Real-World Efficacy and Safety of Thoracic Radiotherapy after First-Line Chemo-Immunotherapy in Extensive-Stage Small-Cell Lung Cancer
Abstract
:1. Introduction
2. Methods
2.1. Patients
2.2. Treatment
2.3. Information Collection and Follow-Up
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
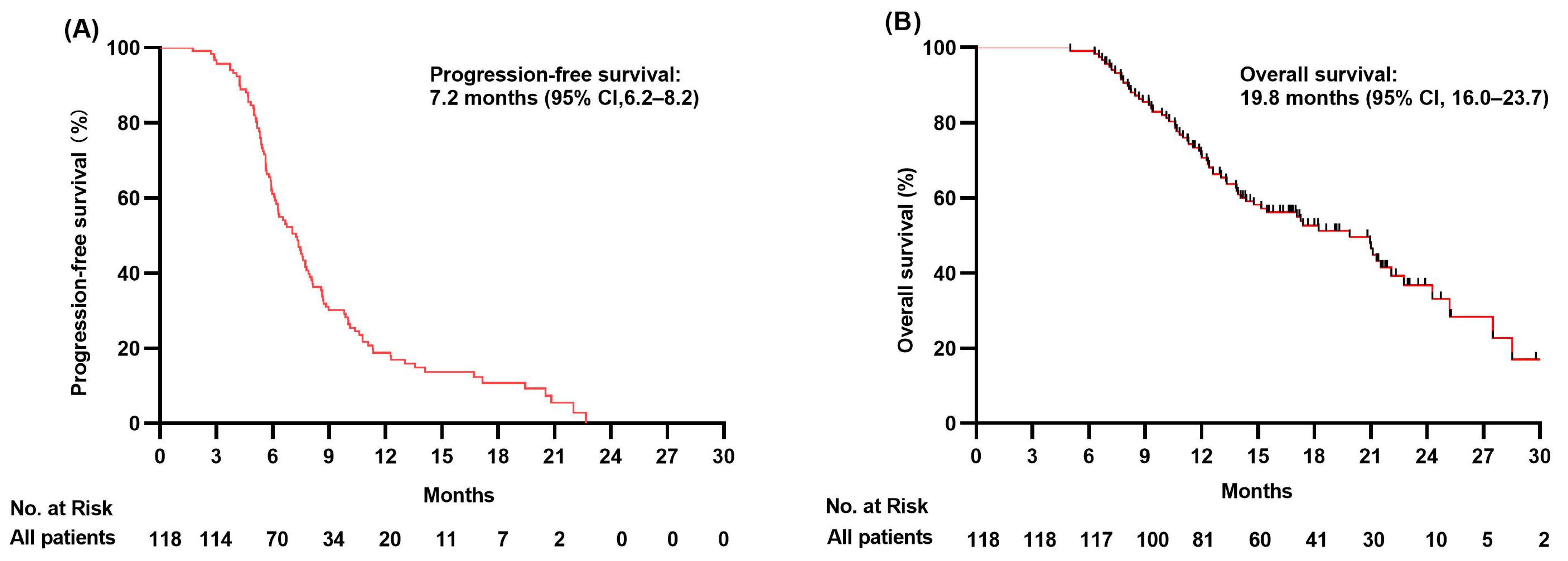
3.2. Progression-Free Survival Analysis
3.3. Overall Survival Analysis
3.4. Survival Outcomes in Selected Patient Subgroups
3.5. Safety
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Govindan, R.; Page, N.; Morgensztern, D.; Read, W.; Tierney, R.; Vlahiotis, A.; Spitznagel, E.L.; Piccirillo, J. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: Analysis of the surveillance, epidemiologic, and end results database. J. Clin. Oncol. 2006, 24, 4539–4544. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Reid, T.R.; Oronsky, A.; Carter, C.A. What’s New in SCLC? A Review. Neoplasia 2017, 19, 842–847. [Google Scholar] [CrossRef] [PubMed]
- BByers, L.A.; Rudin, C.M. Small cell lung cancer: Where do we go from here? Cancer 2015, 121, 664–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudin, C.M.; Ismaila, N.; Hann, C.L.; Malhotra, N.; Movsas, B.; Norris, K.; Pietanza, M.C.; Ramalingam, S.S.; Turrisi, A.T.; Giaccone, G. Treatment of Small-Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians Guideline. J. Clin. Oncol. 2015, 33, 4106–4111. [Google Scholar] [CrossRef]
- Horn, L.; Mansfield, A.S.; Szczęsna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; et al. Durvalumab plus platinum–etoposide versus platinum–etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomised, controlled, open-label, phase 3 trial. Lancet 2019, 394, 1929–1939. [Google Scholar] [CrossRef]
- Zugazagoitia, J.; Paz-Ares, L. Extensive-Stage Small-Cell Lung Cancer: First-Line and Second-Line Treatment Options. J. Clin. Oncol. 2022, 40, 671–680. [Google Scholar] [CrossRef]
- Ganti, A.K.P.; Loo, B.W.; Bassetti, M.; Blakely, C.; Chiang, A.; D’Amico, T.A.; D’Avella, C.; Dowlati, A.; Downey, R.J.; Edelman, M.; et al. Small Cell Lung Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 1441–1464. [Google Scholar] [CrossRef]
- Simone, C.B., 2nd; Bogart, J.A.; Cabrera, A.R.; Daly, M.E.; DeNunzio, N.J.; Detterbeck, F.; Faivre-Finn, C.; Gatschet, N.; Gore, E.; Jabbour, S.K.; et al. Radiation Therapy for Small Cell Lung Cancer: An ASTRO Clinical Practice Guideline. Pract. Radiat. Oncol. 2020, 10, 158–173. [Google Scholar] [CrossRef]
