Red Wine and Sexual Function in Men: An Original Point of View
Abstract
:1. Introduction
2. Red Wine and Gonadal Function
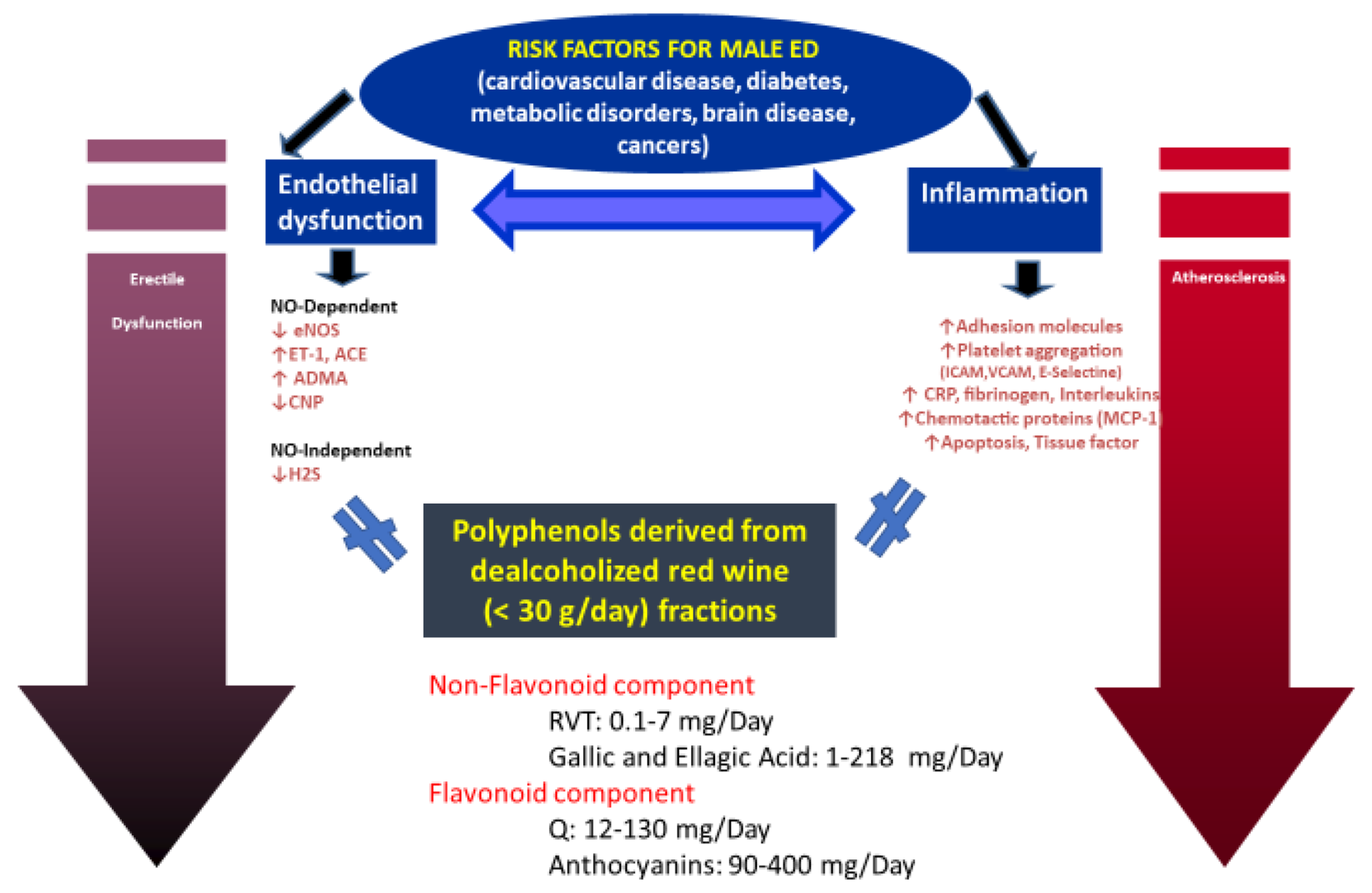
3. Red Wine Polyphenols and Erectile Dysfunction
4. Red Wine Polyphenols and Gonad-Related Hormones
5. Red Wine and Female Sexual Function
6. Effects of Alcohol
7. Limitations of the Study and Caution in Data Interpretation
8. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fernandes, L.; Pérez-Gregorio, R.; Soares, S.; Mateus, N.; de Freitas, V. Wine Flavonoids in Health and Disease Prevention. Molecules 2017, 22, 292. [Google Scholar] [CrossRef] [PubMed]
- Nemzer, B.; Kalita, D.; Yashin, A.Y.; Yashin, Y.I. Chemical Composition and Polyphenolic Compounds of Red Wines: Their Antioxidant Activities and Effects on Human Health—A Review. Beverages 2022, 8, 1. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouysegu, L. Plant polyphenols: Chemical properties, biological activities and synthesis. Angew. Chem. Int. Ed. Engl. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Hano, C.; Tungmunnithum, D. Plant Polyphenols, more than just simple natural antioxidants: Oxidative stress, aging and age-related diseases. Medicines 2020, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Guo, H.; Fang, Y.; Zhou, G. The mechanisms of wine phenolic compounds for preclinical anticancer therapeutics. Food Nutr. Res. 2021, 65, 6507. [Google Scholar] [CrossRef]
- Markoski, M.M.; Garavaglia, J.; Oliveira, A.; Olivaes, J.; Marcadenti, A. Molecular Properties of Red Wine Compounds and Cardiometabolic Benefits. Nutr. Metab. Insights 2016, 9, 51–57. [Google Scholar] [CrossRef]
- Cordova, A.C.; Sumpio, B.E. Polyphenols are medicine: Is it time to prescribe red wine for our patients? Int. J. Angiol. 2009, 18, 111–117. [Google Scholar] [CrossRef]
- Bell, J.; Donnovan, J.; Wong, R.; Waterhouse, A.L.; German, J.B.; Walzem, R.L.; Kasim-Karakas, S.E. (+)-catechin in human plasma after ingestion of a single serving of reconstituted red wine. Am. J. Clin. Nutr. 2000, 71, 103–108. [Google Scholar] [CrossRef]
- Di-Castelnuovo, A.; Rotondo, S.; Lacoviello, L.; Donati, M.B.; De Gaetano, G. Meta-analysis of wine and beer consumption in relation to vascular risk. Circulation 2002, 105, 2836–2844. [Google Scholar] [CrossRef]
- Corrao, G.; Rubbiati, L.; Bagnardi, V.; Zambon, A.; Poikolamen, C. Alcohol and coronary heart disease: A meta-analysis. Addiction 2000, 95, 1505–1523. [Google Scholar] [CrossRef]
- Maclure, M. Demonstration of deductive meta-analysis: Ethanol intake and risk of myocardiac infarction. Epidemiol. Rev. 1993, 15, 328–351. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, B.; Aviram, M. Flavonoids protect LDL from oxidation and attenuate atherosclerosis. Curr. Opin. Lipidol. 2001, 12, 41–48. [Google Scholar] [CrossRef]
