Evaluation of the Factors Associated with Reinfections towards SARS-CoV-2 Using a Case Control Design
Abstract
1. Introduction
- Alpha (B.1.1.7): first variant of concern described in the United Kingdom (UK) in late December 2020
- Beta (B.1.351): first reported in South Africa in December 2020
- Gamma (P.1): first reported in Brazil in early January 2021
- Delta (B.1.617.2): first reported in India in December 2020
- Omicron (B.1.1.529): first reported in South Africa in November 2021 [2]
- A subsequent RT-PCR positive to SARS-CoV-2 > 45 days after the initial presentation if the second test is accompanied by compatible symptoms or epidemiological exposure;
- A subsequent RT-PCR positive to SARS-CoV-2 > 90 days after the initial presentation if the second test is performed among an asymptomatic HSM with close contact with a person known to have a laboratory-confirmed COVID-19. Thereafter, ‘probable reinfection’, defined by clinical context (symptoms, risk exposure) plus a Cycle Threshold (Ct) < 37 and the absence of other diagnoses, was assessed by an infectious disease specialist and a microbiologist [6].
- In subjects with the first diagnosis of COVID-19 notified for more than 210 days compared to those who had the first diagnosis of COVID-19 between the previous 90 and 210 days;
- In subjects not vaccinated or vaccinated with at least one dose for over 120 days compared to vaccinated with at least one dose within 120 days;
- In females compared to males. The greater risk in female subjects may probably be due to the greater presence of women in the school setting (>80%), where intense screening activity is carried out, and to the fact that women perform the role of caregiver in the field more frequently;
- In the younger age groups (from 12 to 49 years) compared to people with the first diagnosis between the ages of 50–59 years. Probably the greater risk of reinfection in the younger age groups is attributable to behaviors and exposures at greater risk, compared to the age groups over 60 years;
- In healthcare workers compared to the rest of the population [8].
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Variables
2.4. Statistical Analysis
- -
- Confidence level: 95%
- -
- Power: 80%
- -
- Expected frequency of chronic disease among controls: 20%
- -
- Odds ratio to detect: 2.
3. Results
- 142 physicians (35.3%);
- 182 nurses (45.3%);
- 10 technicians (2.5%);
- 17 administrative (4.2%);
- 51 healthcare assistants (12.7%).
3.1. Univariate Analysis
3.1.1. Reinfection vs. All Controls
3.1.2. Reinfection vs. Never Positive
3.1.3. Reinfection vs. Single Positivity
3.2. Multivariate Analysis
3.2.1. Reinfection vs. All Controls
3.2.2. Reinfection vs. Never Positive
3.2.3. Reinfection vs. Single Positivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Resende, P.C.; Bezerra, J.F.; Vasconcelos, R.H.T.; Arantes, I.; Appolinario, L.; Mendonça, A.C.; Paixao, A.C.; Duarte, A.C.; Silva, T.; Rocha, A.S.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 P.2 Lineage Associated with Reinfection Case, Brazil, June–October 2020. Emerg. Infect. Dis. 2021, 27, 1789–1794. [Google Scholar] [CrossRef] [PubMed]
- Aleem, A.; Akbar Samad, A.B.; Slenker, A.K. Emerging Variants of SARS-CoV-2 and Novel Therapeutics Against Coronavirus (COVID-19); StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Gopinath, S.; Ishak, A.; Dhawan, N.; Poudel, S.; Shrestha, P.S.; Singh, P.; Xie, E.; Tahir, P.; Marzaban, S.; Michel, J.; et al. Characteristics of COVID-19 Breakthrough Infections among Vaccinated Individuals and Associated Risk Factors: A Systematic Review. Trop. Med. Infect. Dis. 2022, 7, 81. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Zamora, E.; Trujillo, X.; Huerta, M.; Ríos-Silva, M.; Aguilar-Sollano, F.; Mendoza-Cano, O. Symptomatic SARS-COV-2 reinfection: Healthcare workers and immunosuppressed individuals at high risk. BMC Infect. Dis. 2021, 21, 923. [Google Scholar] [CrossRef] [PubMed]
- Guedes, A.R.; Oliveira, M.S.; Tavares, B.M.; Luna-Muschi, A.; Lazari, C.D.S.; Montal, A.C.; de Faria, E.; Maia, F.L.; Barboza, A.D.S.; Leme, M.D.; et al. Reinfection rate in a cohort of healthcare workers over 2 years of the COVID-19 pandemic. Sci. Rep. 2023, 13, 712. [Google Scholar] [CrossRef]
- Davido, B.; De Truchis, P.; Lawrence, C.; Annane, D.; Domart-Rancon, M.; Gault, E.; Saleh-Mghir, A.; Delarocque-Astagneau, E.; Gautier, S. SARS-CoV-2 reinfections among hospital staff in the greater Paris area. J. Travel Med. 2021, 28, taab058. [Google Scholar] [CrossRef]
- Ren, X.; Zhou, J.; Guo, J.; Hao, C.; Zheng, M.; Zhang, R.; Huang, Q.; Yao, X.; Li, R.; Jin, Y. Reinfection in patients with COVID-19: A systematic review. Glob. Health Res. Policy 2022, 7, 12. [Google Scholar] [CrossRef]
- Istituto Superiore di Sanità. Report Esteso ISS, “COVID-19: Sorveglianza, Impatto delle Infezioni ed Efficacia delle Vaccinazioni”, Rome, 17 August 2022. Available online: https://www.epicentro.iss.it/coronavirus/bollettino/Bollettino-sorveglianza-integrata-COVID-19_17-agosto-2022.pdf (accessed on 1 May 2023).