- Slotman, B.J.; van Tinteren, H.; Praag, J.O.; Knegjens, J.L.; El Sharouni, S.Y.; Hatton, M.; Keijser, A.; Faivre-Finn, C.; Senan, S. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: A phase 3 randomised controlled trial. Lancet 2015, 385, 36–42. [Google Scholar] [CrossRef]
- Yee, D.; Butts, C.; Reiman, A.; Joy, A.; Smylie, M.; Fenton, D.; Chu, Q.; Hanson, J.; Roa, W. Clinical trial of post-chemotherapy consolidation thoracic radiotherapy for extensive-stage small cell lung cancer. Radiother. Oncol. 2012, 102, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, M.E.; Atallah, S.; Sun, A.; Bezjak, A.; Le, L.W.; Brade, A.; Cho, J.; Leighl, N.B.; Shepherd, F.A.; Hope, A.J. Clinical outcomes of extensive stage small cell lung carcinoma patients treated with consolidative thoracic radiotherapy. Clin. Lung Cancer 2011, 12, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Kalemkerian, G.P.; Loo, B.W.; Akerley, W.; Attia, A.; Bassetti, M.; Boumber, Y.; Decker, R.; Dobelbower, M.C.; Dowlati, A.; Downey, R.J.; et al. NCCN Guidelines Insights: Small Cell Lung Cancer, Version 2.2018. J. Natl. Compr. Cancer Netw. 2018, 16, 1171–1182. [Google Scholar] [CrossRef]
- Schwartz, L.H.; Litière, S.; De Vries, E.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1-Update and clarification: From the RECIST committee. Eur. J. Cancer 2016, 62, 132–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanna, N.; Bunn, P.A., Jr.; Langer, C.; Einhorn, L.; Guthrie, T., Jr.; Beck, T.; Ansari, R.; Ellis, P.; Byrne, M.; Morrison, M.; et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J. Clin. Oncol. 2006, 24, 2038–2043. [Google Scholar] [CrossRef] [PubMed]
- JJeremic, B.; Shibamoto, Y.; Nikolic, N.; Milicic, B.; Milisavljevic, S.; Dagovic, A.; Aleksandrovic, J.; Radosavljevic-Asic, G. Role of radiation therapy in the combined-modality treatment of patients with extensive disease small-cell lung cancer: A randomized study. J. Clin. Oncol. 1999, 17, 2092–2099. [Google Scholar] [CrossRef]
- Gore, E.M.; Hu, C.; Sun, A.Y.; Grimm, D.F.; Ramalingam, S.S.; Dunlap, N.E.; Higgins, K.A.; Werner-Wasik, M.; Allen, A.M.; Iyengar, P.; et al. Randomized Phase II Study Comparing Prophylactic Cranial Irradiation Alone to Prophylactic Cranial Irradiation and Consolidative Extracranial Irradiation for Extensive-Disease Small Cell Lung Cancer (ED SCLC): NRG Oncology RTOG 0937. J. Thorac. Oncol. 2017, 12, 1561–1570. [Google Scholar] [CrossRef] [Green Version]
- CSCO Clinical Practice Guidelines in Oncology: Small Cell Lung Cancer, v.2.2022. Available online: www.csco.org.cn/professionals (accessed on 1 December 2022).
