]. |
| Carrot-test | Carrot slices | Always available, hardness might be difficult to control, easy to evaluate (scale) [80]. |
| Bite force | Force gauge | Force gauges for bite force are often not available. Bite force, however, is a good predictor of chewing function [47]. |
| Occlusal contacts in the four supporting zones (Eichner Classification) | Needs light and a good overview | Easiest way to extrapolate on chewing function. Main predictor of chewing function [8]. |
6. Chewing Ability
7. Improvement the Masticatory Performance in Frail Older People
8. Conclusions
- To fully encompass oral frailty, oro-facial hypofunction, or oro-facial fitness, dental patient reported outcomes (dPROs) should be included.
- Currently, there are few evidence-based rehabilitation approaches apart from prosthodontics to ameliorate oro-facial hypofunction.
- Older adults may have decreased neuroplasticity, which may hinder the effectiveness of such interventions, thus necessitating functional training and nutritional counseling to complement these strategies.
- The concept of oral frailty, oro-facial hypofunction or oro-facial fitness should involve dental patient reported outcomes (dPROs).
- Reduced neuro-plastic capacity in old individuals might preclude a positive outcome of these strategies that might need to be accompanied by functional training and nutritional counseling.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 22 April 2023).
- Neue Zürcher Zeitung. Available online: https://www.nzz.ch/die_babyboomer_kommen_ins_rentenalter-ld.492953?reduced=true (accessed on 22 April 2023).
- Schimmel, M.; Müller, F.; Suter, V.; Buser, D. Implants for Elderly Patients. Periodontology 2000 2017, 73, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Müller, F.; Srinivasan, M.; Krause, K.H.; Schimmel, M. Periodontitis and Peri-Implantitis in Elderly People Experiencing Institutional and Hospital Confinement. Periodontology 2000 2022, 90, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Laslett, P. The Emergence of the Third Age. Ageing Soc. 2008, 7, 133–160. [Google Scholar] [CrossRef]
- Suzman, R.M.; Willis, D.P.; Manton, K.G. (Eds.) Social Gerontology: The Oldest Old; Oxford University Press: New York, NY, USA, 1992. [Google Scholar]
- Gilleard, C.; Higgs, P. The Fourth Age and the Concept of a “Social Imaginary”: A Theoretical Excursus. J. Aging Stud. 2013, 27, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Dibello, V.; Zupo, R.; Sardone, R.; Lozupone, M.; Castellana, F.; Dibello, A.; Daniele, A.; De Pergola, G.; Bortone, I.; Lampignano, L.; et al. Oral Frailty and Its Determinants in Older Age: A Systematic Review. Lancet Health Longev. 2021, 2, e507–e520. [Google Scholar] [CrossRef]
- Strandberg, T.E.; Nieminen, T. Future Perspectives on the Role of Frailty in Cardiovascular Diseases; Springer: Berlin/Heidelberg, Germany, 2020; Volume 1216, ISBN 9783030333294. [Google Scholar]
- Minakuchi, S.; Tsuga, K.; Ikebe, K.; Ueda, T.; Tamura, F.; Nagao, K.; Furuya, J.; Matsuo, K.; Yamamoto, K.; Kanazawa, M.; et al. Oral Hypofunction in the Older Population: Position Paper of the Japanese Society of Gerodontology in 2016. Gerodontology 2018, 35, 317–324. [Google Scholar] [CrossRef]
- Kosaka, T.; Kida, M.; Kikui, M.; Hashimoto, S.; Fujii, K.; Yamamoto, M.; Nokubi, T.; Maeda, Y.; Hasegawa, Y.; Kokubo, Y.; et al. Factors Influencing the Changes in Masticatory Performance: The Suita Study. JDR Clin. Transl. Res. 2018, 3, 405–412. [Google Scholar] [CrossRef]