- Murthy, P.; Narasimha, V.L. Effects of the COVID-19 pandemic and lockdown on alcohol use disorders and complications. Curr. Opin. Psychiatry 2021, 34, 376–385. [Google Scholar] [CrossRef]
- Piazza, M.F.; Amicizia, D.; Marchini, F.; Astengo, M.; Grammatico, F.; Battaglini, A.; Sticchi, C.; Paganino, C.; Lavieri, R.; Andreoli, G.B.; et al. Who Is at Higher Risk of SARS-CoV-2 Reinfection? Results from a Northern Region of Italy. Vaccines 2022, 10, 1885. [Google Scholar] [CrossRef]
- Ochoa-Hein, E.; Leal-Morán, P.E.; Nava-Guzmán, K.A.; Vargas-Fernández, A.T.; Vargas-Fernández, J.F.; Díaz-Rodríguez, F.; Rayas-Bernal, J.A.; González-González, R.; Vázquez-González, P.; Huertas-Jiménez, M.A.; et al. Significant rise in SARS-CoV-2 reinfection rate in vaccinated Hospital workers during the omicron wave: A prospective cohort study. Rev. Investig. Clin. 2022, 74, 175–180. [Google Scholar] [CrossRef]
- Boule, L.A.; Kovacs, E.J. Alcohol, aging, and innate immunity. J. Leukoc. Biol. 2017, 102, 41–55. [Google Scholar] [CrossRef]
- Pal, R.; Banerjee, M. Are people with uncontrolled diabetes mellitus at high risk of reinfections with COVID-19? Prim. Care Diabetes 2020, 15, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Peleg, A.Y.; Weerarathna, T.; McCarthy, J.S.; Davis, T.M.E. Common infections in diabetes: Pathogenesis, management and relationship to glycaemic control. Diabetes/Metab. Res. Rev. 2006, 23, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Martinez, P.J.; Mathews, C.; Actor, J.K.; Hwang, S.-A.; Brown, E.L.; De Santiago, H.K.; Hoch, S.P.F.; McCormick, J.B.; Mirza, S. Impaired CD4+ and T-helper 17 cell memory response to Streptococcus pneumoniae is associated with elevated glucose and percent glycated hemoglobin A1c in Mexican Americans with type 2 diabetes mellitus. Transl. Res. 2013, 163, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Azam, M.; Sulistiana, R.; Ratnawati, M.; Fibriana, A.I.; Bahrudin, U.; Widyaningrum, D.; Aljunid, S.M. Recurrent SARS-CoV-2 RNA positivity after COVID-19: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 20692. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.N.; Nguyen, Y.N.; Hoang, V.T.; Million, M.; Gautret, P. SARS-CoV-2 Reinfection and Severity of the Disease: A Systematic Review and Meta-Analysis. Viruses 2023, 15, 967. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Zamora, E.; Mendoza-Cano, O.; Delgado-Enciso, I.; Hernandez-Suarez, C. Predictors of severe symptomatic laboratory-confirmed SARS-CoV-2 reinfection. Public Health 2021, 193, 113–115. [Google Scholar] [CrossRef]
- Ghorbani, S.S.; Taherpour, N.; Bayat, S.; Ghajari, H.; Mohseni, P.; Nazari, S.S.H. Epidemiologic characteristics of cases with reinfection, recurrence, and hospital readmission due to COVID-19: A systematic review and meta-analysis. J. Med. Virol. 2021, 94, 44–53. [Google Scholar] [CrossRef]
- OECD. Women at the Core of the Fight against COVID-19 Crisis. 2020. Available online: https://read.oecd-ilibrary.org/view/?ref=127_127000-awfnqj80me&title=Women-at-the-core-of-the-fight-against-COVID-19-crisis (accessed on 1 May 2023).