- Nigdikar, S.V.; Williams, N.R.; Griffin, B.A.; Howard, A.N. Consumption of red wine polyphenols reduces the susceptibility of low-density lipoproteins to oxidation in vivo. Am. J. Clin. Nutr. 1998, 68, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Oczkowski, M.; Średnicka-Tober, D.; Stachon, M.; Kołota, A.; Wolińska-Witort, E.; Malik, A.; Hallmann, E.; Rusaczonek, A.; Gromadzka-Ostrowska, J. The effect of red wine consumption on hormonal reproductive parameters and total antioxidant status in young adult male rats. Food Funct. 2014, 5, 2096–2105. [Google Scholar] [CrossRef] [PubMed]
- Artero, A.; Artero, A.; Tarín, J.J.; Cano, A. The impact of moderate wine consumption on health. Maturitas 2015, 80, 3–13. [Google Scholar] [CrossRef]
- Tekos, F.; Makri, S.; Skaperda, Z.-V.; Patouna, A.; Kalliroi, T.; Kyriazis, I.D.; Kotseridis, Y.; Mikropoulou, E.V.; Papaefstathiou, G.; Halabalaki, M.; et al. Assessment of Antioxidant and Antimutagenic Properties of Red and White Wine Extracts In Vitro. Metabolites 2021, 11, 436. [Google Scholar] [CrossRef]
- Xiang, L.; Xiao, L.; Wang, Y.; Li, H.; Huang, Z.; He, X. Health benefits of wine: Don’t expect resveratrol too much. Food Chem. 2014, 156, 258–263. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Urpi-Sarda, M.; Llorach, R.; Rotches-Ribalta, M.; Guillen, M.; Casas, R.; Arranz, S.; Valderas-Martinez, P.; Portoles, O.; Corella, D. Differential Effects of Polyphenols and Alcohol of Red Wine on the Expression of Adhesion Molecules and Inflammatory Cytokines Related to Atherosclerosis: A Randomized Clinical Trial. Am. J. Clin. Nutr. 2011, 95, 326–334. [Google Scholar] [CrossRef]
- Renaud, S.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Epidemiology 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Catalgol, B.; Batirel, S.; Taga, Y.; Ozer, N.K. Resveratrol: French Paradox Revisited. Front. Pharmacol. 2012, 3, 141–159. [Google Scholar] [CrossRef]
- Kaur, G.; Rao, L.V.M.; Agrawal, A.; Pendurthi, U.R. Effect of wine phenolics on cytokine-induced C-reactive protein expression. J. Thromb. Haemost. 2007, 5, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, I.; Spagnuolo, C.; Russo, G.; Russo, M.; Cervellera, C.; Moccia, S. The Pro-Oxidant Activity of Red Wine Polyphenols Induces an Adaptive Antioxidant Response in Human Erythrocytes. Antioxidants 2021, 10, 800. [Google Scholar] [CrossRef] [PubMed]
- Shafreen, R.M.B.; Lakshmi, S.A.; Pandian, S.K.; Kim, Y.-M.; Deutsch, J.; Katrich, E.; Gorinstein, S. In Vitro and In Silico Interaction Studies with Red Wine Polyphenols against Different Proteins from Human Serum. Molecules 2021, 26, 6686. [Google Scholar] [CrossRef] [PubMed]
- Snopec, L.; Mlcek, J.; Sochorova, L.; Baron, M.; Hlavacova, I.; Jurikova, T.; Kizek, R.; Sedlackova, E.; Sochor, J. Contribution of red wine consumption to human health protection. Molecules 2018, 23, 1684. [Google Scholar] [CrossRef]
- Cavallini, G.; Straniero, S.; Donati, A.; Bergamini, E. Resveratrol requires red wine polyphenols for optimum antioxidant activity. J. Nutr. Health Aging 2016, 20, 540–545. [Google Scholar] [CrossRef]