- Dingemans, A.-M.C.; Früh, M.; Ardizzoni, A.; Besse, B.; Faivre-Finn, C.; Hendriks, L.; Lantuejoul, S.; Peters, S.; Reguart, N.; Rudin, C.; et al. Small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 839–853. [Google Scholar] [CrossRef]
- Cheng, Y.; Han, L.; Wu, L.; Chen, J.; Sun, H.; Wen, G.; Ji, Y.; Dvorkin, M.; Shi, J.; Pan, Z.; et al. Effect of First-Line Serplulimab vs Placebo Added to Chemotherapy on Survival in Patients With Extensive-Stage Small Cell Lung Cancer: The ASTRUM-005 Randomized Clinical Trial. JAMA 2022, 328, 1223–1232. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, C.; Yao, W.; Wang, Q.; Min, X.; Chen, G.; Xu, X.; Li, X.; Xu, F.; Fang, Y.; et al. Adebrelimab or placebo plus carboplatin and etoposide as first-line treatment for extensive-stage small-cell lung cancer (CAPSTONE-1): A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022, 23, 739–747. [Google Scholar] [CrossRef]
- Golden, E.B.; Chhabra, A.; Chachoua, A.; Adams, S.; Donach, M.; Fenton-Kerimian, M.; Friedman, K.; Ponzo, F.; Babb, J.S.; Goldberg, J.; et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: A proof-of-principle trial. Lancet Oncol. 2015, 16, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Formenti, S.C.; Rudqvist, N.-P.; Golden, E.; Cooper, B.; Wennerberg, E.; Lhuillier, C.; Vanpouille-Box, C.; Friedman, K.; Ferrari de Andrade, L.; Wucherpfennig, K.W.; et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat. Med. 2018, 24, 1845–1851. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Chen, D.; Yu, J. Radiotherapy combined with immunotherapy: The dawn of cancer treatment. Signal Transduct. Target. Ther. 2022, 7, 258. [Google Scholar] [CrossRef]
- Dovedi, S.J.; Cheadle, E.J.; Popple, A.L.; Poon, E.; Morrow, M.; Stewart, R.; Yusko, E.C.; Sanders, C.M.; Vignali, M.; Emerson, R.O.; et al. Fractionated Radiation Therapy Stimulates Antitumor Immunity Mediated by Both Resident and Infiltrating Polyclonal T-cell Populations when Combined with PD-1 Blockade. Clin. Cancer Res. 2017, 23, 5514–5526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, L.; Liang, H.; Burnette, B.; Beckett, M.; Darga, T.; Weichselbaum, R.R.; Fu, Y.-X. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Investig. 2014, 124, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Shaverdian, N.; Lisberg, A.E.; Bornazyan, K.; Veruttipong, D.; Goldman, J.W.; Formenti, S.C.; Garon, E.B.; Lee, P. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: A secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017, 18, 895–903. [Google Scholar] [CrossRef]
- Theelen, W.S.; Peulen, H.M.; Lalezari, F.; van der Noort, V.; De Vries, J.F.; Aerts, J.G.; Dumoulin, D.W.; Bahce, I.; Niemeijer, A.L.N.; De Langen, A.J.; et al. Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients with Advanced Non-Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019, 5, 1276–1282. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; De Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef] [Green Version]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; De Wit, M.; et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. Non-Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 497–530. [Google Scholar] [CrossRef]
- The LEAD Trial. Available online: https://clinicaltricals.gov/ct2/show/NCT05092412 (accessed on 1 April 2022).
- The TRIPLEX Trial. Available online: https://clinicaltricals.gov/ct2/show/NCT05223647 (accessed on 1 April 2022).
- The RAPTOR Trial. Available online: https://clinicaltricals.gov/ct2/show/NCT04402788 (accessed on 1 April 2022).