- Schneider, C.; Zemp, E.; Zitzmann, N.U. Oral Health Improvements in Switzerland over 20 Years. Eur. J. Oral Sci. 2017, 125, 55–62. [Google Scholar] [CrossRef]
- Slade, G.D.; Akinkugbe, A.A.; Sanders, A.E. Projections of U.S. Edentulism Prevalence Following 5 Decades of Decline. J. Dent. Res. 2014, 93, 959–965. [Google Scholar] [CrossRef]
- Bousiou, A.; Konstantopoulou, K.; Polychronopoulou, A.; Halazonetis, D.J.; Schimmel, M.; Kossioni, A.E. Sociomedical and Oral Factors Affecting Masticatory Performance in an Older Population. Clin. Oral Investig. 2022, 26, 3477–3486. [Google Scholar] [CrossRef]
- Müller, F. Oral Hygiene Reduces the Mortality from Aspiration Pneumonia in Frail Elders. J. Dent. Res. 2015, 94, 14S–16S. [Google Scholar] [CrossRef] [PubMed]
- Schimmel, M. Preventive Strategies in Geriatric Dental Medicine. Oral Health Prev. Dent. 2016, 14, 291–292. [Google Scholar] [CrossRef] [PubMed]
- Brocklehurst, P.R.; McKenna, G.; Schimmel, M.; Kossioni, A.; Jerković-Ćosić, K.; Hayes, M.; da Mata, C.; Müller, F. How Do We Incorporate Patient Views into the Design of Healthcare Services for Older People: A Discussion Paper. BMC Oral Health 2018, 18, 61. [Google Scholar] [CrossRef]
- Ship, J.A. Oral Health in the Elderly—What’s Missing? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2004, 98, 625–626. [Google Scholar] [CrossRef] [PubMed]
- Kossioni, A.; McKenna, G.; Müller, F.; Schimmel, M.; Vanobbergen, J. Higher Education in Gerodontology in European Universities. BMC Oral Health 2017, 17, 71. [Google Scholar] [CrossRef]
- Bircher, J.; Kuruvilla, S. Defining Health by Addressing Individual, Social, and Environmental Determinants: New Opportunities for Health Care and Public Health. J. Public Health Policy 2014, 35, 363–386. [Google Scholar] [CrossRef]
- Bircher, J.; Hahn, E.G. Will the Meikirch Model, a New Framework for Health, Induce a Paradigm Shift in Healthcare? Cureus 2017, 9, e1081. [Google Scholar] [CrossRef]
- Szczesniak, M.M.; Maclean, J.; Zhang, T.; Graham, P.H.; Cook, I.J. Persistent Dysphagia after Head and Neck Radiotherapy: A Common and under-Reported Complication with Significant Effect on Non-Cancer-Related Mortality. Clin. Oncol. 2014, 26, 697–703. [Google Scholar] [CrossRef]
- Chebib, N.; Abou-Ayash, S.; Maniewicz, S.; Srinivasan, M.; Hill, H.; McKenna, G.; Holmes, E.; Schimmel, M.; Brocklehurst, P.; Müller, F. Exploring Older Swiss People’s Preferred Dental Services for When They Become Dependent. Swiss Dent. J. 2020, 130, 876–884. [Google Scholar]
- Schimmel, M.; Aarab, G.; Baad-Hansen, L.; Lobbezoo, F.; Svensson, P. A Conceptual Model of Oro-Facial Health with an Emphasis on Function. J. Oral Rehabil. 2021, 48, 1283–1294. [Google Scholar] [CrossRef]
- Reissmann, D.R. Dental Patient-Reported Outcome Measures Are Essential for Evidence-Based Prosthetic Dentistry. J. Evid. Based. Dent. Pract. 2019, 19, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Reissmann, D.R. Methodological Considerations When Measuring Oral Health–Related Quality of Life. J. Oral Rehabil. 2021, 48, 233–245. [Google Scholar] [CrossRef] [PubMed]
- John, M.T.; Rener-Sitar, K.; Baba, K.; Čelebić, A.; Larsson, P.; Szabo, G.; Norton, W.E.; Reissmann, D.R.; Rashighi, M.; Harris, J.E. Patterns of Impaired Oral Health-Related Quality of Life Dimensions. Physiol. Behav. 2017, 43, 519–527. [Google Scholar] [CrossRef]