- Montorsi, P.; Montorsi, F.; Schulman, C.C. Is erectile dysfunction the “tip of the iceberg” of a systemic vascular disorder? Eur. Urol. 2003, 44, 352–354. [Google Scholar] [CrossRef]
- Torres, A.; Cachofeiro, V.; Millán, J.; Lahera, V.; Nieto, M.; Martin, R.; Bello, E.; Alvarez-Sala, L.; Nieto, M. Red wine intake but not other alcoholic beverages increases total antioxidant capacity and improves pro-inflammatory profile after an oral fat diet in healthy volunteers. Rev. Clínica Española 2015, 215, 486–494. [Google Scholar] [CrossRef]
- Nova, E.; San Mauro-Martín, I.; Díaz-Prieto, L.E.; Marcos, A. Wine and beer within a moderate alcohol intake is associated with higher levels of HDL-c and adiponectin. Nutr. Res. 2019, 63, 42–50. [Google Scholar] [CrossRef]
- Castaldo, L.; Narváez, A.; Izzo, L.; Graziani, G.; Gaspari, A.; Minno, G.D.; Ritieni, A. Red Wine Consumption and Cardiovascular Health. Molecules 2019, 24, 3626. [Google Scholar] [CrossRef]
- Muñoz-Bernal, Ó.A.; Coria-Oliveros, A.J.; de la Rosa, L.A.; Rodrigo-García, J.; Del Rocío Martínez-Ruiz, N.; Sayago-Ayerdi, S.G.; Alvarez-Parrilla, E. Cardioprotective effect of red wine and grape pomace. Food Res. Int. 2021, 140, 110069–110076. [Google Scholar] [CrossRef]
- De Paula, G.C.; de Oliveira, J.; Engel, D.F.; Lopes, S.C.; Moreira, E.L.G.; Figueiredo, C.P.; Prediger, R.D.; Fabro de Bem, A. Red wine consumption mitigates the cognitive impairments in low-density lipoprotein receptor knockout (LDLr-/-) mice. Nutr. Neurosci. 2021, 24, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhao, Y. Absorption and metabolism of red wine polyphenols and their potential health benefits in cardiovascular function. Am. J. Clin. Nutr. 2012, 95, 1496–1497, author reply 1497–1498. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Arranz, S.; Lamuela-Raventos, R.M.; Estruch, R. Effects of Wine, Alcohol and Polyphenols on Cardiovascular Disease Risk Factors: Evidences from Human Studies. Alcohol Alcohol. 2013, 48, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Duluc, L.; Jacques, C.; Soleti, R.; Iacobazzi, F.; Simard, G.; Andriantsitohaina, R. Modulation of mitochondrial capacity and angiogenesis by red wine polyphenols via estrogen receptor, NADPH oxidase and nitric oxide synthase pathways. Int. J. Biochem. Cell Biol. 2013, 45, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.K.; Pace-Asciak, C.R. Vasorelaxing activity of resveratrol and quercetin in isolated rat aorta. Gen. Pharmacol. 1996, 27, 363–366. [Google Scholar] [CrossRef]
- Shen, M.; Zhao, L.; Wu, R.X.; Yue, S.Q.; Pei, J.M. The vasorelaxing effect of resveratrol on abdominal aorta from rats and its underlying mechanisms. Vascul. Pharmacol. 2013, 58, 64–70. [Google Scholar] [CrossRef]