- Daher, S.N.; Allen, A.; Rottenberg, Y.; Nasrallah, H.; Yosef, L.; Blumenfeld, P.; Wollner, M.; Appel, S.; Nechushtan, H.; Moskovitz, M.; et al. Real-world data of consolidative radiotherapy for extensive stage (ES)-SCLC treated by chemo-immunotherapy (chemo-IO). Ann. Oncol. 2022, 33 (Suppl. S2), S97–S104. [Google Scholar] [CrossRef]
- Bruni, A.; Bertolini, F.; D’Angelo, E.; Barbieri, F.; Imbrescia, J.; Trudu, L.; Cappelli, A.; Lohr, F.; Dominici, M.; Guaitoli, G. Chemo-immunotherapy with or without consolidative radiotherapy in extensive-stage small cell lung cancer: An initial report of clinical outcome and safety. Ann. Oncol. 2022, 33 (Suppl. S2), S100–S101. [Google Scholar] [CrossRef]
- Wu, J.-J.; Huang, J.-W.; Hsu, K.-H.; Huang, Y.-H.; Chen, K.-C.; Tseng, J.-S.; Yang, T.-Y.; Chang, G.-C. Thoracic radiotherapy may improve the outcome of extensive stage small cell lung carcinoma patients treated with first-line immunotherapy plus chemotherapy. Anticancer. Drugs 2022, 33, e842–e849. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Cushman, T.R.; Selek, U.; Tang, C.; Welsh, J.W. Safety of Combined Immunotherapy and Thoracic Radiation Therapy: Analysis of 3 Single-Institutional Phase I/II Trials. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 1141–1148. [Google Scholar] [CrossRef]



| Characteristics | Total | CT-IT + TRT (%) | CT-IT (%) | p-Value |
|---|---|---|---|---|
| N = 118 | N = 45 | N = 73 | ||
| Median age (range) | 62 (45–90) | 62 (45–79) | 63 (45–90) | |
| Age (years) | 0.80 | |||
| >65 | 41 (34.7) | 15 (33.3) | 26 (35.6) | |
| ≤65 | 77 (65.3) | 30 (66.7) | 47 (64.4) | |
| Gender | 0.43 | |||
| Male | 96 (81.3) | 35 (77.8) | 61 (83.6) | |
| Female | 22 (18.7) | 10 (22.2) | 12 (16.4) | |
| Smoking status | 0.02 | |||
| Smoker | 98 (83.0) | 33 (73.3) | 65 (89.0) | |
| Never smoker | 20 (17.0) | 12 (26.7) | 8 (11.0) | |
| Drinking | 0.05 | |||
| Yes | 39 (33.1) | 10 (22.2) | 29 (39.7) | |
| No | 79 (66.9) | 35 (77.8) | 44 (60.3) | |
| ECOG PS | 0.94 | |||
| 0–1 | 94 (79.7) | 36 (80.0) | 58 (79.5) | |
| 2 | 24 (20.3) | 9 (20.0) | 15 (20.5) | |
| Lung metastasis | 0.24 | |||
| Yes | 16 (13.5) | 4 (8.9) | 12 (16.4) | |
| No | 102 (86.5) | 41 (91.1) | 61 (83.6) | |
| Extrathoracic metastasis | 0.98 | |||
| Yes | 105 (89.0) | 40 (88.9) | 65 (89.0) | |
| No | 13 (11.0) | 5 (11.1) | 8 (11.0) | |
| Bone metastasis | 0.02 | |||
| Yes | 35 (29.6) | 6 (13.3) | 29 (39.7) | |
| No | 83 (70.4) | 39 (86.7) | 44 (60.3) | |
| Brain metastasis | 0.66 | |||
| Yes | 34 (28.8) | 14 (31.1) | 20 (27.3) | |
| No | 84 (71.2) | 31 (68.9) | 53 (72.7) | |
| Liver metastasis | 0.98 | |||
| Yes | 34 (28.8) | 13 (28.8) | 21 (28.8) | |
| No | 84 (71.2) | 32 (71.2) | 52 (71.2) | |