- John, M.T.; Sekulić, S.; Bekes, K.; Al-Harthy, M.H.; Michelotti, A.; Reissmann, D.R.; Nikolovska, J.; Sanivarapu, S.; Lawal, F.B.; List, T.; et al. Why Patients Visit Dentists—A Study in All World Health Organization Regions. J. Evid. Based. Dent. Pract. 2020, 20, 101459. [Google Scholar] [CrossRef] [PubMed]
- Bader, J.D.; Shugars, D.A.; White, B.A.; Rindal, D.B. Evaluation of Audit-Based Performance Measures for Dental Care Plans. J. Public Health Dent. 1999, 59, 150–157. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Wong, N.S.M. The Historical Roots of Visual Analog Scale in Psychology as Revealed by Reference Publication Year Spectroscopy. Front. Hum. Neurosci. 2019, 13, 86. [Google Scholar] [CrossRef]
- Slade, G.; Spencer, A. Development and Evaluation of the Oral Health Impact Profile. Community Dent. Health 1994, 11, 3–11. [Google Scholar] [PubMed]
- Hassel, A.J.; Rolko, C.; Koke, U.; Leisen, J.; Rammelsberg, P. A German Version of the GOHAI. Community Dent. Oral Epidemiol. 2008, 36, 34–42. [Google Scholar] [CrossRef]
- Allyson Ross, D.; Ware, J.E. Development of a Dental Satisfaction Questionnaire; The Rand Corporation: Santa Monica, CA, USA, 1982; pp. 298–309. [Google Scholar]
- Elgestad Stjernfeldt, P.; Faxén-Irving, G.; Wårdh, I. Masticatory Ability in Older Individuals: A Qualitative Interview Study. Gerodontology 2021, 38, 199–208. [Google Scholar] [CrossRef]
- Anliker, N.; Molinero-Mourelle, P.; Weijers, M.; Bukvic, H.; Bornstein, M.M.; Schimmel, M. Dental Status and Its Correlation with Polypharmacy and Multimorbidity in a Swiss Nursing Home Population: A Cross-Sectional Study. Clin. Oral Investig. 2023; in press. [Google Scholar] [CrossRef]
- Woda, A.; Hennequin, M.; Peyron, M.A. Mastication in Humans: Finding a Rationale. J. Oral Rehabil. 2011, 38, 781–784. [Google Scholar] [CrossRef] [PubMed]
- Imamura, Y.; Chebib, N.; Ohta, M.; Mojon, P.; Schulte-Eickhoff, R.M.; Schimmel, M.; Graf, C.; Sato, Y.; Müller, F. Masticatory Performance in Oral Function Assessment: Alternative Methods. J. Oral Rehabil. 2023, 50, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.E.L. Fundamentals of Eating and Sensory Perception; Wiley: Hoboken, NJ, USA, 2012; ISBN 9781444330120. [Google Scholar]
- Micheelis, W.; Schiffner, U.; Hoffmann, T.; Reis, U.; Schroeder, E. Vierte Deutsche Mundgesundheitsstudie (DMS IV): Neue Ergebnisse Zu Oralen Erkrankungsprävalenzen, Risikogruppen Und Zum Zahnärztlichen Versorgungsgrad in Deutschland 2005; Institut der Deutschen Zahnärzte: Köln, Germany, 2007; Volume 101, ISBN 3934280943. [Google Scholar]
- Da, D.; Ge, S.; Zhang, H.; Zeng, X. Association between Occlusal Support and Cognitive Impairment in Older Chinese Adults: A Community-Based Study. Front. Aging Neurosci. 2023, 15, 1146335. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.E.; Mohan, J.; Parithimar, K.; Kandasamy, S.; Raju, R.; Champakesan, B. Influence of Dental Prostheses on Cognitive Functioning in Elderly Population: A Systematic Review. J. Pharm. Bioallied Sci. 2021, 13 (Suppl. S1), S788–S794. [Google Scholar] [CrossRef]
- Nakamura, T.; Zou, K.; Shibuya, Y.; Michikawa, M. Oral Dysfunctions and Cognitive Impairment/Dementia. J. Neurosci. Res. 2021, 99, 518–528. [Google Scholar] [CrossRef]