- Boydens, C.; Pauwels, B.; Decaluwé, K.; Brouckaert, P.; Van de Voorde, J. Relaxant and Antioxidant Capacity of the Red Wine Polyphenols, Resveratrol and Quercetin, on Isolated Mice Corpora Cavernosa. J. Sex. Med. 2014, 12, 303–312. [Google Scholar] [CrossRef]
- Neves, D.R.G.L.M.; Tomada, I.M.A.S.C.M.; Assunção, M.M.B.; Marques, F.A.P.; Almeida, H.M.N.; Andrade, J.P.A.V. Effects of Chronic Red Wine Consumption on the Expression of Vascular Endothelial Growth Factor, Angiopoietin 1, Angiopoietin 2, and Its Receptors in Rat Erectile Tissue. J. Food Sci. 2010, 75, 79–86. [Google Scholar] [CrossRef]
- Yetik-Anacak, G.; Dereli, M.V.; Sevin, G.; Ozzayım, O.; Erac, Y.; Ahmed, A. Resveratrol Stimulates Hydrogen Sulfide (H2S) Formation to Relax Murine Corpus Cavernosum. J. Sex. Med. 2015, 12, 2004–2012. [Google Scholar] [CrossRef]
- Monteiro, R.; Azevedo, I.; Calhau, C. Modulation of aromatase activity by diet polyphenolic compounds. J. Agric. Food Chem. 2006, 54, 3535–3540. [Google Scholar] [CrossRef]
- Jenkinson, C.; Petroczi, A.; Naughton, D.P. Red wine and component flavonoids inhibit UGT2B17 in vitro. Nutr. J. 2012, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Radisavljevic, Z.M.; Siroky, M.B.; Azadzoi, K.M. Dietary antioxidants improve arteriogenic erectile dysfunction. Int. J. Androl. 2010, 34, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Gholami, S.S.; Rogers, R.; Chang, J.; Ho, H.C.; Grazziottin, T.; Lin, C.S.; Lue, T.F. The effect of vascular endothelial growth factor and adeno-associated virus mediated brain derived neurotrophic factor on neurogenic and vasculogenic erectile dysfunction induced by hyperlipidemia. J. Urol. 2003, 169, 1577–1581. [Google Scholar] [CrossRef]
- Kwangsung, P.; Kyu, Y.A.; Min-Kyung, K.; Song, E.L.; Taek, W.K.; Soo-Bang, R. Intracavernosal Injection of Vascular Endothelial Growth Factor Improves Erectile Function in Aged Rats. Eur. Urol. 2004, 46, 403–407. [Google Scholar] [CrossRef]
- Isaac, U.E.; Obeten, K.E.; Igiri, A.O. Effects of regular administration of red wine on testicular profile and body weight in adult male experimental model. J. Clin. Med. Kaz. 2021, 18, 75–80. [Google Scholar] [CrossRef]
- Kaya, C.; Uslu, Z.; Karaman, I. Is endothelial function impaired in erectile dysfunction patients? Int. J. Impot. Res. 2006, 18, 55–60. [Google Scholar] [CrossRef]
- Mondaini, N.; Cai, T.; Gontero, P.; Gavazzi, A.; Lombardi, G.; Boddi, V.; Bartoletti, R. Regular Moderate Intake of Red Wine Is Linked to a Better Women’s Sexual Health. J. Sex. Med. 2009, 6, 2772–2777. [Google Scholar] [CrossRef]
- Chew, K.K.; Bremner, A.; Stuckey, B.; Earle, C.; Jamrozik, K. Alcohol consumption and male erectile dysfunction: An unfounded reputation for risk? J. Sex. Med. 2009, 6, 1386–1394. [Google Scholar] [CrossRef]