- Bechmann, N.; Barthel, A.; Schedl, A.; Herzig, S.; Varga, Z.; Gebhard, C.; Mayr, M.; Hantel, C.; Beuschlein, F.; Wolfrum, C.; et al. Sexual dimorphism in COVID-19: Potential clinical and public health implications. Lancet Diabetes Endocrinol. 2022, 10, 221–230. [Google Scholar] [CrossRef]
- Hall, V.J.; Foulkes, S.; Charlett, A.; Atti, A.; Monk, E.J.M.; Simmons, R.; Wellington, E.; Cole, M.J.; Saei, A.; Oguti, B.; et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: A large, multicentre, prospective cohort study (SIREN). Lancet 2021, 397, 1459–1469. [Google Scholar] [CrossRef]
- Encabo, H.H.; Grey, W.; Garcia-Albornoz, M.; Wood, H.; Ulferts, R.; Aramburu, I.V.; Kulasekararaj, A.G.; Mufti, G.; Papayannopoulos, V.; Beale, R.; et al. Human Erythroid Progenitors Are Directly Infected by SARS-CoV-2: Implications for Emerging Erythropoiesis in Severe COVID-19 Patients. Stem Cell Rep. 2021, 16, 428–436. [Google Scholar] [CrossRef]
- Taneri, P.E.; Gómez-Ochoa, S.A.; Llanaj, E.; Raguindin, P.F.; Rojas, L.Z.; Roa-Díaz, Z.M.; Salvador, D., Jr.; Groothof, D.; Minder, B.; Kopp-Heim, D.; et al. Anemia and iron metabolism in COVID-19: A systematic review and meta-analysis. Eur. J. Epidemiol. 2020, 35, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Misiti, F. SARS-CoV-2 infection and red blood cells: Implications for long term symptoms during exercise. Sports Med. Health Sci. 2021, 3, 181–182. [Google Scholar] [CrossRef] [PubMed]
- Kubánková, M.; Hohberger, B.; Hoffmanns, J.; Fürst, J.; Herrmann, M.; Guck, J.; Kräter, M. Physical phenotype of blood cells is altered in COVID-19. Biophys. J. 2021, 120, 2838–2847. [Google Scholar] [CrossRef] [PubMed]
- Piri, S.M.; Edalatfar, M.; Shool, S.; Jalalian, M.N.; Tavakolpour, S. A systematic review on the recurrence of SARS-CoV-2 virus: Frequency, risk factors, and possible explanations. Infect. Dis. 2021, 53, 315–324. [Google Scholar] [CrossRef]
- Deng, L.; Li, P.; Zhang, X.; Jiang, Q.; Turner, D.; Zhou, C.; Gao, Y.; Qian, F.; Zhang, C.; Lu, H.; et al. Risk of SARS-CoV-2 reinfection: A systematic review and meta-analysis. Sci. Rep. 2022, 12, 20763. [Google Scholar] [CrossRef] [PubMed]
| Variables | N (%) or Mean (SD) [Range] |
|---|---|
| Gender | |
| Females | 250 (62.3) |
| Males | 151 (37.7) |
| Age | 43.03 (12.33) |
| Profession | |
| Physicians | 142 (35.3) |
| Nurses | 182 (45.3) |
| Technicians | 10 (2.5) |
| Administrative | 17 (4.2) |
| Healthcare Assistants | 51 (12.7) |
