Infection Control among Healthcare Workers and Management of a Scabies Outbreak in a Large Italian University Hospital
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting and Participants
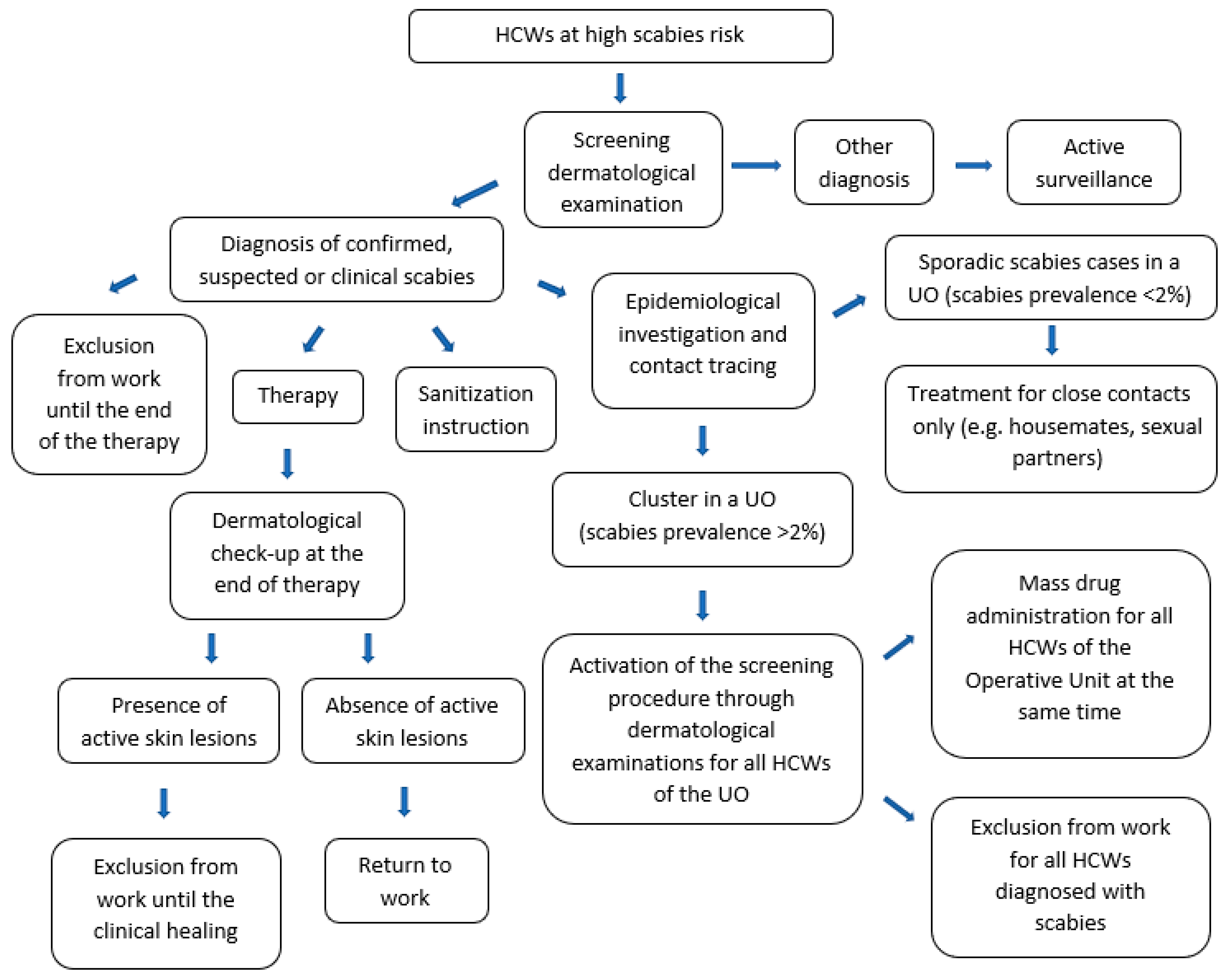
2.2. Case Definitions
2.3. Epidemiologic Investigation and Notification System
2.4. Prevention and Protection Measures
2.5. Statistical Analysis
3. Results
3.1. Scabies Cases: Frequency and Distribution
3.2. Characteristics of The Sample and Risk Factors of Scabies
3.3. The Index Case and Outbreak Reconstruction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Newsroom, Fact Sheets, Details, Scabies. Available online: https://www.who.int/news-room/fact-sheets/detail/scabies (accessed on 30 March 2023).