- Ki, S.; Yun, J.; Kim, J.; Lee, Y. Association between Dental Implants and Cognitive Function in Community-Dwelling Older Adults in Korea. J. Prev. Med. Public Health 2019, 52, 333–343. [Google Scholar] [CrossRef]
- Klotz, A.L.; Hassel, A.J.; Schröder, J.; Rammelsberg, P.; Zenthöfer, A. Oral Health-Related Quality of Life and Prosthetic Status of Nursing Home Residents with or without Dementia. Clin. Interv. Aging 2017, 12, 659–665. [Google Scholar] [CrossRef]
- Kamiya, K.; Narita, N.; Iwaki, S. Improved Prefrontal Activity and Chewing Performance as Function of Wearing Denture in Partially Edentulous Elderly Individuals: Functional near-Infrared Spectroscopy Study. PLoS ONE 2016, 11, e0158070. [Google Scholar] [CrossRef]
- Chuhuaicura, P.; Dias, F.J.; Arias, A.; Lezcano, M.F.; Fuentes, R. Mastication as a Protective Factor of the Cognitive Decline in Adults: A Qualitative Systematic Review. Int. Dent. J. 2019, 69, 334–340. [Google Scholar] [CrossRef]
- Ikebe, K.; Matsuda, K.; Morii, K.; Furuya-Yoshinaka, M.; Nokubi, T.; Renner, R.P. Association of Masticatory Performance with Age, Posterior Occlusal Contacts, Occlusal Force, and Salivary Flow in Older Adults. Int. J. Prosthodont. 2006, 19, 475–481. [Google Scholar]
- Kikutani, T.; Tamura, F.; Nishiwaki, K.; Kodama, M.; Suda, M.; Fukui, T.; Takahashi, N.; Yoshida, M.; Akagawa, Y.; Kimura, M. Oral Motor Function and Masticatory Performance in the Community-Dwelling Elderly. Odontology 2009, 97, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Mazari, A.; Heath, M.R.; Prinz, J.F. Contribution of the Cheeks to the Intraoral Manipulation of Food. Dysphagia 2007, 22, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Schimmel, M.; Leemann, B.; Christou, P.; Kiliaridis, S.; Herrmann, F.R.; Müller, F. Quantitative Assessment of Facial Muscle Impairment in Patients with Hemispheric Stroke. J. Oral Rehabil. 2011, 38, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Weijenberg, R.A.F.; Scherder, E.J.A.; Lobbezoo, F. Mastication for the Mind-The Relationship between Mastication and Cognition in Ageing and Dementia. Neurosci. Biobehav. Rev. 2011, 35, 483–497. [Google Scholar] [CrossRef] [PubMed]
- Miura, H.; Yamasaki, K.; Kariyasu, M.; Miura, K.; Sumi, Y. Relationship between Cognitive Function and Mastication in Elderly Females. J. Oral Rehabil. 2003, 30, 808–811. [Google Scholar] [CrossRef]
- Chang, C.C.; Roberts, B.L. Feeding Difficulty in Older Adults with Dementia. J. Clin. Nurs. 2008, 17, 2266–2274. [Google Scholar] [CrossRef]
- Ikeda, M.; Brown, J.; Holland, A.J.; Fukuhara, R.; Hodges, J.R. Changes in Appetite, Food Preference, and Eating Habits in Frontotemporal Dementia and Alzheimer’s Disease. J. Neurol. Neurosurg. Psychiatry 2002, 73, 371–376. [Google Scholar] [CrossRef]
- Newton, J.P.; Yemm, R.; Abel, R.W.; Menhinick, S. Changes in Human Jaw Muscles with Age and Dental State. Gerodontology 1993, 10, 16–22. [Google Scholar] [CrossRef]
- Newton, J.P.; McManus, F.C.; Menhenick, S. Jaw Muscles in Older Overdenture Patients. Gerodontology 2004, 21, 37–42. [Google Scholar] [CrossRef]
- Müller, F.; Duvernay, E.; Loup, A.; Vazquez, L.; Herrmann, F.R.; Schimmel, M. Implant-Supported Mandibular Overdentures in Very Old Adults: A Randomized Controlled Trial. J. Dent. Res. 2013, 92, 446–450. [Google Scholar] [CrossRef]