| Distant LN | 0.72 | |||
| Yes | 31 (26.3) | 11 (24.4) | 20 (27.4) | |
| No | 87 (73.7) | 34 (75.6) | 53 (72.6) | |
| BMI | 0.36 | |||
| BIM ≥ 24 | 60 (50.8) | 21 (46.7) | 39 (53.4) | |
| 18.5 ≤ BIM < 24 | 56 (47.5) | 24 (53.3) | 32 (43.9) | |
| BIM < 18.5 | 2 (1.7) | 0 (0.0) | 2 (2.7) | |
| T Stage | 0.29 | |||
| T = 4 | 48 (40.7) | 21 (46.7) | 27 (37.0) | |
| T= 1–3 | 70 (59.3) | 24 (53.3) | 46 (63.0) | |
| N Stage | 0.07 | |||
| N = 3 | 70 (59.3) | 22 (48.9) | 48 (65.8) | |
| N = 0–2 | 48 (40.7) | 23 (51.1) | 25 (34.2) | |
| Anti-PD-L1 drugs | 0.93 | |||
| Atezolizumab | 39 (33.0) | 15 (33.3) | 24 (32.9) | |
| Durvalumab | 52 (44.0) | 19 (42.2) | 33 (45.2) | |
| Other PD-L1 antibodies | 27 (23.0) | 11 (24.5) | 16 (21.9) | |
| Treatment cycles | 0.17 | |||
| >6 cycles | 1 (0.9) | 1 (2.2) | 0 (0.0) | |
| 4–6 cycles | 114 (96.6) | 44 (97.8) | 70 (95.9) | |
| <4 cycles | 3 (2.5) | 0 (0.0) | 3 (4.1) | |
| Response to CT-IT | ||||
| CR/PR | 85 (72.0) | 38 (84.4) | 47 (64.4) | 0.01 |
| SD/PD | 33 (28.0) | 7 (15.6) | 26 (35.6) |
| Characteristics | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Clinicopathology | ||||||
| Gender (Male vs. Female) | 0.857 | 0.508–1.444 | 0.562 | - | - | - |
| Age (>65 vs. ≤65; years) | 0.700 | 0.458–1.071 | 0.100 | - | - | - |
| Smoking (Yes vs. No) | 0.882 | 0.522–1.488 | 0.637 | - | - | - |
| Alcohol (Yes vs. No) | 0.908 | 0.605–1.364 | 0.643 | - | - | - |
| T stage (4 vs. <4) | 1.065 | 0.716–1.583 | 0.757 | - | - | - |
| N stage (3 vs. <3) | 1.364 | 0.915–2.035 | 0.128 | - | - | - |
| BMI (≥23.5 vs. <23.5) | 1.441 | 0.968–2.145 | 0.072 | - | - | - |
| Lung metastasis (Yes vs. No) | 0.678 | 0.341–1.348 | 0.268 | - | - | - |
| Extrathoracic metastasis (Yes vs. No) | 1.510 | 0.802–2.842 | 0.201 | - | - | - |
| Bone metastasis (Yes vs. No) | 1.424 | 0.932–2.176 | 0.102 | - | - | - |
| Brain metastasis (Yes vs. No) | 1.429 | 0.927–2.204 | 0.106 | - | - | |
| Liver metastasis (Yes vs. No) | 1.573 | 1.030–2.402 | 0.036 * | 1.601 | 1.048–2.445 | 0.029 * |
| Distant LN (Yes vs. No) | 0.831 | 0.528–1.306 | 0.422 | - | - | - |
| TRT (Yes vs. No) | 0.634 | 0.423–0.951 | 0.028 * | 0.683 | 0.455–1.026 | 0.066 |
| Response to CT-IT (CR/PR vs. SD/PD) | 0.456 | 0.298–0.697 | 0.000 * | 0.481 | 0.314–0.736 | 0.001 * |
| Hematology | - | - | - | |||
| NLR (≥1.5 vs. <1.5) | 1.497 | 0.778–2.879 | 0.227 | - | - | - |
| MLR (≥0.5 vs. <0.5) | 0.893 | 0.556–1.435 | 0.640 | - | - | - |
| PLR (≥194.4 vs. <194.4) | 0.885 | 0.593–1.323 | 0.553 | - | - | |
| LDH (≥231.0 vs. <231.0) | 1.225 | 0.823–1.821 | 0.317 | - | - | - |
| Characteristics | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Clinicopathology | ||||||
| Gender (Male vs. Female) | 0.814 | 0.425–1.557 | 0.533 | - | - | - |