| Reinfection | |
| Yes | 134 (33.4) |
| No | 267 (66.6) |
| Smoking | |
| No | 244 (62.4) |
| Yes | 147 (37.6) |
| Audit C | |
| 0 | 120 (31.2) |
| 1 | 105 (27.3) |
| 2 | 108 (28.1) |
| 3 | 36 (9.4) |
| 4 | 11 (2.9) |
| 5 | 4 (1.0) |
| 6 | 1 (0.3) |
| Physical activity | |
| No | 173 (44.9) |
| <4 h per week | 146 (37.9) |
| 4 < h < 8 per week | 55 (14.3) |
| >8 h per week | 11 (2.9) |
| Diabetes | |
| No | 369 (93.4) |
| Yes | 26 (6.6) |
| Hypertension | |
| No | 337 (86.0) |
| Yes | 55 (14.0) |
| Drugs use | |
| No | 213 (54.6) |
| Yes | 177 (45.4) |
| Biological hazard | |
| No | 14 (3.6) |
| Yes | 378 (96.4) |
| Manual handling of loads | |
| No | 200 (51.0) |
| Yes | 192 (49.0) |
| Night shift | |
| No | 88 (22.6) |
| Yes | 302 (77.4) |
| Low-dose ionizing radiations exposure | |
| No | 336 (85.7) |
| Yes | 56 (14.3) |
| BMI | 24.53 (4.68) |
| Red blood cells (×1012/L) | 4.40 (1.40) [3.77–6.61] |
| Haemoglobin (g/dL) | 14.18 (1.38) [8.00–18.03] |
| Platelets (×109/L) | 242.71 (98.67) [94–1785] |
| White blood cells (×109/L) | 6.04 (2.53) [3.12–13.50] |
| Reinfection vs. All Controls | |||
|---|---|---|---|
| Variable | Reinfection (%) | All Controls (%) | p |
| Gender | |||
| Females | 48 (35.8) | 103 (38.6) | 0.59 |
| Males | 86 (64.2) | 164 (61.4) | |
| Age | 43.13 (12.51) | 42.99 (12.25) | 0.91 |
| Profession | |||
| Physicians | 46 (34.3) | 96 (35.8) | 0.98 |
| Nurses | 62 (46.3) | 120 (44.8) | |
| Technicians | 4 (3.0) | 6 (2.2) | |
| Administrative | 6 (4.5) | 11 (4.1) | |
| Healthcare Assistants | 16 (11.9) | 35 (13.1) | |
| Smoking | |||
| No | 76 (60.3) | 168 (63.4) | 0.55 |
| Yes | 50 (39.7) | 97 (36.6) | |
| Audit C | |||
| 0 | 27 (21.4) | 93 (35.9) | 0.002 |
| 1 | 29 (23.0) | 76 (29.3) | |
| 2 | 43 (34.1) | 65 (25.1) | |
| 3 | 21 (16.7) | 15 (5.8) | |
| 4 | 4 (3.2) | 7 (2.7) | |
| 5 | 2 (1.6) | 2 (0.8) | |
| 6 | 0 (0.0) | 1 (0.4) | |
| Physical activity | |||
| No | 61 (48.8) | 112 (43.1) | 0.48 |
| <4 h per week | 42 (33.6) | 104 (40.0) | |
| 4 < h < 8 per week | 17 (13.6) | 38 (14.6) | |
| >8 h per week | 5 (4.0) | 6 (2.3) | |
| Diabetes | |||
| No | 114 (87.0) | 255 (96.6) | <0.001 |
| Yes | 17 (13.0) | 9 (3.4) | |
| Hypertension | |||
| No | 111 (85.4) | 226 (86.3) | 0.81 |
| Yes | 19 (14.6) | 36 (13.7) | |
| Obesity | |||
| No | 115 (87.8) | 238 (90.5) | 0.40 |
| Yes | 16 (14.6) | 25 (9.5) | |
| Biological hazard | |||
| No | 6 (4.6) | 8 (3.1) | 0.43 |