- van Deursen, B.; Hooiveld, M.; Marks, S.; Snijdewind, I.; van den Kerkhof, H.; Wintermans, B.; Bom, B.; Schimmer, B.; Fanoy, E. Increasing incidence of reported scabies infestations in the Netherlands, 2011–2021. PLoS ONE 2022, 17, e0268865. [Google Scholar] [CrossRef] [PubMed]
- Amato, E.; Dansie, L.S.; Grøneng, G.M.; Blix, H.S.; Bentele, H.; Veneti, L.; Stefanoff, P.; MacDonald, E.; Blystad, H.H.; Soleng, A. Increase of scabies infestations, Norway, 2006 to 2018. Euro Surveill. 2019, 24, 190020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lugović-Mihić, L.; Aždajić, M.D.; Filipović, S.K.; Bukvić, I.; Prkačin, I.; Grbić, D.Š.; Ličina, M.L.K. An increasing scabies incidence in Croatia: A call for coordinated action among dermatologists, physicians and epidemiologists. Zdr. Varst. 2020, 59, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Bravo, L.; Fernandez-Martinez, B.; Gómez-Barroso, D.; Gherasim, A.; García-Gómez, M.; Benito, A.; Herrador, Z. Scabies in Spain? A comprehensive epidemiological picture. PLoS ONE 2021, 16, e0258780. [Google Scholar] [CrossRef] [PubMed]
- Reichert, F.; Schulz, M.; Mertens, E.; Lachmann, R.; Aebischer, A. Reemergence of scabies driven by adolescents and young adults, Germany, 2009–2018. Emerg. Infect. Dis. 2021, 27, 1693–1696. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Global Health, Division of Parasitic Diseases and Malaria. Parasites—Scabies. Available online: https://www.cdc.gov/parasites/scabies/index.html (accessed on 30 March 2023).
- Scanni, G. The mite-gallery unit: A new concept for describing scabies through entodermoscopy. Trop. Med. Infect. Dis. 2019, 4, 48. [Google Scholar] [CrossRef] [Green Version]
- Bedoya del Campillo, A.; Lleopart, N.; ChQR, G.; Álvarez, M.; Montilla, M.; Martínez-Carpio, P.A. Intervention protocol to improve scabies control in enclosed communities: A case report. Rev. Esp. Sanid. Penit. 2021, 23, 37–42. [Google Scholar] [CrossRef]
- Khan, A.; O’Grady, S.; Muller, M.P. Rapid control of a scabies outbreak at a tertiary care hospital without ward closure. Am. J. Infect. Control 2012, 40, 451–455. [Google Scholar] [CrossRef]
- Leistner, R.; Buchwald, D.; Beyer, M.; Philipp, S. Scabies outbreak among healthcare workers in a German acute care hospital. J. Infect. Prev. 2017, 18, 189–192. [Google Scholar] [CrossRef]
- Meyer, E.P.; Heranney, D.; Foeglé, J.; Chamouard, V.; Hernandez, C.; Mechkour, S.; Passemard, R.; Berthel, M.; Kaltenbach, G.; Lipsker, D.; et al. Gestion d’une épidémie de gale aux hôpitaux universitaires de Strasbourg [Management of a scabies epidemic in the Strasbourg teaching hospital, France]. Med. Mal. Infect. 2011, 41, 92–96. [Google Scholar] [CrossRef]
- Ozdamar, M.; Turkoglu, S. A nosocomial scabies outbreak originating from immunocompromised transplant patients in Turkey: Upholstery as a possible cause. Transpl. Infect. Dis. 2020, 22, e13284. [Google Scholar] [CrossRef]
- Jungbauer, F.H.; Veenstra-Kyuchukova, Y.K.; Koeze, J.; Kruijt Spanjer, M.R.; Kardaun, S.H. Management of nosocomial scabies, an outbreak of occupational disease. Am. J. Ind. Med. 2015, 58, 577–582. [Google Scholar] [CrossRef]
