Endoleak Detection after Endovascular Aortic Repair via Coded-Excitation Ultrasound—A Feasibility Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Recruitment and Study Design
2.2. Computed Tomography
2.3. Ultrasound Examinations
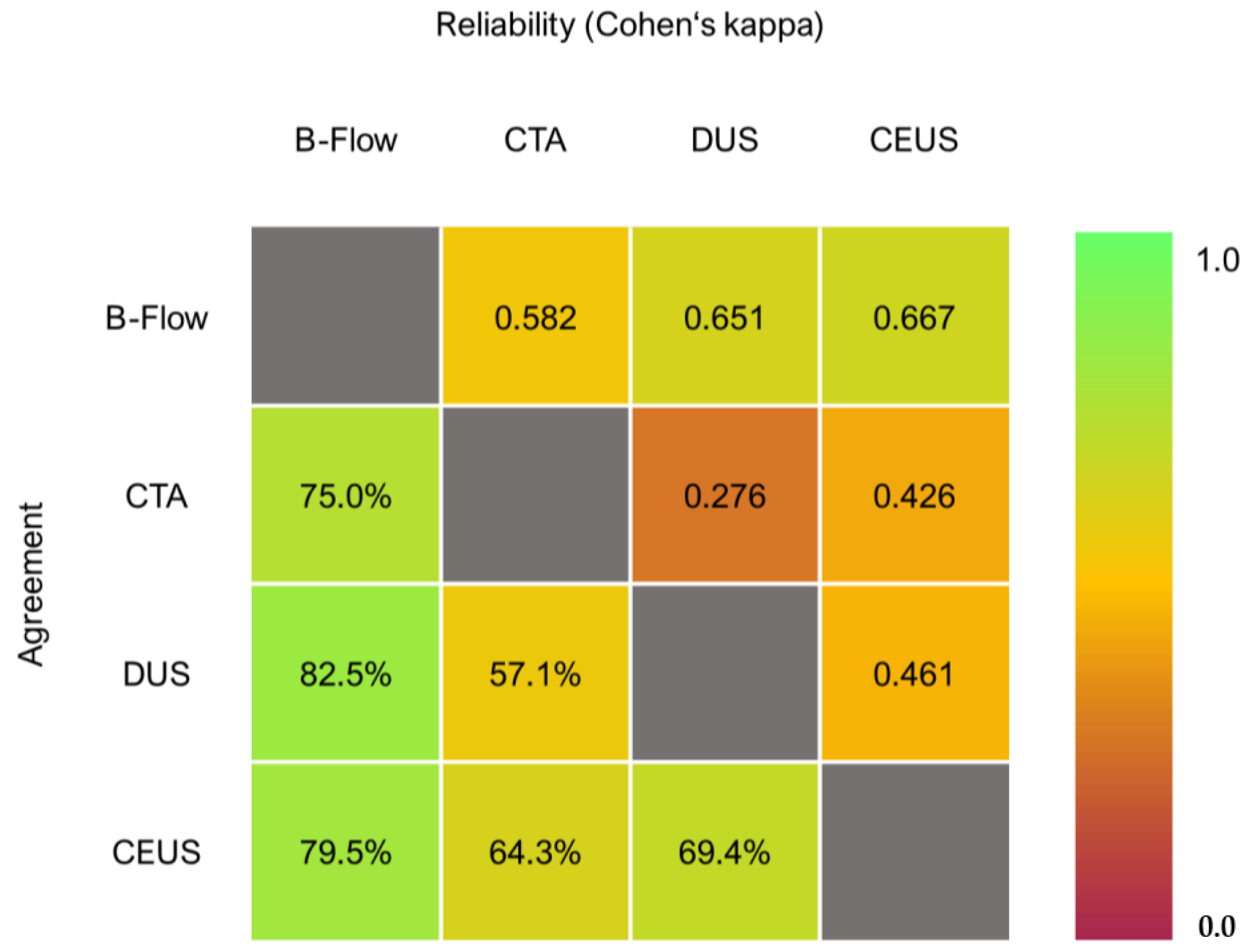
2.4. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Aneurysm Diameter
3.3. Endoleak Detection
3.4. Endoleak Classification
3.5. Sensitivity Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ivancev, K.; Vogelzang, R. A 35 Year History of Stent Grafting, and How EVAR Conquered the World. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.O.; Yim, N.Y.; Kim, J.K.; Kang, Y.J.; Lee, B.C. Endovascular Aneurysm Repair for Abdominal Aortic Aneurysm: A Comprehensive Review. Korean J. Radiol. 2019, 20, 1247–1265. [Google Scholar] [CrossRef]
- Burgers, L.T.; Vahl, A.C.; Severens, J.L.; Wiersema, A.M.; Cuypers, P.W.M.; Verhagen, H.J.M.; Redekop, W.K. Cost-effectiveness of Elective Endovascular Aneurysm Repair Versus Open Surgical Repair of Abdominal Aortic Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2016, 52, 29–40. [Google Scholar] [CrossRef]
- AlOthman, O.; Bobat, S. Comparison of the Short and Long-Term Outcomes of Endovascular Repair and Open Surgical Repair in the Treatment of Unruptured Abdominal Aortic Aneurysms: Meta-Analysis and Systematic Review. Cureus 2020, 12, e9683. [Google Scholar] [CrossRef]
- Wanhainen, A.; Verzini, F.; Van Herzeele, I.; Allaire, E.; Bown, M.; Cohnert, T.; Dick, F.; van Herwaarden, J.; Karkos, C.; Koelemay, M.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 8–93. [Google Scholar] [CrossRef]
- Sweeting, M.J.; Balm, R.; Desgranges, P.; Ulug, P.; Powell, J.T.; Ruptured Aneurysm Trialists. Individual-patient meta-analysis of three randomized trials comparing endovascular versus open repair for ruptured abdominal aortic aneurysm. Br. J. Surg. 2015, 102, 1229–1239. [Google Scholar] [CrossRef]
- IMPROVE Trial Investigators. Comparative clinical effectiveness and cost effectiveness of endovascular strategy v open repair for ruptured abdominal aortic aneurysm: Three year results of the IMPROVE randomised trial. BMJ 2017, 359, j4859. [Google Scholar]
- Daye, D.; Walker, T.G. Complications of endovascular aneurysm repair of the thoracic and abdominal aorta: Evaluation and management. Cardiovasc. Diagn. Ther. 2018, 8, S138–S156. [Google Scholar] [CrossRef]
- Schlösser, F.J.V.; Muhs, B.E. Endoleaks after endovascular abdominal aortic aneurysm repair: What one needs to know. Curr. Opin. Cardiol. 2012, 27, 598–603. [Google Scholar] [CrossRef]
- Cao, P.; De Rango, P.; Verzini, F.; Parlani, G. Endoleak after endovascular aortic repair: Classification, diagnosis and management following endovascular thoracic and abdominal aortic repair. J. Cardiovasc. Surg. 2010, 51, 53–69. [Google Scholar]
