The Non-Invasive Diagnosis of Chronic Coronary Syndrome: A Focus on Stress Computed Tomography Perfusion and Stress Cardiac Magnetic Resonance
Abstract
:1. Introduction
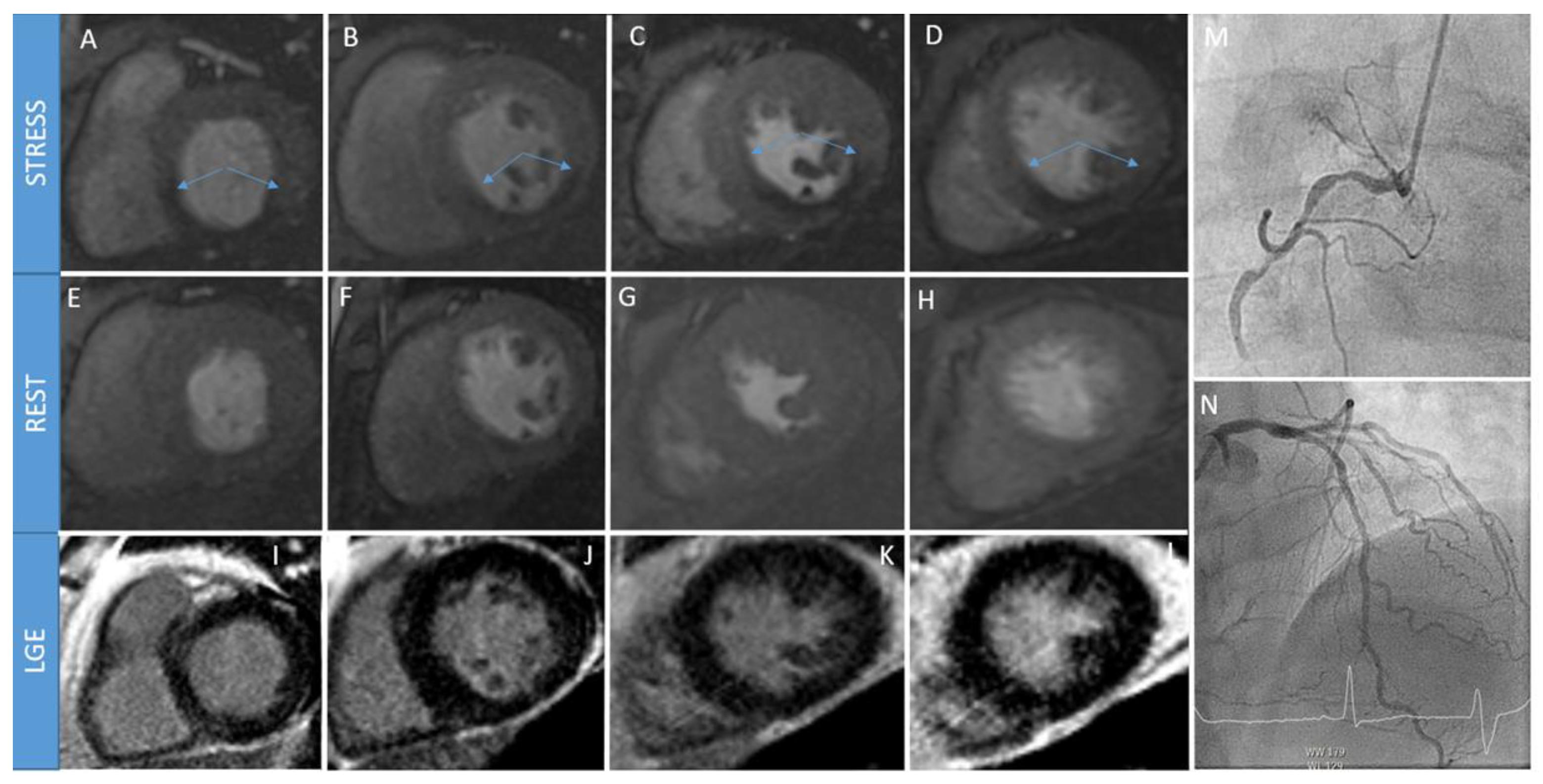
2. Stress Cardiac Magnetic Resonance
2.1. Why S-CMR
2.2. How S-CMR
- uptake model: this model measures myocardial blood flow by analyzing the uptake of a contrast agent in the myocardium. It involves calculating the rate of contrast agent accumulation in the tissue.
- one-compartment model: this model assumes that the myocardium can be represented as a single compartment. It estimates myocardial blood flow by analyzing the contrast agent concentration over time within this compartment.
- Fermi model: the Fermi model is a mathematical model used to analyze the contrast agent concentration data in the myocardium. It incorporates both the arterial input function and the tissue residue function to estimate myocardial blood flow.