| Age (>65 vs. ≤65; years) | 0.873 | 0.518–1.473 | 0.612 | - | - | - |
| Smoking (Yes vs. No) | 1.278 | 0.663–2.464 | 0.464 | - | - | - |
| Alcohol (Yes vs. No) | 1.266 | 0.761–2.106 | 0.363 | - | - | - |
| T stage (4 vs. <4) | 0.726 | 0.434–1.214 | 0.222 | - | - | - |
| N stage (3 vs. <3) | 2.136 | 1.262–3.616 | 0.005 * | 1.281 | 0.723–2.267 | 0.396 |
| BMI (≥31.9 vs. <31.9) | 3.188 | 0.771–13.179 | 0.109 | - | - | - |
| Lung metastasis (Yes vs. No) | 1.274 | 0.662–2.451 | 0.468 | - | - | - |
| Extrathoracic metastasis (Yes vs. No) | 3.618 | 1.293–10.128 | 0.014 * | 2.012 | 0.641–6.314 | 0.231 |
| Bone metastasis (Yes vs. No) | 3.101 | 1.874–5.132 | 0.000 * | 2.292 | 1.353–3.884 | 0.002 * |
| Brain metastasis (Yes vs. No) | 1.381 | 0.832–2.293 | 0.212 | - | - | |
| Liver metastasis (Yes vs. No) | 3.366 | 2.027–5.591 | 0.000 * | 2.889 | 1.710–4.879 | 0.000 * |
| Distant LN (Yes vs. No) | 1.388 | 0.800–2.407 | 0.243 | - | - | - |
| TRT (Yes vs. No) | 0.522 | 0.305–0.893 | 0.018 * | 0.564 | 0.317–1.004 | 0.052 |
| Response to CT-IT (CR/PR vs. SD/PD) | 0.638 | 0.383–1.064 | 0.085 | |||
| Hematology | - | - | - | |||
| NLR (≥1.6 vs. <1.6) | 1.808 | 0.824–3.968 | 0.140 | - | - | - |
| MLR (≥0.5 vs. <0.5) | 0.995 | 0.566–1.750 | 0.987 | - | - | - |
| PLR (≥175.5 vs. <175.5) | 1.252 | 0.767–2.043 | 0.369 | - | - | |
| LDH (≥231.0 vs. <231.0) | 1.939 | 1.160–3.241 | 0.011 * | 1.421 | 0.833–2.422 | 0.197 |
| Adverse Event | CT-IT + TRT Group | CT-IT Group |
|---|---|---|
| (n = 45) | (n = 73) | |
| Esophagitis | 1 (2.2%) | 0 (0%) |
| Pneumonia | 2 (4.4%) | 1 (1.3%) |
| Leukopenia | 3 (6.6%) | 6 (8.2%) |
| Neutropenia | 10 (22.2%) | 17 (23.2%) |
| Anemia | 4 (8.8%) | 6 (8.2%) |
| Thrombocytopenia | 3 (6.6%) | 5 (6.8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Z.; Liu, J.; Wu, M.; Wang, X.; Lu, Y.; Han, C.; Cong, L.; Li, J.; Meng, X. Real-World Efficacy and Safety of Thoracic Radiotherapy after First-Line Chemo-Immunotherapy in Extensive-Stage Small-Cell Lung Cancer. J. Clin. Med. 2023, 12, 3828. https://doi.org/10.3390/jcm12113828
Xie Z, Liu J, Wu M, Wang X, Lu Y, Han C, Cong L, Li J, Meng X. Real-World Efficacy and Safety of Thoracic Radiotherapy after First-Line Chemo-Immunotherapy in Extensive-Stage Small-Cell Lung Cancer. Journal of Clinical Medicine. 2023; 12(11):3828. https://doi.org/10.3390/jcm12113828
Chicago/Turabian StyleXie, Zhaoliang, Jingru Liu, Min Wu, Xiaohan Wang, Yuhan Lu, Chunyan Han, Lei Cong, Jisheng Li, and Xue Meng. 2023. "Real-World Efficacy and Safety of Thoracic Radiotherapy after First-Line Chemo-Immunotherapy in Extensive-Stage Small-Cell Lung Cancer" Journal of Clinical Medicine 12, no. 11: 3828. https://doi.org/10.3390/jcm12113828