- Müller, F.; Nitschke, I. Mundgesundheit, Zahnstatus Und Ernährung Im Alter. Z. Gerontol. Geriatr. 2005, 38, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Dormenval, V.; Budtz-Jørgensen, E.; Mojon, P.; Bruyère, A.; Rapin, C.H. Nutrition, General Health Status and Oral Health Status in Hospitalised Elders. Gerodontology 1995, 12, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Nordström, G. The Impact of Socio- Medical Factors and Oral Status on Dietary Intake in the Eighth Decade of Life. Aging Clin. Exp. Res. 1990, 2, 371–385. [Google Scholar] [CrossRef] [PubMed]
- Boumendjel, N.; Herrmann, F.; Girod, V.; Sieber, C.; Rapin, C. Refrigerator Content and Hospital Admission in Old People for Personal Use Only. Not to Be Reproduced without Permission of The Lancet. Lancet 2000, 356, 2000. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Ono, T.; Kon, H.; Sakurai, N.; Kohno, S.; Yoshihara, A.; Miyazaki, H. Ten-Year Longitudinal Study on the State of Dentition and Subjective Masticatory Ability in Community-Dwelling Elderly People. J. Prosthodont. Res. 2016, 60, 177–184. [Google Scholar] [CrossRef]
- Schimmel, M.; Leemann, B.; Schnider, A.; Herrmann, F.R.; Kiliaridis, S.; Müller, F. Changes in Oro-Facial Function and Hand-Grip Strength during a 2-Year Observation Period after Stroke. Clin. Oral Investig. 2013, 17, 867–876. [Google Scholar] [CrossRef]
- Gonçalves, T.M.S.V.; Schimmel, M.; van der Bilt, A.; Chen, J.; van der Glas, H.W.; Kohyama, K.; Hennequin, M.; Peyron, M.A.; Woda, A.; Leles, C.R.; et al. Consensus on the Terminologies and Methodologies for Masticatory Assessment. J. Oral Rehabil. 2021, 48, 745–761. [Google Scholar] [CrossRef]
- Peyron, M.A.; Blanc, O.; Lund, J.P.; Woda, A. Influence of Age on Adaptability of Human Mastication. J. Neurophysiol. 2004, 92, 773–779. [Google Scholar] [CrossRef]
- Manly, R.S.; Braley, L.C. Masticatory Performance and Efficiency. J. Dent. Res. 1950, 29, 448–462. [Google Scholar] [CrossRef]
- Rosin, P.; Rammler, E. The Rosin-Rammler Particle Size Distribution. J. Inst. Fuel 1933, 5, 275–277. [Google Scholar]
- Liedberg, B.; Öwall, B. Masticatory Ability in Experimentally Induced Xerostomia. Dysphagia 1991, 6, 211–213. [Google Scholar] [CrossRef] [PubMed]
- Prinz, J.F. Quantitative Evaluation of the Effect of Bolus Size and Number of Chewing Strokes on the Intra-Oral Mixing of a Two-Colour Chewing Gum. J. Oral Rehabil. 1999, 26, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Schimmel, M.; Christou, P.; Herrmann, F.; Müller, F. A Two-Colour Chewing Gum Test for Masticatory Efficiency: Development of Different Assessment Methods. J. Oral Rehabil. 2007, 34, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Speksnijder, C.M.; Abbink, J.H.; Van Der Glas, H.W.; Janssen, N.G.; Van Der Bilt, A. Mixing Ability Test Compared with a Comminution Test in Persons with Normal and Compromised Masticatory Performance. Eur. J. Oral Sci. 2009, 117, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Van Der Bilt, A.; Mojet, J.; Tekamp, F.A.; Abbink, J.H. Comparing Masticatory Performance and Mixing Ability. J. Oral Rehabil. 2010, 37, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Schimmel, M.; Leemann, B.; Christou, P.; Kiliaridis, S.; Schnider, A.; Herrmann, F.R.; Müller, F. Oral Health-Related Quality of Life in Hospitalised Stroke Patients. Gerodontology 2011, 28, 3–11. [Google Scholar] [CrossRef]
- Viewgum. Available online: www.dhal.com (accessed on 23 April 2023).