- deconvolution method: this method involves deconvolving the contrast agent concentration curve in the myocardium with the arterial input function. It aims to extract the impulse response function, which represents the transfer of the contrast agent through the tissue, thus allowing for the estimation of myocardial blood flow.
2.3. When S-CMR
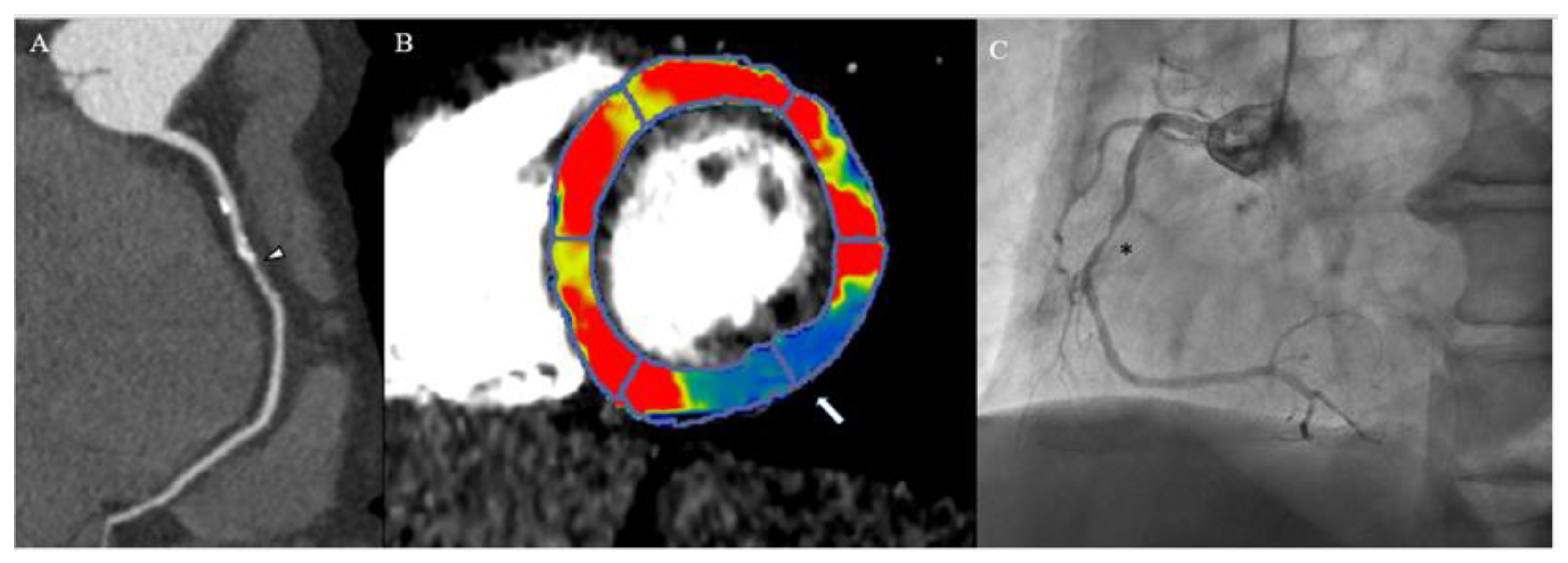
3. Myocardial Computed Tomography Perfusion
3.1. Why CTP
3.2. How CTP
3.3. When CTP
4. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malakar, A.K.; Choudhury, D.; Halder, B.; Paul, P.; Uddin, A.; Chakraborty, S. A review on coronary artery disease, its risk factors, and therapeutics. J. Cell. Physiol. 2019, 234, 16812–16823. [Google Scholar] [CrossRef] [PubMed]
- Pezel, T.; Silva, L.M.; Bau, A.A.; Teixiera, A.; Jerosch-Herold, M.; Coelho-Filho, O.R. What Is the Clinical Impact of Stress CMR After the ISCHEMIA Trial? Front. Cardiovasc. Med. 2021, 8, 683434. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Ballo, H.; Juarez-Orozco, L.E.; Saraste, A.; Kolh, P.; Rutjes, A.W.S.; Jüni, P.; Windecker, S.; Bax, J.J.; Wijns, W. The performance of non-invasive tests to rule-in and rule-out significant coronary artery stenosis in patients with stable angina: A meta-analysis focused on post-test disease probability. Eur. Heart J. 2018, 39, 3322–3330. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, S.; Conte, E.; Pontone, G.; Baggiano, A.; Annoni, A.; Formenti, A.; Mancini, M.E.; Guglielmo, M.; Muscogiuri, G.; Tanzilli, A.; et al. State-of-the-art-myocardial perfusion stress testing: Static CT perfusion. J. Cardiovasc. Comput. Tomogr. 2020, 14, 294–302. [Google Scholar] [CrossRef]
- Pontone, G.; Baggiano, A.; Andreini, D.; Guaricci, A.I.; Guglielmo, M.; Muscogiuri, G.; Fusini, L.; Fazzari, F.; Mushtaq, S.; Conte, E.; et al. Stress Computed Tomography Perfusion Versus Fractional Flow Reserve CT Derived in Suspected Coronary Artery Disease: The PERFECTION Study. JACC Cardiovasc. Imaging 2019, 12, 1487–1497. [Google Scholar] [CrossRef]
- Patel, A.R.; Salerno, M.; Kwong, R.Y.; Singh, A.; Heydari, B.; Kramer, C.M. Stress Cardiac Magnetic Resonance Myocardial Perfusion Imaging: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 78, 1655–1668. [Google Scholar] [CrossRef]
- Bruder, O.; Wagner, A.; Lombardi, M.; Schwitter, J.; van Rossum, A.; Pilz, G.; Nothnagel, D.; Steen, H.; Petersen, S.; Nagel, E.; et al. European Cardiovascular Magnetic Resonance (EuroCMR) registry—multi national results from 57 centers in 15 countries. J. Cardiovasc. Magn. Reson. 2013, 15, 9. [Google Scholar] [CrossRef]
- Schwitter, J.; Wacker, C.M.; van Rossum, A.C.; Lombardi, M.; Al-Saadi, N.; Ahlstrom, H.; Dill, T.; Larsson, H.B.; Flamm, S.D.; Marquardt, M.; et al. MR-IMPACT: Comparison of perfusion-cardiac magnetic resonance with single-photon emission computed tomography for the detection of coronary artery disease in a multicentre, multivendor, randomized trial. Eur. Heart J. 2008, 29, 480–489. [Google Scholar] [CrossRef]
- Schwitter, J.; Wacker, C.M.; Wilke, N.; Al-Saadi, N.; Sauer, E.; Huettle, K.; Schönberg, S.O.; Luchner, A.; Strohm, O.; Ahlstrom, H.; et al. MR-IMPACT II: Magnetic Resonance Imaging for Myocardial Perfusion Assessment in Coronary artery disease Trial: Perfusion-cardiac magnetic resonance vs. single-photon emission computed tomography for the detection of coronary artery disease: A comparative multicentre, multivendor trial. Eur. Heart J. 2013, 34, 775–781. [Google Scholar] [CrossRef]