| Yes | 124 (95.4) | 254 (96.9) | |
| Manual handling of loads | |||
| No | 67 (51.5) | 133 (50.8) | 0.88 |
| Yes | 63 (48.5) | 129 (49.2) | |
| Night shift | |||
| No | 32 (24.8) | 56 (21.5) | 0.45 |
| Yes | 97 (75.2) | 205 (78.5) | |
| BMI | 24.66 (4.5) | 24.45 (4.7) | 0.68 |
| Red blood cells (×1012/L) | 4.80 (0.49) | 4.17 (1.66) | <0.001 |
| Haemoglobin (g/dL) | 14.13 (1.18) | 14·21 (60.26) | 0.56 |
| Platelets (×109/L) | 245.50 (147.52) | 241·30 (60.26) | 0.69 |
| White blood cells (×109/L) | 6.52 (1.74) | 5.78 (2.83) | 0.007 |
| Drugs | |||
| No | 72 (55.4) | 141 (54.2) | 0.82 |
| Yes | 58 (44.6) | 119 (45.8) | |
| Vaccination against COVID-19 | |||
| No | 14 (10.4%) | 19 (7.1) | 0.248 |
| Yes | 120 (89.6) | 249 (92.9%) | |
| Reinfection vs. Never Positive | |||
|---|---|---|---|
| Variable | Reinfection (%) | Never Positive (%) | p |
| Gender | |||
| Females | 48 (35.8) | 53 (39.3) | 0.56 |
| Males | 86 (64.2) | 82 (60.7) | |
| Age | 43.13 (12.51) | 43.16 (12.29) | 0.98 |
| Profession | 0.97 | ||
| Physicians | 46 (34.3) | 48 (35.6) | |
| Nurses | 62 (46.3) | 61 (45.2) | |
| Technicians | 4 (3.0) | 3 (2.2) | |
| Administrative | 6 (4.5) | 6 (4.4) | |
| Healthcare Assistants | 16 (11.9) | 17 (12.6) | |
| Smoking | 0.27 | ||
| No | 76 (60.3) | 81 (60.0) | |
| Yes | 50 (39.7) | 54 (40.0) | |
| Audit C | <0.001 | ||
| 0 | 27 (21.4) | 48 (35.8) | |
| 1 | 29 (23.0) | 44 (32.8) | |
| 2 | 43 (34.1) | 35 (26.1) | |
| 3 | 21 (16.7) | 3 (2.2) | |
| 4 | 4 (3.2) | 3 (2.2) | |
| 5 | 2 (1.6) | 0 (0.0) | |
| 6 | 0 (0.0) | 1 (0.7) | |
| Physical activity | 0.63 | ||
| No | 61 (48.8) | 65 (48.5) | |
| <4 h per week | 42 (33.6) | 46 (34.3) | |
| 4 < h < 8 per week | 17 (13.6) | 21 (15.7) | |
| >8 h per week | 5 (4.0) | 2 (1.5) | |
| Diabetes | <0.001 | ||
| No | 114 (87.0) | 134 (99.3) | |
| Yes | 17 (13.0) | 1 (0.7) | |
| Hypertension | 0.14 | ||
| No | 111 (85.4) | 123 (91.1) | |
| Yes | 19 (14.6) | 12 (8.9) | |
| Obesity | 0.19 | ||
| No | 115 (87.8) | 124 (92.5) | |
| Yes | 16 (12.2) | 10 (7.5) | |
| Biological hazard | 0.95 | ||
| No | 6 (4.6) | 6 (4.5) | |
| Yes | 124 (95.4) | 128 (95.5) | |
| Manual handling of loads | 0.89 | ||
| No | 67 (51.5) | 68 (50.7) | |
| Yes | 63 (48.5) | 66 (49.3) | |
| Night shift | 0.45 | ||
| No | 32 (24.8) | 28 (20.9) | |
| Yes | 97 (75.2) | 106 (79.1) | |
| Ionizing radiations | 0.32 | ||
| No | 109 (83.8) | 118 (88.1) | |
| Si | 21 (16.2) | 16 (11.9) | |
| BMI | 24.66 (4.5) | 24·28 (4.76) | 0.49 |