- Halazonetis, D.J.; Schimmel, M.; Antonarakis, G.S.; Christou, P. Novel Software for Quantitative Evaluation and Graphical Representation of Masticatory Efficiency. J. Oral Rehabil. 2013, 40, 329–335. [Google Scholar] [CrossRef]
- Kaya, M.S.; Güçlü, B.; Schimmel, M.; Akyüz, S. Two-Colour Chewing Gum Mixing Ability Test for Evaluating Masticatory Performance in Children with Mixed Dentition: Validity and Reliability Study. J. Oral Rehabil. 2017, 44, 827–834. [Google Scholar] [CrossRef]
- Schimmel, M.; Katsoulis, J.; Genton, L.; Müller, F.; Bern, C.; Kosaka, T.; Kida, M.; Kikui, M.; Hashimoto, S.; Fujii, K.; et al. Masticatory Function and Nutrition in Old Age. JDR Clin. Transl. Res. 2018, 3, 449–454. [Google Scholar]
- Buser, R.; Ziltener, V.; Samietz, S.; Fontolliet, M.; Nef, T.; Schimmel, M. Validation of a Purpose-Built Chewing Gum and Smartphone Application to Evaluate Chewing Efficiency. J. Oral Rehabil. 2018, 45, 845–853. [Google Scholar] [CrossRef]
- Heath, M.R. The Effect of Maximum Biting Force and Bone Loss upon Masticatory Function and Dietary Selection of the Elderly. Int. Dent. J. 1982, 32, 345–356. [Google Scholar] [PubMed]
- Wöstmann, B.; Brinkert, B.; Melchheier, A.; Zenginel, M.R.P. Chewing Efficiency Screening Test for Non-Dental-Professionals. J. Dent. Res. 2011, 90, 1598. [Google Scholar]
- Schimmel, M.; Rachais, E.; Al-Haj Husain, N.; Müller, F.; Srinivasan, M.; Abou-Ayash, S. Assessing Masticatory Performance with a Colour-Mixing Ability Test Using Smartphone Camera Images. J. Oral Rehabil. 2022, 49, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Liedberg, B.; Öwall, B. Oral Bolus Kneading and Shaping Measured with Chewing Gum. Dysphagia 1995, 10, 101–106. [Google Scholar] [CrossRef]
- Takeshima, T.; Fujita, Y.; Maki, K. Factors Associated with Masticatory Performance and Swallowing Threshold According to Dental Formula Development. Arch. Oral Biol. 2019, 99, 51–57. [Google Scholar] [CrossRef]
- TMJ Disability Index. Available online: http://www.nppt.com/files/forms/tmj-03-2016.pdf (accessed on 23 April 2023).
- Moynihan, P.; Chu, M.; Moores, C.; Ibrahim, A. Eating-Related Quality of Life in People Who Wear Complete Dentures. Proc. Nutr. Soc. 2023, 82, 2023. [Google Scholar] [CrossRef]
- Moynihan, P.; Varghese, R. Impact of Wearing Dentures on Dietary Intake, Nutritional Status, and Eating: A Systematic Review. JDR Clin. Transl. Res. 2022, 7, 334–351. [Google Scholar] [CrossRef]
- de Souza, R.F.; Ribeiro, A.B.; Oates, T.W.; Feine, J.S. The McGill Denture Satisfaction Questionnaire Revisited: Exploratory Factor Analysis of a Binational Sample. Gerodontology 2020, 37, 233–243. [Google Scholar] [CrossRef]
- McKenna, G.; Allen, P.F.; O’Mahony, D.; Flynn, A.; Cronin, M.; Damata, C.; Woods, N. Comparison of Functionally Orientated Tooth Replacement and Removable Partial Dentures on the Nutritional Status of Partially Dentate Older Patients: A Randomised Controlled Clinical Trial. J. Dent. 2014, 42, 653–659. [Google Scholar] [CrossRef]
- Bousiou, A.; Konstantopoulou, K.; Martimianaki, G.; Peppa, E.; Trichopoulou, A.; Polychronopoulou, A.; Halazonetis, D.J.; Schimmel, M.; Kossioni, A.E. Oral Factors and Adherence to Mediterranean Diet in an Older Greek Population. Aging Clin. Exp. Res. 2021, 33, 3237–3244. [Google Scholar] [CrossRef]