- Sophiea, B.; Oliviera, C. Scabies in healthcare settings. Curr. Opin. Infect. Dis. 2010, 23, 111–118. [Google Scholar] [CrossRef]
- FitzGerald, D.; Grainger, R.J.; Reid, A. Interventions for preventing the spread of infestation in close contacts of people with scabies. Cochrane Database Syst. Rev. 2014, 2, CD009943. [Google Scholar] [CrossRef]
- De Maria, L.; Sponselli, S.; Caputi, A.; Pipoli, A.; Giannelli, G.; Delvecchio, G.; Zagaria, S.; Cavone, D.; Stefanizzi, P.; Bianchi, F.P.; et al. Comparison of three different waves in healthcare workers during the COVID-19 pandemic: A retrospective observational study in an Italian university hospital. J. Clin. Med. 2022, 11, 3074. [Google Scholar] [CrossRef]
- Engelman, D.; Yoshizumi, J.; Hay, R.J.; Osti, M.; Micali, G.; Norton, S.; Walton, S.; Boralevi, F.; Bernigaud, C.; Bowen, A.C.; et al. The 2020 International Alliance for the Control of Scabies consensus criteria for the diagnosis of scabies. Br. J. Dermatol. 2020, 183, 808–820. [Google Scholar] [CrossRef] [Green Version]
- Salavastru, C.M.; Chosidow, O.; Boffa, M.J.; Janier, M.; Tiplica, G.S. European guideline for the management of scabies. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1248–1253. [Google Scholar] [CrossRef] [Green Version]
- Official Gazette of the Italian Republic. Italian Ministry of Health. Decreto 15 Dicembre 1990. Sistema Informativo Delle Malattie Infettive e Diffusive. (GU Serie Generale n.6 del 08-01-1991). Available online: https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=1991-01-08&atto.codiceRedazionale=091A0055 (accessed on 30 March 2023).
- Italian Ministry of Health. Circolare n.4 of 13 Marzo 1998 Protocollo 400.3/26/1189. Misure di Profilassi Per Esigenze di Sanità Pubblica. Provvedimenti da Adottare Nei Confronti di Soggetti Affetti da Alcune Malattie Infettive e Nei Confronti di Loro Conviventi o Contatti. Available online: https://www.trovanorme.salute.gov.it/norme/dettaglioAtto?id=25185 (accessed on 30 March 2023).
- Vimercati, L.; De Maria, L.; Quarato, M.; Caputi, A.; Stefanizzi, P.; Gesualdo, L.; Migliore, G.; Fucilli, F.I.M.; Cavone, D.; Delfino, M.C.; et al. COVID-19 hospital outbreaks: Protecting healthcare workers to protect frail patients. An Italian observational cohort study. Int. J. Infect. Dis. 2021, 102, 532–537. [Google Scholar] [CrossRef]
- De Maria, L.; Sponselli, S.; Caputi, A.; Stefanizzi, P.; Pipoli, A.; Giannelli, G.; Delvecchio, G.; Tafuri, S.; Inchingolo, F.; Migliore, G.; et al. SARS-CoV-2 breakthrough infections in health care workers: An Italian retrospective cohort study on characteristics, clinical course and outcomes. J. Clin. Med. 2023, 12, 628. [Google Scholar] [CrossRef]
- Sunderkötter, C.; Wohlrab, J.; Hamm, H. Scabies: Epidemiology, diagnosis, and treatment. Dtsch. Arztebl. Int. 2021, 118, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Parasites—Scabies. Prevention & Control. Available online: https://www.cdc.gov/parasites/scabies/prevent.html (accessed on 30 March 2023).