- Anil, K.B.; Shalini, M.; Sanjai, R.J.; Prasannakumar, D.R. Sexual dysfunction in women with alcohol dependence syndrome: A study from India. Asian J. Psychiatr. 2017, 28, 9–14. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Urpi-Sarda, M.; Ros, E.; Arranz, S.; Valderas-Martínez, P.; Casas, R.; Sacanella, E.; Llorach, R.; Lamuela-Raventos, R.M.; Andres-Lacueva, C.; et al. Dealcoholized Red Wine Decreases Systolic and Diastolic Blood Pressure and Increases Plasma Nitric Oxide. Circ. Res. 2012, 111, 1065–1068. [Google Scholar] [CrossRef]
- Golan, R.; Gepner, Y.; Shai, I. Wine and Health—New Evidence. Eur. J. Clin. Nutr. 2018, 72, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Corder, R.; Mullen, W.; Khan, N.Q.; Marks, S.C.; Wood, E.G.; Carrier, M.J.; Crozier, A. Red wine procyanidins and vascular health. Nature 2006, 444, 566. [Google Scholar] [CrossRef]
- Bartoletti, R.; Mondaini, N.; Cai, T. Red Wine, Sex, and a Genius. Eur. Urol. 2008, 53, 231–233. [Google Scholar] [CrossRef] [PubMed]
- Yuyun, M.F.; Ng, L.L.; Ng, G.A. Endothelial dysfunction, endothelial nitric oxide bioavailability, tetrahydrobiopterin, and 5-methyltetrahydrofolate in cardiovascular disease. Where are we with therapy? Microvasc. Res. 2018, 119, 7–12. [Google Scholar] [CrossRef]
- Corder, R. Endothelin and Its Inhibitors. In Handbook of Experimental Pharmacology; Warner, T.D., Ed.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 35–67. [Google Scholar]
- Burnett, A.L. The Role of Nitric Oxide in Erectile Dysfunction: Implications for Medical Therapy. J. Clin. Hypertens. 2006, 8, 53–62. [Google Scholar] [CrossRef]
- Little, P.J.; Askew, C.D.; Xu, S.; Kamato, D. Endothelial Dysfunction and Cardiovascular Disease: History and Analysis of the Clinical Utility of the Relationship. Biomedicines 2021, 9, 699. [Google Scholar] [CrossRef]
- Cheng, Y.-C.; Sheen, J.-M.; Hu, W.L.; Hung, Y.-C. Polyphenols and Oxidative Stress in Atherosclerosis-Related Ischemic Heart Disease and Stroke. Oxid. Med. Cell Longev. 2017, 2017, 8526438. [Google Scholar] [CrossRef]
- Feinman, L.; Lieber, C.S. Ethanol and lipid metabolism. Am. J. Clin. Nutr. 1999, 70, 791–792. [Google Scholar] [CrossRef]
- Li, J.M.; Mukamal, K.J. An update on alcohol and atherosclerosis. Curr. Opin. Lipidol. 2004, 15, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Saremi, A.; Rohit, A. The Cardiovascular Implications of Alcohol and Red Wine. Am. J. Ther. 2008, 15, 265–277. [Google Scholar] [CrossRef]
- Wollin, S.D.; Jones, P.J.H. Alcohol, Red Wine and Cardiovascular Disease. J. Nutr. 2001, 131, 1401–1404. [Google Scholar] [CrossRef] [PubMed]
- Arranz, S.; Chiva-Blanch, G.; Valderas-Martínez, P.; Medina-Remón, A.; Lamuela-Raventós, R.M.; Estruch, R. Wine, beer, alcohol and polyphenols on cardiovascular disease and cancer. Nutrients 2012, 4, 759–781. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Esumi, H. Reciprocal regulation between nitric oxide and vascular endothelial growth factor in angiogenesis. Acta Biochim. Pol. 2003, 50, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H. Hydrogen sulfide as a neuromodulator. Mol. Neurobiol. 