- Schlösser, F.J.V.; Gusberg, R.J.; Dardik, A.; Lin, P.H.; Verhagen, H.J.M.; Moll, F.L.; Muhs, B.E. Aneurysm Rupture after EVAR: Can the Ultimate Failure be Predicted? Eur. J. Vasc. Endovasc. Surg. 2009, 37, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Pandey, N.; Litt, H.I. Surveillance imaging following endovascular aneurysm repair. Semin Interv. Radiol. 2015, 32, 239–248. [Google Scholar] [CrossRef]
- Tse, D.M.L.; Tapping, C.R.; Patel, R.; Morgan, R.; Bratby, M.J.; Anthony, S.; Uberoi, R. Surveillance after endovascular abdominal aortic aneurysm repair. Cardiovasc. Interv. Radiol. 2014, 37, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Andreucci, M.; Solomon, R.; Tasanarong, A. Side effects of radiographic contrast media: Pathogenesis, risk factors, and prevention. Biomed. Res. Int. 2014, 2014, 741018. [Google Scholar] [CrossRef] [PubMed]
- Motaganahalli, R.; Martin, A.; Feliciano, B.; Murphy, M.P.; Slaven, J.; Dalsing, M.C. Estimating the risk of solid organ malignancy in patients undergoing routine computed tomography scans after endovascular aneurysm repair. J. Vasc. Surg. 2012, 56, 929–937. [Google Scholar] [CrossRef]
- Ayuso, J.R.; de Caralt, T.M.; Pages, M.; Riambau, V.; Ayuso, C.; Sanchez, M.; Real, M.I.; Montaña, X. MRA is useful as a follow-up technique after endovascular repair of aortic aneurysms with nitinol endoprostheses: MRA After EVAR With Nitinol Stents. J. Magn. Reson. Imaging 2004, 20, 803–810. [Google Scholar] [CrossRef]
- Zaiem, F.; Almasri, J.; Tello, M.; Prokop, L.J.; Chaikof, E.L.; Murad, M.H. A systematic review of surveillance after endovascular aortic repair. J. Vasc. Surg. 2018, 67, 320–331.e37. [Google Scholar] [CrossRef]
- Ashoke, R.; Brown, L.C.; Rodway, A.; Choke, E.; Thompson, M.M.; Greenhalgh, R.M.; Powell, J.T. Color duplex ultrasonography is insensitive for the detection of endoleak after aortic endografting: A systematic review. J. Endovasc. Ther. 2005, 12, 297–305. [Google Scholar] [CrossRef]
- Mirza, T.A.; Karthikesalingam, A.; Jackson, D.; Walsh, S.R.; Holt, P.J.; Hayes, P.D.; Boyle, J.R. Duplex ultrasound and contrast-enhanced ultrasound versus computed tomography for the detection of endoleak after EVAR: Systematic review and bivariate meta-analysis. Eur. J. Vasc. Endovasc. Surg. 2010, 39, 418–428. [Google Scholar] [CrossRef]
- Guo, Q.; Zhao, J.; Huang, B.; Yuan, D.; Yang, Y.; Zeng, G.; Xiong, F.; Du, X. A Systematic Review of Ultrasound or Magnetic Resonance Imaging Compared With Computed Tomography for Endoleak Detection and Aneurysm Diameter Measurement After Endovascular Aneurysm Repair. J. Endovasc. Ther. 2016, 23, 936–943. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Y.; Sun, M.; Wang, Y. Efficacy of contrast-enhanced ultrasound in detection of type II endoleak after abdominal aortic aneurysm surgery: A prospective cohort study. J. Clin. Ultrasound. 2022, 50, 474–479. [Google Scholar] [CrossRef]