| Red blood cells (×1012/L) | 4.80 (0.49) | 3·52 (2.13) | <0.001 |
| Haemoglobin (g/dL) | 14.3 (1.18) | 14·23 (1.47) | 0.55 |
| Platelets (×109/L) | 245.50 (147.52) | 241·83 (56.21) | 0.79 |
| White blood cells (×109/L) | 6.52 (1.74) | 5·06 (3.44) | <0.001 |
| Drugs | 0.47 | ||
| No | 72 (55.4) | 80 (59.7) | |
| Si | 58 (44.6) | 54 (40.3) | |
| Vaccination against COVID-19 | 0.516 | ||
| No | 14 (10.4%) | 11 (8.1) | |
| Yes | 120 (89.6) | 124 (91.9%) | |
| Reinfection vs. Single Positivity | |||
|---|---|---|---|
| Variable | Reinfection (%) | Single Positivity (%) | p |
| Gender | 0.72 | ||
| Females | 48 (35.8) | 50 (37.9) | |
| Males | 86 (64.2) | 82 (62.1) | |
| Age | 43.13 (12.51) | 42.80 (12.26) | 0.83 |
| Profession | 0.97 | ||
| Physicians | 46 (343) | 48 (36.1) | |
| Nurses | 62 (46.3) | 59 (44.4) | |
| Technicians | 4 (3.0) | 3 (2.3) | |
| Administrative | 6 (4.5) | 5 (3.8) | |
| Healthcare Assistants | 16 (11.9) | 18 (13.5) | |
| Smoking | 0.27 | ||
| No | 76 (60.3) | 87 (66.9) | |
| Yes | 50 (39.7) | 43 (33.1) | |
| Audit C | 0.09 | ||
| 0 | 27 (21.4) | 45 (36.0) | |
| 1 | 29 (23.0) | 32 (25.6) | |
| 2 | 43 (34.1) | 30 (24.0) | |
| 3 | 21 (16.7) | 12 (9.6) | |
| 4 | 4 (3.2) | 4 (3.2) | |
| 5 | 2 (1.6) | 2 (1.6) | |
| 6 | 0 (0.0) | 0 (0.0) | |
| Physical activity | 0.21 | ||
| No | 61 (48.8) | 47 (37.3) | |
| <4 h per week | 42 (33.6) | 58 (46.0) | |
| 4 < h < 8 per week | 17 (13.6) | 17 (13.5) | |
| >8 h per week | 5 (4.0) | 4 (3.2) | |
| Diabetes | 0.064 | ||
| No | 114 (87.0) | 121 (93.8) | |
| Yes | 17 (13.0) | 8 (6.2) | |
| Hypertension | 0.35 | ||
| No | 111 (85.4) | 103 (81.1) | |
| Yes | 19 (14.6) | 24 (18.9) | |
| Obesity | 0.88 | ||
| No | 115 (87.8) | 114 (88.4) | |
| Yes | 16 (12.2) | 15 (11.6) | |
| Biological hazard | 0.15 | ||
| No | 6 (4.6) | 2 (1.6) | |
| Yes | 124 (95.4) | 126 (98.4) | |
| Manual handling of loads | 0.90 | ||
| No | 67 (51.5) | 65 (50.8) | |
| Yes | 63 (48.5) | 63 (49.2) | |
| Night shift | 0.60 | ||
| No | 32 (24.8) | 28 (22.0) | |
| Yes | 97 (75.2) | 99 (78.0) | |
| Ionizing radiations | 0.77 | ||
| No | 109 (83.8) | 109 (85.2) | |
| Si | 21 (16.2) | 19 (14.8) | |
| BMI | 24.66 (4.5) | 24.64 (4.80) | 0.97 |
| Red blood cells (×1012/L) | 4.80 (0.49) | 4.81 (0.46) | 0.87 |
| Haemoglobin (g/dL) | 14.13 (1.18) | 14.20 (1.48) | 0.66 |
| Platelets (×109/L) | 245.50 (147.52) | 240.74 (64.53) | 0.74 |
| White blood cells (×109/L) | 6·52 (1.74) | 6.52 (1.77) | 0.99 |