- Greenwood, J.P.; Maredia, N.; Younger, J.F.; Brown, J.M.; Nixon, J.; Everett, C.C.; Bijsterveld, P.; Ridgway, J.P.; Radjenovic, A.; Dickinson, C.J.; et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): A prospective trial. Lancet 2012, 379, 453–460. [Google Scholar] [CrossRef]
- Greenwood, J.P.; Ripley, D.P.; Berry, C.; McCann, G.P.; Plein, S.; Bucciarelli-Ducci, C.; Dall’Armellina, E.; Prasad, A.; Bijsterveld, P.; Foley, J.R.; et al. Effect of Care Guided by Cardiovascular Magnetic Resonance, Myocardial Perfusion Scintigraphy, or NICE Guidelines on Subsequent Unnecessary Angiography Rates: The CE-MARC 2 Randomized Clinical Trial. JAMA 2016, 316, 1051–1060. [Google Scholar] [CrossRef]
- Arai, A.E.; Schulz-Menger, J.; Berman, D.; Mahrholdt, H.; Han, Y.; Bandettini, W.P.; Gutberlet, M.; Abraham, A.; Woodard, P.K.; Selvanayagam, J.B.; et al. Gadobutrol-Enhanced Cardiac Magnetic Resonance Imaging for Detection of Coronary Artery Disease. J. Am. Coll. Cardiol. 2020, 76, 1536–1547. [Google Scholar] [CrossRef]
- Danad, I.; Szymonifka, J.; Twisk, J.W.R.; Norgaard, B.L.; Zarins, C.K.; Knaapen, P.; Min, J.K. Diagnostic performance of cardiac imaging methods to diagnose ischaemia-causing coronary artery disease when directly compared with fractional flow reserve as a reference standard: A meta-analysis. Eur. Heart J. 2017, 38, 991–998. [Google Scholar] [CrossRef]
- Pontone, G.; Guaricci, A.I.; Palmer, S.C.; Andreini, D.; Verdecchia, M.; Fusini, L.; Lorenzoni, V.; Guglielmo, M.; Muscogiuri, G.; Baggiano, A.; et al. Diagnostic performance of non-invasive imaging for stable coronary artery disease: A meta-analysis. Int. J. Cardiol. 2020, 300, 276–281. [Google Scholar] [CrossRef]
- Kwong, R.Y.; Ge, Y.; Steel, K.; Bingham, S.; Abdullah, S.; Fujikura, K.; Wang, W.; Pandya, A.; Chen, Y.Y.; Mikolich, J.R.; et al. Cardiac Magnetic Resonance Stress Perfusion Imaging for Evaluation of Patients with Chest Pain. J. Am. Coll. Cardiol. 2019, 74, 1741–1755. [Google Scholar] [CrossRef]
- Pezel, T.; Hovasse, T.; Lefèvre, T.; Sanguineti, F.; Unterseeh, T.; Champagne, S.; Benamer, H.; Neylon, A.; Toupin, S.; Garot, P.; et al. Prognostic Value of Stress CMR in Symptomatic Patients with Coronary Stenosis on CCTA. JACC Cardiovasc. Imaging 2022, 15, 1408–1422. [Google Scholar] [CrossRef]
- Heilmaier, C.; Bruder, O.; Meier, F.; Jochims, M.; Forsting, M.; Sabin, G.V.; Barkhausen, J.; Schlosser, T.W. Dobutamine stress cardiovascular magnetic resonance imaging in patients after invasive coronary revascularization with stent placement. Acta Radiol. 2009, 50, 1134–1141. [Google Scholar] [CrossRef]
- Nanni, S.; Lovato, L.; Ghetti, G.; Vagnarelli, F.; Mineo, G.; Fattori, R.; Saia, F.; Marzocchi, A.; Marrozzini, C.; Zompatori, M.; et al. Utility of stress perfusion-cardiac magnetic resonance in follow-up of patients undergoing percutaneous coronary interventions of the left main coronary artery. Int. J. Cardiovasc. Imaging 2017, 33, 1589–1597. [Google Scholar] [CrossRef]
- Leiner, T.; Bogaert, J.; Friedrich, M.G.; Mohiaddin, R.; Muthurangu, V.; Myerson, S.; Powell, A.J.; Raman, S.V.; Pennell, D.J. SCMR Position Paper (2020) on clinical indications for cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2020, 22, 76. [Google Scholar] [CrossRef]
- Kramer, C.M.; Barkhausen, J.; Bucciarelli-Ducci, C.; Flamm, S.D.; Kim, R.J.; Nagel, E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 17. [Google Scholar] [CrossRef] [PubMed]