- Romani, L.; Whitfeld, M.J.; Koroivueta, J.; Kama, M.; Wand, H.; Tikoduadua, L.; Tuicakau, M.; Koroi, A.; Andrews, R.; Kaldor, J.; et al. Mass drug administration for scabies control in a population with endemic disease. N. Engl. J. Med. 2015, 373, 2305–2313. [Google Scholar] [CrossRef] [PubMed]
- Engelman, D.; Marks, M.; Steer, A.C.; Beshah, A.; Biswas, G.; Chosidow, O.; Coffeng, L.E.; Dofitas, B.L.; Enbiale, W.; Fallah, M.; et al. A framework for scabies control. PLoS Negl. Trop. Dis. 2021, 15, e0009661. [Google Scholar] [CrossRef] [PubMed]
- Vorou, R.; Remoudaki, H.D.; Maltezou, H.C. Nosocomial scabies. J. Hosp. Infect. 2007, 65, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Gomes, N.; Guedes, P.M.; Norton, P.; Azevedo, F.; Lisboa, C. Dois surtos de escabiose num hospital terciário em Portugal [Two Scabies Outbreaks at a Tertiary Care Hospital in Portugal]. Acta Med. Port. 2020, 33, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Stoevesandt, J.; Carlé, L.; Leverkus, M.; Hamm, H. Control of large institutional scabies outbreaks: Institutional scabies outbreaks. J. Dtsch. Dermatol. Ges. 2012, 10, 637–647. [Google Scholar] [CrossRef]
- Karaca Ural, Z.; Çatak, B.; Ağaoğlu, E. Prevalence of scabies in the Covid-19 pandemic period and determination of risk factors for scabies: A hospital-based cross-sectional study in northeast Turkey. Acta Parasitol. 2022, 67, 802–808. [Google Scholar] [CrossRef]
- Dobner, J.; Kaser, S. Body mass index and the risk of infection—From underweight to obesity. Clin. Microbiol. Infect. 2018, 24, 24–28. [Google Scholar] [CrossRef] [Green Version]
- Capobussi, M.; Sabatino, G.; Donadini, A.; Tersalvi, C.A.; Castaldi, S. Control of scabies outbreaks in an Italian hospital: An information-centered management strategy. Am. J. Infect. Control 2014, 42, 316–320. [Google Scholar] [CrossRef]
- Hay, R.J.; Steer, A.C.; Chosidow, O.; Currie, B.J. Scabies: A suitable case for a global control initiative. Curr. Opin. Infect. Dis. 2013, 26, 107–109. [Google Scholar] [CrossRef]
- Engelman, D.; Steer, A.C. Diagnosis, treatment, and control of scabies: Can we do better? Lancet Infect. Dis. 2018, 18, 822–823. [Google Scholar] [CrossRef]

| Number of Cases of Confirmed or Suspected Scabies | ||||||||
|---|---|---|---|---|---|---|---|---|
| Month | Internal Medicine Division F. | Internal Medicine Division B. | Pneumology | Emergency Medicine and Surgery | Transfusion Medicine | Pediatric Oncology | Rheumatology | Cardiac Surgery |
| October 2022 | 9 | - | 1 | - | - | - | - | - |
| November 2022 | 3 | - | - | 1 | 1 | 1 | 1 | 1 |
| December 2022 | - | - | - | - | - | - | - | 1 |
| January 2023 | 1 | 1 | - | - | - | - | - | - |
| February 2023 | - | - | - | - | - | - | - | - |
| March 2023 | - | - | - | - | - | - | - | - |
| Variable | Scabei (n = 21) | Control (n = 162) | Total (n = 183) | p-Value |
|---|---|---|---|---|
| Age; mean ± SD (range) | 37.81 ± 12.56 (24–65) | 39.29 ± 13.07 (23–70) | 39.12 ± 12.99 (23–70) | 0.493 |
| Males; n (%) | 11 (52.38) | 60 (37.04) | 71 (38.80) | 0.175 |
| Internal Medicine F. Unit; n (%) | 13 (61.90) | 157 (96.91) | 170 (92.90) | <0.0001 |
| Job category; n (%) • Physician • Nurse • Other | 8 (38.10) 7 (33.33) 6 (28.57) | 106 (65.43) 29 (17.90) 27 (16.67) | 114 (62.30) 36 (19.67) 33 (18.03) | 0.051 |