- Cantisani, V.; David, E.; Ferrari, D.; Fanelli, F.; Di Marzo, L.; Catalano, C.; Benedetto, F.; Spinelli, D.; Katsargyris, A.; Blandino, A.; et al. Color Doppler Ultrasound with Superb Microvascular Imaging Compared to Contrast-enhanced Ultrasound and Computed Tomography Angiography to Identify and Classify Endoleaks in Patients Undergoing EVAR. Ann. Vasc. Surg. 2017, 40, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, M.; Tomczak, J.; Snoch-Ziółkiewicz, M.; Dzieciuchowicz, Ł.; Strauss, E.; Pawlaczyk, K.; Wojtusik, D.; Oszkinis, G. Superb Micro-vascular Imaging (SMI): A Doppler ultrasound technique with potential to identify, classify, and follow up endoleaks in patients after Endovascular Aneurysm Repair (EVAR). Abdom Radiol. 2018, 43, 3479–3486. [Google Scholar] [CrossRef]
- Curti, M.; Piacentino, F.; Fontana, F.; Ossola, C.; Coppola, A.; Marra, P.; Basile, A.; Ierardi, A.M.; Carrafiello, G.; Carcano, G.; et al. EVAR Follow-Up with Ultrasound Superb Microvascular Imaging (SMI) Compared to CEUS and CT Angiography for Detection of Type II Endoleak. Diagnostics 2022, 12, 526. [Google Scholar] [CrossRef]
- O’Donnell, M.; Wang, Y. Coded excitation for synthetic aperture ultrasound imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2005, 52, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Jibiki, T. Coded excitation medical ultrasound imaging. Igaku Butsuri 2001, 21, 136–141. [Google Scholar]
- Hofmann, A.G.; Mlekusch, I.; Wickenhauser, G.; Assadian, A.; Taher, F. Clinical Applications of B-Flow Ultrasound: A Scoping Review of the Literature. Diagnostics 2023, 13, 397. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159. [Google Scholar] [CrossRef] [PubMed]
- van Stralen, K.; Dekker, F.; Zoccali, C.; Jager, K. Measuring agreement, more complicated than it seems. Nephron. Clin. Pract. 2012, 120, c162–c167. [Google Scholar] [CrossRef]
- de Oliveira Guirro, E.C.; Leite, G.D.P.M.F.; Dibai-Filho, A.V.; de Souza Borges, N.C.; de Jesus Guirro, R.R. Intra- and Inter-rater Reliability of Peripheral Arterial Blood Flow Velocity by Means of Doppler Ultrasound. J. Manip. Physiol. Ther. 2017, 40, 236–240. [Google Scholar] [CrossRef]
- Li, J.; Chen, M.; Wang, Z.-J.; Li, S.-G.; Jiang, M.; Shi, L.; Cao, C.-L.; Sang, T.; Cui, X.-W.; Dietrich, C.F. Interobserver agreement for contrast-enhanced ultrasound of liver imaging reporting and data system: A systematic review and meta-analysis. World J. Clin. Cases 2020, 8, 5589–5602. [Google Scholar] [CrossRef]
- Bucek, R.A.; Reiter, M.; Koppensteiner, I.; Ahmadi, R.; Minar, E.; Lammer, J. B-flow evaluation of carotid arterial stenosis: Initial experience. Radiology 2002, 225, 295–299. [Google Scholar] [CrossRef]
- Karthikesalingam, A.; Al-Jundi, W.; Jackson, D.; Boyle, J.R.; Beard, J.D.; Holt, P.; Thompson, M.M. Systematic review and meta-analysis of duplex ultrasonography, contrast-enhanced ultrasonography or computed tomography for surveillance after endovascular aneurysm repair. Br. J. Surg. 2012, 99, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Bredahl, K.K.; Taudorf, M.; Lönn, L.; Vogt, K.C.; Sillesen, H.; Eiberg, J.P. Contrast Enhanced Ultrasound can Replace Computed Tomography Angiography for Surveillance After Endovascular Aortic Aneurysm Repair. Eur. J. Vasc. Endovasc. Surg. 2016, 52, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Kapetanios, D.; Kontopodis, N.; Mavridis, D.; McWilliams, R.G.; Giannoukas, A.D.; Antoniou, G.A. Meta-analysis of the accuracy of contrast-enhanced ultrasound for the detection of endoleak after endovascular aneurysm repair. J. Vasc. Surg. 2019, 69, 280–294.e6. [Google Scholar] [CrossRef] [PubMed]