- Baessato, F.; Guglielmo, M.; Muscogiuri, G.; Baggiano, A.; Fusini, L.; Scafuri, S.; Babbaro, M.; Mollace, R.; Collevecchio, A.; Guaricci, A.I.; et al. Stress CMR in Known or Suspected CAD: Diagnostic and Prognostic Role. Biomed. Res. Int. 2021, 2021, 6678029. [Google Scholar] [CrossRef] [PubMed]
- Baritussio, A.; Scatteia, A.; Dellegrottaglie, S.; Bucciarelli-Ducci, C. Evidence and Applicability of Stress Cardiovascular Magnetic Resonance in Detecting Coronary Artery Disease: State of the Art. J. Clin. Med. 2021, 10, 3279. [Google Scholar] [CrossRef] [PubMed]
- Heiberg, J.; Asschenfeldt, B.; Maagaard, M.; Ringgaard, S. Dynamic bicycle exercise to assess cardiac output at multiple exercise levels during magnetic resonance imaging. Clin. Imaging 2017, 46, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Craven, T.P.; Jex, N.; Chew, P.G.; Higgins, D.M.; Bissell, M.M.; Brown, L.A.E.; Saunderson, C.E.D.; Das, A.; Chowdhary, A.; Dall’Armellina, E.; et al. Exercise cardiovascular magnetic resonance: Feasibility and development of biventricular function and great vessel flow assessment, during continuous exercise accelerated by Compressed SENSE: Preliminary results in healthy volunteers. Int. J. Cardiovasc. Imaging 2021, 37, 685–698. [Google Scholar] [CrossRef]
- Schulz-Menger, J.; Bluemke, D.A.; Bremerich, J.; Flamm, S.D.; Fogel, M.A.; Friedrich, M.G.; Kim, R.J.; von Knobelsdorff-Brenkenhoff, F.; Kramer, C.M.; Pennell, D.J.; et al. Standardized image interpretation and post-processing in cardiovascular magnetic resonance–2020 update: Society for Cardiovascular Magnetic Resonance (SCMR): Board of Trustees Task Force on Standardized Post-Processing. J. Cardiovasc. Magn. Reson. 2020, 22, 19. [Google Scholar] [CrossRef]
- Patel, A.R.; Antkowiak, P.F.; Nandalur, K.R.; West, A.M.; Salerno, M.; Arora, V.; Christopher, J.; Epstein, F.H.; Kramer, C.M. Assessment of advanced coronary artery disease: Advantages of quantitative cardiac magnetic resonance perfusion analysis. J. Am. Coll. Cardiol. 2010, 56, 561–569. [Google Scholar] [CrossRef]
- Kotecha, T.; Martinez-Naharro, A.; Boldrini, M.; Knight, D.; Hawkins, P.; Kalra, S.; Patel, D.; Coghlan, G.; Moon, J.; Plein, S.; et al. Automated Pixel-Wise Quantitative Myocardial Perfusion Mapping by CMR to Detect Obstructive Coronary Artery Disease and Coronary Microvascular Dysfunction: Validation Against Invasive Coronary Physiology. JACC Cardiovasc. Imaging 2019, 12, 1958–1969. [Google Scholar] [CrossRef]
- Kotecha, T.; Chacko, L.; Chehab, O.; O’Reilly, N.; Martinez-Naharro, A.; Lazari, J.; Knott, K.D.; Brown, J.; Knight, D.; Muthurangu, V.; et al. Assessment of Multivessel Coronary Artery Disease Using Cardiovascular Magnetic Resonance Pixelwise Quantitative Perfusion Mapping. JACC Cardiovasc. Imaging 2020, 13, 2546–2557. [Google Scholar] [CrossRef]
- Sammut, E.C.; Villa, A.D.M.; Di Giovine, G.; Dancy, L.; Bosio, F.; Gibbs, T.; Jeyabraba, S.; Schwenke, S.; Williams, S.E.; Marber, M.; et al. Prognostic Value of Quantitative Stress Perfusion Cardiac Magnetic Resonance. JACC Cardiovasc. Imaging 2018, 11, 686–694. [Google Scholar] [CrossRef]
- Pepine, C.J.; Anderson, R.D.; Sharaf, B.L.; Reis, S.E.; Smith, K.M.; Handberg, E.M.; Johnson, B.D.; Sopko, G.; Bairey Merz, C.N. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J. Am. Coll. Cardiol. 2010, 55, 2825–2832. [Google Scholar] [CrossRef]