| Drugs | 0.26 | ||
| No | 72 (55.4) | 61 (48.4) | |
| Si | 58 (44.6) | 65 (51.6) | |
| Vaccination against COVID-19 | 0.188 | ||
| No | 14 (10.4%) | 8 (6) | |
| Yes | 120 (89.6) | 125 (914%) | |
| Reinfection vs. All Controls | ||
|---|---|---|
| Reinfection | All Controls | |
| Variable | Full Model OR [IC 95%] | Stepwise Model OR [IC 95%] |
| Gender | ||
| Males (ref.) | 1 | 1 |
| Females | 2.05 (1.01–4.20) | 2.42 (1.38–4.25) |
| Age | 1.01 (0.99–1.04) | |
| BMI | 0.98 (0.92–1.05) | |
| Profession | ||
| Physicians | 0 | |
| Nurses | 0 | |
| Technicians | 0 | |
| Administrative | 0 | |
| Healthcare Assistants | 0 | |
| Other healthcare workers (ref) | 1 | |
| Drugs | ||
| Yes | 0.75 (0.42–1.34) | |
| No (ref) | 1 | |
| Smoking | ||
| Yes | ||
| No (ref) | 1.25 (0.74–2.11) | |
| Audit C | 1.53 (1.20–1.95) | 1.49 (1.19–1.87) |
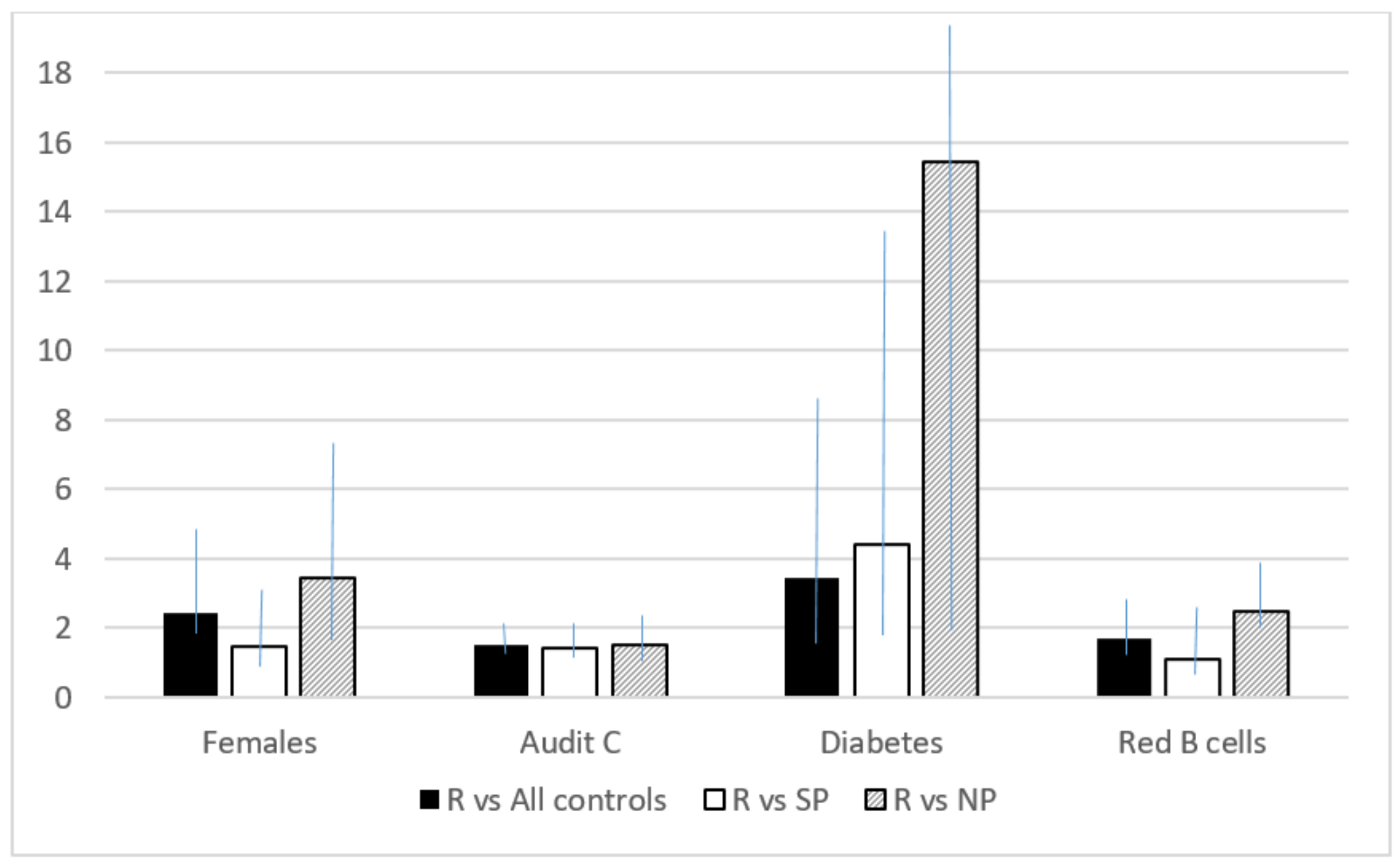
| Diabetes | 3.45 (1.41–8.46) | |
| Yes | 5.82 (1.99–17.06) | |
| No (ref) | 1 | |
| Hypertension | ||
| Yes | 0.58 (0.21–1.64) | |
| No (ref) | 1 | |
| Weekly physical activity | 0.99 (0.91–1.37) | |
| Red blood cells (×1012/L) | 1.99 (1.35–2.94) | 1.69 (1.21–2.25) |
| Haemoglobin g/dL | 0.85 (0.67–1.08) | |
| White blood cells (×109/L) | 0.91 (0.79–1.05) | |
| Reinfection vs. Never Positive | ||
|---|---|---|
| Reinfection | Never Positive | |
| Variable | Full Model OR [IC 95%] | Stepwise Model OR [IC 95%] |
| Gender | ||
| Males (ref.) | 1 | 1 |
| Females | 2.96 (1.22–7.22) | 3.42 (1.66–7.03) |
| Age | 1.00 (0.98–1.04) | |
| BMI | 1.00 (0.92–1.09) | |
| Profession | ||
| Physicians | 0 | |
| Nurses | 0 | |
| Technicians | 0 | |
| Administrative | 0 | |
| Healthcare Assistants | 0 | |
| Other healthcare workers (ref) | 1 | |
| Drugs | ||
| Yes | 0.76 (0.38–1.54) | |
| No (ref) | 1 | |
| Smoking | ||
| Yes | 1.16 (0.61–2.22) | |
| No (ref) | 1 | |
| Audit C | 1.58 (1.14–2.18) | 1.51 (1.13–2.01) |
| Diabetes | ||
| Yes | 16.10 (1.88–137.58) | 15.42 (1.92–123.6) |
| No (ref) | 1 | |
| Hypertension | ||
| Yes | 1.58 (0.39–6.46) | |
| No (ref) | 1 | |
| Weekly physical activity | 1.07 (0.72–1.57) | |
| Red blood cells (×1012/L) | 2.66 (1.69–4.20) | 2.47 (1.63–3.74) |
| Haemoglobin g/dL | 0.88 (0.65–1.19) | |
| White blood cells (×109/L) | 0.84 (0.70–1.02) | |
| Reinfection vs. Single Positivity | ||
|---|---|---|
| Reinfection | Single Positivity | |
| Variable | Full Model OR [IC 95%] | Stepwise Model OR [IC 95%] |