- Nagre, S.B.; Taylor, S.M.; Passman, M.A.; Patterson, M.A.; Combs, B.R.; Lowman, B.G.; Jordan, W.D. Evaluating outcomes of endoleak discrepancies between computed tomography scan and ultrasound imaging after endovascular abdominal aneurysm repair. Ann. Vasc. Surg. 2011, 25, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Clevert, D.; Helck, A.; D’Anastasi, M.; Gürtler, V.; Sommer, W.; Meimarakis, G.; Weidenhagen, R.; Reiser, M. Improving the follow up after EVAR by using ultrasound image fusion of CEUS and MS-CT. Clin. Hemorheol. Microcirc. 2011, 49, 91–104. [Google Scholar] [CrossRef] [PubMed]




| Baseline Characteristics | |
|---|---|
| N | 34 |
| Age at surgery (in years) | 78.1 (73.8–78.3) |
| Sex (female:male) | 3 (8.8%):31 (91.2%) |
| BMI (at surgery) | 27.7 (24.2–31.4) |
| Follow-up appointments | 43 |
| Imaging studies | 132 |
| Graft type | |
| EVAR | 29 |
| BEVAR | 2 |
| FEVAR after failed EVAR | 3 |
| Follow-Ups | |
|---|---|
| B-Flow | |
| Examinations | 43 |
| With Endoleaks | 19 |
| CTA | |
| Examinations | 18 |
| With Endoleaks | 12 |
| DUS | |
| Examinations | 40 |
| With Endoleaks | 11 |
| CEUS | |
| Examinations | 41 |
| With Endoleaks | 22 |
| B-Flow | CEUS | CTA | Angiography | |
|---|---|---|---|---|
| Case 1 | Type II | Type II | Type II | Type II |
| Case 2 | Type II | Type II | - | Type II |
| Case 3 | Type II | - | - | Type II |
| Case 4 | Type II | Type II | - | Type II |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hofmann, A.G.; Klosz, F.R.; Mlekusch, I.; Wickenhauser, G.; Walter, C.; Assadian, A.; Taher, F. Endoleak Detection after Endovascular Aortic Repair via Coded-Excitation Ultrasound—A Feasibility Study. J. Clin. Med. 2023, 12, 3775. https://doi.org/10.3390/jcm12113775
Hofmann AG, Klosz FR, Mlekusch I, Wickenhauser G, Walter C, Assadian A, Taher F. Endoleak Detection after Endovascular Aortic Repair via Coded-Excitation Ultrasound—A Feasibility Study. Journal of Clinical Medicine. 2023; 12(11):3775. https://doi.org/10.3390/jcm12113775
Chicago/Turabian StyleHofmann, Amun G., Fabian R. Klosz, Irene Mlekusch, Georg Wickenhauser, Corinna Walter, Afshin Assadian, and Fadi Taher. 2023. "Endoleak Detection after Endovascular Aortic Repair via Coded-Excitation Ultrasound—A Feasibility Study" Journal of Clinical Medicine 12, no. 11: 3775. https://doi.org/10.3390/jcm12113775
APA StyleHofmann, A. G., Klosz, F. R., Mlekusch, I., Wickenhauser, G., Walter, C., Assadian, A., & Taher, F. (2023). Endoleak Detection after Endovascular Aortic Repair via Coded-Excitation Ultrasound—A Feasibility Study. Journal of Clinical Medicine, 12(11), 3775. https://doi.org/10.3390/jcm12113775