- Liu, A.; Wijesurendra, R.S.; Liu, J.M.; Forfar, J.C.; Channon, K.M.; Jerosch-Herold, M.; Piechnik, S.K.; Neubauer, S.; Kharbanda, R.K.; Ferreira, V.M. Diagnosis of Microvascular Angina Using Cardiac Magnetic Resonance. J. Am. Coll. Cardiol. 2018, 71, 969–979. [Google Scholar] [CrossRef]
- Zhou, W.; Lee, J.C.Y.; Leung, S.T.; Lai, A.; Lee, T.F.; Chiang, J.B.; Cheng, Y.W.; Chan, H.L.; Yiu, K.H.; Goh, V.K.; et al. Long-Term Prognosis of Patients With Coronary Microvascular Disease Using Stress Perfusion Cardiac Magnetic Resonance. JACC Cardiovasc. Imaging 2021, 14, 602–611. [Google Scholar] [CrossRef]
- Nissen, L.; Winther, S.; Westra, J.; Ejlersen, J.A.; Isaksen, C.; Rossi, A.; Holm, N.R.; Urbonaviciene, G.; Gormsen, L.C.; Madsen, L.H.; et al. Diagnosing coronary artery disease after a positive coronary computed tomography angiography: The Dan-NICAD open label, parallel, head to head, randomized controlled diagnostic accuracy trial of cardiovascular magnetic resonance and myocardial perfusion scintigraphy. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 369–377. [Google Scholar] [CrossRef]
- Montalescot, G.; Sechtem, U.; Achenbach, S.; Andreotti, F.; Arden, C.; Budaj, A.; Bugiardini, R.; Crea, F.; Cuisset, T.; Di Mario, C.; et al. 2013 ESC guidelines on the management of stable coronary artery disease: The Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur. Heart J. 2013, 34, 2949–3003. [Google Scholar] [CrossRef]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2022, 24, 4–131. [Google Scholar] [CrossRef]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Neglia, D.; Liga, R.; Caselli, C.; Carpeggiani, C.; Lorenzoni, V.; Sicari, R.; Lombardi, M.; Gaemperli, O.; Kaufmann, P.A.; Scholte, A.; et al. Anatomical and functional coronary imaging to predict long-term outcome in patients with suspected coronary artery disease: The EVINCI-outcome study. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 1273–1282. [Google Scholar] [CrossRef]
- Lorenzoni, V.; Bellelli, S.; Caselli, C.; Knuuti, J.; Underwood, S.R.; Neglia, D.; Turchetti, G. Cost-effectiveness analysis of stand-alone or combined non-invasive imaging tests for the diagnosis of stable coronary artery disease: Results from the EVINCI study. Eur. J. Health Econ. 2019, 20, 1437–1449. [Google Scholar] [CrossRef]
- Cury, R.C.; Kitt, T.M.; Feaheny, K.; Blankstein, R.; Ghoshhajra, B.B.; Budoff, M.J.; Leipsic, J.; Min, J.K.; Akin, J.; George, R.T. A randomized, multicenter, multivendor study of myocardial perfusion imaging with regadenoson CT perfusion vs single photon emission CT. J. Cardiovasc. Comput. Tomogr. 2015, 9, 103–112.e2. [Google Scholar] [CrossRef] [PubMed]
- Rochitte, C.E.; George, R.T.; Chen, M.Y.; Arbab-Zadeh, A.; Dewey, M.; Miller, J.M.; Niinuma, H.; Yoshioka, K.; Kitagawa, K.; Nakamori, S.; et al. Computed tomography angiography and perfusion to assess coronary artery stenosis causing perfusion defects by single photon emission computed tomography: The CORE320 study. Eur. Heart J. 2014, 35, 1120–1130. [Google Scholar] [CrossRef] [PubMed]
- George, R.T.; Mehra, V.C.; Chen, M.Y.; Kitagawa, K.; Arbab-Zadeh, A.; Miller, J.M.; Matheson, M.B.; Vavere, A.L.; Kofoed, K.F.; Rochitte, C.E.; et al. Myocardial CT perfusion imaging and SPECT for the diagnosis of coronary artery disease: A head-to-head comparison from the CORE320 multicenter diagnostic performance study. Radiology 2014, 272, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; Arbab-Zadeh, A.; Kishi, S.; Chen, M.Y.; Magalhães, T.A.; George, R.T.; Dewey, M.; Rybicki, F.J.; Kofoed, K.F.; de Roos, A.; et al. Incremental diagnostic accuracy of computed tomography myocardial perfusion imaging over coronary angiography stratified by pre-test probability of coronary artery disease and severity of coronary artery calcification: The CORE320 study. Int. J. Cardiol. 