| Gender | ||
| Males (ref.) | 1 | |
| Females | 1.48 (0.63–3.46) | |
| Age | 1.01 (0.98–1.04) | |
| BMI | 0.98 (0.91–1.06) | |
| Profession | ||
| Physicians | 0 | |
| Nurses | 0 | |
| Technicians | 0 | |
| Administrative | 0 | |
| Healthcare Assistants | 0 | |
| Other healthcare workers (ref) | 1 | |
| Drugs | ||
| Yes | 0.70 (0.35–1.38) | |
| No (ref) | 1 | |
| Smoking | ||
| Yes | 1.31 (0.72–2.41) | |
| No (ref) | 1 | |
| Audit C | 1.54 (1.16–2.04) | 1.40 (1.09–1.79) |
| Diabetes | ||
| Yes | 4.51 (1.40–14.61) | 4.39 (1.41–13.67) |
| No (ref) | 1 | 1 |
| Hypertension | ||
| Yes | 0.33 (0.10–1.08) | 0.28 (0.11–0.74) |
| No (ref) | 1 | 1 |
| Weekly physical activity | 0.83 (0.56–1.23) | |
| Red blood cells (×1012/L) | 1.09 (0.49–2.43) | |
| Haemoglobin g/dL | 0.91 (0.67–1.25) | |
| White blood cells (×109/L) | 0.96 (0.80–1.14) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Torre, G.; Paglione, G.; Barone, L.C.; Cammalleri, V.; Faticoni, A.; Marte, M.; Pocino, R.N.; Previte, C.M.; Bongiovanni, A.; Colaprico, C.; et al. Evaluation of the Factors Associated with Reinfections towards SARS-CoV-2 Using a Case Control Design. J. Clin. Med. 2023, 12, 3861. https://doi.org/10.3390/jcm12113861
La Torre G, Paglione G, Barone LC, Cammalleri V, Faticoni A, Marte M, Pocino RN, Previte CM, Bongiovanni A, Colaprico C, et al. Evaluation of the Factors Associated with Reinfections towards SARS-CoV-2 Using a Case Control Design. Journal of Clinical Medicine. 2023; 12(11):3861. https://doi.org/10.3390/jcm12113861
Chicago/Turabian StyleLa Torre, Giuseppe, Gianluca Paglione, Lavinia Camilla Barone, Vittoria Cammalleri, Augusto Faticoni, Mattia Marte, Roberta Noemi Pocino, Carlo Maria Previte, Andrea Bongiovanni, Corrado Colaprico, and et al. 2023. "Evaluation of the Factors Associated with Reinfections towards SARS-CoV-2 Using a Case Control Design" Journal of Clinical Medicine 12, no. 11: 3861. https://doi.org/10.3390/jcm12113861
APA StyleLa Torre, G., Paglione, G., Barone, L. C., Cammalleri, V., Faticoni, A., Marte, M., Pocino, R. N., Previte, C. M., Bongiovanni, A., Colaprico, C., Ricci, E., Imeshtari, V., Manai, M. V., Shaholli, D., Barletta, V. I., Carluccio, G., Moretti, L., Vezza, F., Volpicelli, L., ... Sernia, S. (2023). Evaluation of the Factors Associated with Reinfections towards SARS-CoV-2 Using a Case Control Design. Journal of Clinical Medicine, 12(11), 3861. https://doi.org/10.3390/jcm12113861