| BMI; mean ± SD (range) | 24.34 ± 3.03 (19.56–29.23) | 24.11 ± 3.82 (17.04–42.83) | 24.14 ± 3.73 (17.04–42.82) | 0.653 |
| Smoke; n (%) | 6 (28.57) | 42 (25.93) | 48 (26.23) | 0.795 |
| Alcohol; n (%) | 6 (28.57) | 71 (43.83) | 77 (42.08) | 0.183 |
| Allergy: • dust mites; n (%) • other; n (%) | 3 (14.29) 1 (4.76) | 18 (11.11) 40 (24.69) | 21 (11.48) 41 (22.40) | 0.668 0.039 |
| Diabetes; n (%) | 1 (4.76) | 5 (3.09) | 6 (3.28) | 0.685 |
| Hypertension; n (%) | 2 (9.52) | 18 (11.11) | 20 (10.93) | 0.826 |
| Cardiovascular diseases; n (%) | 3 (14.29) | 7 (4.32) | 10 (5.46) | 0.059 |
| Lung diseases; n (%) | 8 (4.94) | 0 (0.00) | 8 (4.37) | 0.298 |
| Nephropathies; n (%) | 3 (1.85) | 0 (0.00) | 3 (1.64) | 0.529 |
| Steroids; n (%) | 8 (4.94) | 0 (0.00) | 8 (4.37) | 0.298 |
| Immunosuppression/depression; n (%) | 1 (4.76) | 2 (1.23) | 3 (1.64) | 0.231 |
| Other therapies; n (%) | 9 (42.86) | 55 (33.95) | 64 (34.97) | 0.421 |
| Determinant | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95%CI) | p-Value | aOR (95%CI) | p-Value | |
| Age | 0.99 (0.96–1.03) | 0.623 | 0.96 (0.91–1.02) | 0.138 |
| Sex (male vs. female) | 1.87 (0.75–4.66) | 0.179 | 3.12 (0.84–11.65) | 0.089 |
| Job category • nurse vs. physicians • other vs. physicians | 3.20 (1.07–9.55) 2.94 (0.94–9.20) | 0.037 0.063 | 7.05 (1.66–29.90) 9.05 (1.79–46.24) | 0.008 0.008 |
| BMI | 1.02 (0.90–1.14) | 0.793 | 0.95 (0.80–1.12) | 0.538 |
| Smoke habit | 1.14 (0.42–3.14) | 0.796 | 0.66 (0.20–2.17) | 0.494 |
| Alcohol | 0.51 (0.19–1.39) | 0.189 | 0.96 (0.29–3.19) | 0.941 |
| Allergies • Dust mites • Other | 1.33 (0.36–4.98) 0.15 (0.02–1.17) | 0.071 <0.0001 | 8.02 (1.10–58.29) 0.05 (0.01–0.57) | 0.040 0.016 |
| Diabetes | 1.57 (0.17–14.13) | 0.687 | 1.30 (0.10–17.60) | 0.843 |
| Hypertension | 0.84 (0.18–3.92) | 0.827 | 0.38 (0.04–4.10) | 0.426 |
| Cardiovascular diseases | 3.69 (0.88–15.54) | 0.075 | 1.63 (0.26–10.10) | 0.599 |
| Other therapies | 1.46 (0.58–3.67) | 0.423 | 2.19 (0.61–7.91) | 0.230 |
| Immunosuppression/depression; n (%) | 8.05 (0.48–133.78) | 0.146 | 1.64 (0.10–27.46) | 0.732 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sponselli, S.; De Maria, L.; Caputi, A.; Stefanizzi, P.; Bianchi, F.P.; Delvecchio, G.; Foti, C.; Romita, P.; Ambrogio, F.; Zagaria, S.; et al. Infection Control among Healthcare Workers and Management of a Scabies Outbreak in a Large Italian University Hospital. J. Clin. Med. 2023, 12, 3830. https://doi.org/10.3390/jcm12113830
Sponselli S, De Maria L, Caputi A, Stefanizzi P, Bianchi FP, Delvecchio G, Foti C, Romita P, Ambrogio F, Zagaria S, et al. Infection Control among Healthcare Workers and Management of a Scabies Outbreak in a Large Italian University Hospital. Journal of Clinical Medicine. 2023; 12(11):3830. https://doi.org/10.3390/jcm12113830
Chicago/Turabian StyleSponselli, Stefania, Luigi De Maria, Antonio Caputi, Pasquale Stefanizzi, Francesco Paolo Bianchi, Giuseppe Delvecchio, Caterina Foti, Paolo Romita, Francesca Ambrogio, Silvia Zagaria, and et al. 2023. "Infection Control among Healthcare Workers and Management of a Scabies Outbreak in a Large Italian University Hospital" Journal of Clinical Medicine 12, no. 11: 3830. https://doi.org/10.3390/jcm12113830