2015, 201, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Andreini, D.; Mushtaq, S.; Pontone, G.; Conte, E.; Collet, C.; Sonck, J.; D’Errico, A.; Di Odoardo, L.; Guglielmo, M.; Baggiano, A.; et al. CT Perfusion Versus Coronary CT Angiography in Patients with Suspected In-Stent Restenosis or CAD Progression. JACC Cardiovasc. Imaging 2020, 13, 732–742. [Google Scholar] [CrossRef]
- Magalhães, T.A.; Cury, R.C.; Pereira, A.C.; Moreira Vde, M.; Lemos, P.A.; Kalil-Filho, R.; Rochitte, C.E. Additional value of dipyridamole stress myocardial perfusion by 64-row computed tomography in patients with coronary stents. J. Cardiovasc. Comput. Tomogr. 2011, 5, 449–458. [Google Scholar] [CrossRef]
- Takx, R.A.; Blomberg, B.A.; El Aidi, H.; Habets, J.; de Jong, P.A.; Nagel, E.; Hoffmann, U.; Leiner, T. Diagnostic accuracy of stress myocardial perfusion imaging compared to invasive coronary angiography with fractional flow reserve meta-analysis. Circ. Cardiovasc. Imaging 2015, 8, e002666. [Google Scholar] [CrossRef]
- Celeng, C.; Leiner, T.; Maurovich-Horvat, P.; Merkely, B.; de Jong, P.; Dankbaar, J.W.; van Es, H.W.; Ghoshhajra, B.B.; Hoffmann, U.; Takx, R.A.P. Anatomical and Functional Computed Tomography for Diagnosing Hemodynamically Significant Coronary Artery Disease: A Meta-Analysis. JACC Cardiovasc. Imaging 2019, 12, 1316–1325. [Google Scholar] [CrossRef]
- Chen, M.Y.; Rochitte, C.E.; Arbab-Zadeh, A.; Dewey, M.; George, R.T.; Miller, J.M.; Niinuma, H.; Yoshioka, K.; Kitagawa, K.; Sakuma, H.; et al. Prognostic Value of Combined CT Angiography and Myocardial Perfusion Imaging versus Invasive Coronary Angiography and Nuclear Stress Perfusion Imaging in the Prediction of Major Adverse Cardiovascular Events: The CORE320 Multicenter Study. Radiology 2017, 284, 55–65. [Google Scholar] [CrossRef]
- van Assen, M.; De Cecco, C.N.; Eid, M.; von Knebel Doeberitz, P.; Scarabello, M.; Lavra, F.; Bauer, M.J.; Mastrodicasa, D.; Duguay, T.M.; Zaki, B.; et al. Prognostic value of CT myocardial perfusion imaging and CT-derived fractional flow reserve for major adverse cardiac events in patients with coronary artery disease. J. Cardiovasc. Comput. Tomogr. 2019, 13, 26–33. [Google Scholar] [CrossRef]
- Patel, A.R.; Bamberg, F.; Branch, K.; Carrascosa, P.; Chen, M.; Cury, R.C.; Ghoshhajra, B.; Ko, B.; Nieman, K.; Pugliese, F.; et al. Society of cardiovascular computed tomography expert consensus document on myocardial computed tomography perfusion imaging. J. Cardiovasc. Comput. Tomogr. 2020, 14, 87–100. [Google Scholar] [CrossRef]
- Branch, K.R.; Haley, R.D.; Bittencourt, M.S.; Patel, A.R.; Hulten, E.; Blankstein, R. Myocardial computed tomography perfusion. Cardiovasc. Diagn. Ther. 2017, 7, 452–462. [Google Scholar] [CrossRef]
- Seitun, S.; De Lorenzi, C.; Cademartiri, F.; Buscaglia, A.; Travaglio, N.; Balbi, M.; Bezante, G.P. CT Myocardial Perfusion Imaging: A New Frontier in Cardiac Imaging. Biomed. Res. Int. 2018, 2018, 7295460. [Google Scholar] [CrossRef]
- Danad, I.; Szymonifka, J.; Schulman-Marcus, J.; Min, J.K. Static and dynamic assessment of myocardial perfusion by computed tomography. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 836–844. [Google Scholar] [CrossRef]