- Heckmann, S.M.; Heußinger, S.; Linke, J.J.; Graef, F.; Pröschel, P. Improvement and Long-Term Stability of Neuromuscular Adaptation in Implant-Supported Overdentures. Clin. Oral Implants Res. 2009, 20, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.S. Functional Adaptation of Oromotor Functions and Aging: A Focused Review of the Evidence From Brain Neuroimaging Research. Front. Aging Neurosci. 2020, 11, 354. [Google Scholar] [CrossRef] [PubMed]
- Lindquist, T.J.; Ettinger, R.L. Patient Management and Decision Making in Complete Denture Fabrication Using a Duplicate Denture Procedure: A Clinical Report. J. Prosthet. Dent. 1999, 82, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Daher, T.; Dermendjian, S.; Morgano, S.M. Obtaining Maxillomandibular Records and Definitive Impressions in a Single Visit for a Completely Edentulous Patient with a History of Combination Syndrome. J. Prosthet. Dent. 2008, 99, 489–491. [Google Scholar] [CrossRef] [PubMed]
- Utz, K.H.; Müller, F.; Kettner, N.; Reppert, G.; Koeck, B. Functional Impression and Jaw Registration: A Single Session Procedure for the Construction of Complete Dentures. J. Oral Rehabil. 2004, 31, 554–561. [Google Scholar] [CrossRef]
- Schimmel, M.; Leuchter, I.; Héritier Barras, A.C.; Leles, C.R.; Abou-Ayash, S.; Viatte, V.; Esteve, F.; Janssens, J.P.; Mueller, F.; Genton, L. Oral Function in Amyotrophic Lateral Sclerosis Patients: A Matched Case–Control Study. Clin. Nutr. 2021, 40, 4904–4911. [Google Scholar] [CrossRef]
| Index | Methodology |
|---|---|
| TMJ Disability Index (TDI) [84] | Various items relate to difficulties in chewing certain examples of food items with varying consistencies. Hence, the TDI might be applicable to older adults with various cultural backgrounds. |
| Open or semi-structured interviews [85] | Individual evaluation of oral health and function, possible compensatory mechanisms, eating habits, and further adaptation processes. i.e., self-reported chewing difficulties, food avoidance, |
| Eating Related Quality of Life ERQoL [85,86] | Enjoyment of eating and social and emotional issues around eating, eating comfort |
| Denture satisfaction index [87] | VAS-scale-based instrument with certain items relating to chewing ability |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schimmel, M.; Anliker, N.; Sabatini, G.P.; De Paula, M.S.; Weber, A.R.; Molinero-Mourelle, P. Assessment and Improvement of Masticatory Performance in Frail Older People: A Narrative Review. J. Clin. Med. 2023, 12, 3760. https://doi.org/10.3390/jcm12113760
Schimmel M, Anliker N, Sabatini GP, De Paula MS, Weber AR, Molinero-Mourelle P. Assessment and Improvement of Masticatory Performance in Frail Older People: A Narrative Review. Journal of Clinical Medicine. 2023; 12(11):3760. https://doi.org/10.3390/jcm12113760
Chicago/Turabian StyleSchimmel, Martin, Noemi Anliker, Gabriela Panca Sabatini, Marcella Silva De Paula, Adrian Roman Weber, and Pedro Molinero-Mourelle. 2023. "Assessment and Improvement of Masticatory Performance in Frail Older People: A Narrative Review" Journal of Clinical Medicine 12, no. 11: 3760. https://doi.org/10.3390/jcm12113760
APA StyleSchimmel, M., Anliker, N., Sabatini, G. P., De Paula, M. S., Weber, A. R., & Molinero-Mourelle, P. (2023). Assessment and Improvement of Masticatory Performance in Frail Older People: A Narrative Review. Journal of Clinical Medicine, 12(11), 3760. https://doi.org/10.3390/jcm12113760