- Pontone, G.; Baggiano, A.; Andreini, D.; Guaricci, A.I.; Guglielmo, M.; Muscogiuri, G.; Fusini, L.; Soldi, M.; Del Torto, A.; Mushtaq, S.; et al. Dynamic Stress Computed Tomography Perfusion with a Whole-Heart Coverage Scanner in Addition to Coronary Computed Tomography Angiography and Fractional Flow Reserve Computed Tomography Derived. JACC Cardiovasc. Imaging 2019, 12, 2460–2471. [Google Scholar] [CrossRef]
- Pontone, G.; Andreini, D.; Guaricci, A.I.; Baggiano, A.; Fazzari, F.; Guglielmo, M.; Muscogiuri, G.; Berzovini, C.M.; Pasquini, A.; Mushtaq, S.; et al. Incremental Diagnostic Value of Stress Computed Tomography Myocardial Perfusion With Whole-Heart Coverage CT Scanner in Intermediate- to High-Risk Symptomatic Patients Suspected of Coronary Artery Disease. JACC Cardiovasc. Imaging 2019, 12, 338–349. [Google Scholar] [CrossRef]
- de Knegt, M.C.; Rossi, A.; Petersen, S.E.; Wragg, A.; Khurram, R.; Westwood, M.; Saberwal, B.; Mathur, A.; Nieman, K.; Bamberg, F.; et al. Stress myocardial perfusion with qualitative magnetic resonance and quantitative dynamic computed tomography: Comparison of diagnostic performance and incremental value over coronary computed tomography angiography. Eur. Heart J. Cardiovasc. Imaging 2020, 22, 1452–1462. [Google Scholar] [CrossRef]
- Arbab-Zadeh, A.; Miller, J.M.; Rochitte, C.E.; Dewey, M.; Niinuma, H.; Gottlieb, I.; Paul, N.; Clouse, M.E.; Shapiro, E.P.; Hoe, J.; et al. Diagnostic accuracy of computed tomography coronary angiography according to pre-test probability of coronary artery disease and severity of coronary arterial calcification. The CORE-64 (Coronary Artery Evaluation Using 64-Row Multidetector Computed Tomography Angiography) International Multicenter Study. J. Am. Coll. Cardiol. 2012, 59, 379–387. [Google Scholar] [CrossRef]
- Wykrzykowska, J.J.; Arbab-Zadeh, A.; Godoy, G.; Miller, J.M.; Lin, S.; Vavere, A.; Paul, N.; Niinuma, H.; Hoe, J.; Brinker, J.; et al. Assessment of in-stent restenosis using 64-MDCT: Analysis of the CORE-64 Multicenter International Trial. AJR Am. J. Roentgenol. 2010, 194, 85–92. [Google Scholar] [CrossRef]
- Piechnik, S.K.; Neubauer, S.; Ferreira, V.M. State-of-the-art review: Stress T1 mapping-technical considerations, pitfalls and emerging clinical applications. Magma 2018, 31, 131–141. [Google Scholar] [CrossRef]
- Gunasekaran, P.; Panaich, S.; Briasoulis, A.; Cardozo, S.; Afonso, L. Incremental Value of Two Dimensional Speckle Tracking Echocardiography in the Functional Assessment and Characterization of Subclinical Left Ventricular Dysfunction. Curr. Cardiol. Rev. 2017, 13, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Aziz, R.; Al Musa, T.; Ripley, D.P.; Haaf, P.; Foley, J.R.J.; Swoboda, P.P.; Fent, G.J.; Dobson, L.E.; Greenwood, J.P.; et al. Effects of hyperaemia on left ventricular longitudinal strain in patients with suspected coronary artery disease: A first-pass stress perfusion cardiovascular magnetic resonance imaging study. Neth. Heart J. 2018, 26, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Palmisano, A.; Giannini, F.; Rancoita, P.; Gallone, G.; Benedetti, G.; Baldetti, L.; Tzanis, G.; Vignale, D.; Monti, C.; Ponticelli, F.; et al. Feature tracking and mapping analysis of myocardial response to improved perfusion reserve in patients with refractory angina treated by coronary sinus Reducer implantation: A CMR study. Int. J. Cardiovasc. Imaging 2021, 37, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Iwanczyk, J.S. Vision 20/20: Single photon counting x-ray detectors in medical imaging. Med. Phys. 2013, 40, 100901. [Google Scholar] [CrossRef]
- Tortora, M.; Gemini, L.; D’Iglio, I.; Ugga, L.; Spadarella, G.; Cuocolo, R. Spectral Photon-Counting Computed Tomography: A Review on Technical Principles and Clinical Applications. J. Imaging 2022, 8, 112. [Google Scholar] [CrossRef]
- Si-Mohamed, S.A.; Boccalini, S.; Lacombe, H.; Diaw, A.; Varasteh, M.; Rodesch, P.A.; Dessouky, R.; Villien, M.; Tatard-Leitman, V.; Bochaton, T.; et al. Coronary CT Angiography with Photon-counting CT: First-In-Human Results. Radiology 2022, 303, 303–313. [Google Scholar] [CrossRef]
| S-TTE | SPECT | S-CMR | PET | CTP | |
|---|---|---|---|---|---|
| Radiation | NA | ++ | NA | ++ | +++ |
| Nephrotoxicity | NA | NA | + | NA | ++ |
| Specificity | ++ | ++ | +++ | +++ | +++ |
| Sensitivity | + | ++ | ++ | +++ | +++ |
| Diagnostic accuracy | + | ++ | +++ | +++ | +++ |
| Temporal resolution | +++ | ++ | ++ | ++ | + |
| Spatial resolution | ++ | ++ | +++ | +++ | +++ |
| Availability | +++ | +++ | ++ | + | + |
| Cost | + | ++ | +++ | +++ | +++ |
| Absolute CI | Relative CI |
|---|---|
| High-risk acute coronary syndrome | Left main stenosis > 50% |
| Decompensated heart failure | Outflow tract obstruction |
| Blood pressure > 200/110 | Electrolytes alterations |
| Hypotension < 90 | Significant tachy-/bradyarrhitimias |
| Bronchospastic lung disease | Recent stroke or seizure |
| Severe aortic stenosis | Heart rate > 100 bpm |
| Uncontrolled arrhythmia | Moderate renal insufficiency |
| Bronchospastic lung disease | Morbid obesity |
| Acute pulmonary embolism | |
| Active cerebrovascular accident | |
| Acute myocarditis or pericarditis | |
| Acute aortic dissection | |
| Severe renal insufficiency | |
| High degree atrioventricular block | |
| Caffeine, chocolate, or theophylline in the last 12 h |
| CTP | Advantages | Disadvantages |
|---|---|---|
| Static | Rapid | No quantitative data |
| Lower radiation dose More data on accuracy and outcomes | More artifacts | |
| CCTA and CTP in a single data set | Time of acquisition critical | |
| Dynamic | Quantitative data possible | Compliance (long breath-hold) |
| Fewer artifacts | Longer analysis | |
| Timing of acquisition less critical | Higher radiation dose | |
| Increased image noise |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Groenhoff, L.; De Zan, G.; Costantini, P.; Siani, A.; Ostillio, E.; Carriero, S.; Muscogiuri, G.; Bergamaschi, L.; Patti, G.; Pizzi, C.; et al. The Non-Invasive Diagnosis of Chronic Coronary Syndrome: A Focus on Stress Computed Tomography Perfusion and Stress Cardiac Magnetic Resonance. J. Clin. Med. 2023, 12, 3793. https://doi.org/10.3390/jcm12113793
Groenhoff L, De Zan G, Costantini P, Siani A, Ostillio E, Carriero S, Muscogiuri G, Bergamaschi L, Patti G, Pizzi C, et al. The Non-Invasive Diagnosis of Chronic Coronary Syndrome: A Focus on Stress Computed Tomography Perfusion and Stress Cardiac Magnetic Resonance. Journal of Clinical Medicine. 2023; 12(11):3793. https://doi.org/10.3390/jcm12113793
Chicago/Turabian StyleGroenhoff, Léon, Giulia De Zan, Pietro Costantini, Agnese Siani, Eleonora Ostillio, Serena Carriero, Giuseppe Muscogiuri, Luca Bergamaschi, Giuseppe Patti, Carmine Pizzi, and et al. 2023. "The Non-Invasive Diagnosis of Chronic Coronary Syndrome: A Focus on Stress Computed Tomography Perfusion and Stress Cardiac Magnetic Resonance" Journal of Clinical Medicine 12, no. 11: 3793. https://doi.org/10.3390/jcm12113793