2002, 26, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Pauls, R.N. Anatomy of the clitoris and the female sexual response. Clin. Anat. 2015, 28, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Mollaioli, D.; Ciocca, G.; Limoncin, E.; Di Sante, S.; Gravina, G.L.; Carosa, E.; Lenzi, A.; Jannini, E.A.F. Lifestyles and sexuality in men and women: The gender perspective in sexual medicine. Reprod. Biol. Endocrinol. 2020, 18, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Peugh, J.; Belenko, S. Alcohol, drugs, and sexual function: A review. J. Psychoact. Drugs 2001, 33, 223–332. [Google Scholar] [CrossRef]
- Condorelli, R.A.; Calogero, A.E.; Vicari, E.; La Vignera, S. Chronic consumption of alcohol and sperm parameters: Ourexperience and the main evidences. Andrologia 2015, 47, 368–379. [Google Scholar] [CrossRef]
- Leiblum, S.R.; Rosen, R.C. Alcohol and human sexual response. In Alcoholism and Sexual Dysfunction: Issues in Clinical Management; Powell, D.J., Ed.; Haworth Press: Binghampton, NY, USA, 1984. [Google Scholar]
- Sam, F.E.; Ma, T.-Z.; Salifu, R.; Wang, J.; Jiang, Y.-M.; Zhang, B.; Han, S.-Y. Techniques for Dealcoholization of Wines: Their Impact on Wine Phenolic Composition, Volatile Composition, and Sensory Characteristics. Foods 2021, 10, 2498. [Google Scholar] [CrossRef]
- Shirai, M.; Miyoshi, Y.; Ogasa, T.; Miyoshi, M.; Ishikawa, K.; Hiramatsu, I.; Uesaka, Y.; Nozaki, T.; Koyama, T.; Tsujimura, A. Oral Testofen, L-Citrulline, Resveratrol, and Caffeine Supplement Drink Improves Sexual Function in Men with Phosphodiesterase 5 Inhibitors: Randomized, Double-Blind, Placebo-Controlled Crossover Pilot Study. World J. Mens Health 2021, 39, 733–739. [Google Scholar] [CrossRef]
- Fusco, G.M.; Cirillo, L.; Abate, M.; Morra, S.; Morgera, V.; Barone, B.; Crocetto, F.; Cacace, G.; Di Bello, F.; Spirito, L.; et al. Male infertility, what Mobile Health Applications “know”: Quality analysis and adherence to European Association of Urology Guidelines. Arch. Ital. Urol. Androl. 2022, 94, 470–475. [Google Scholar] [CrossRef] [PubMed]
| In Vitro | In Vivo | In Humans |
|---|---|---|
| Tekos, F. et al., 2021 Metabolites [16] Kaur, G. et al., 2007 J Thromb Haemost. [21] Tedesco, I. et al., 2021 Antioxidants [22] Shafreen, R.M.B. et al., 2021 Molecules [23] Cavallini, G. et al., 2016 J Nutr Health Aging [25] Duluc, L. et al., 2013 The International Journal of Biochemistry & Cell Biology [34] Chen, C.K. et al., 1996 Gen Pharmacol. [35] Shen, M. et al., 2013 Vascul Pharmacol. [36] Boydens, C. et al., 2014 J Sex Med. [37] Neves, D.R.G.L.M. et al., 2010 J Food Sci. [38] Yetik-Anacak, G. et al., 2015 J Sex Med. [39] Monteiro, R. et al., 2006 J Agric Food Chem. [40] Jenkinson, C. et al., 2012 Nutr. J. [41] | Oczkowski, M. et al., 2014 Food Funct. [14] De Paula, G.C. et al., 2021 Nutr Neurosci. [31] Zhang, Q. et al., 2010 Int J Androl. [42] Gholami, S.S. et al., 2003 J Urol [43] Kwangsung, P. et al., 2004 Eur Urol. [44] Isaac, U.E. et al., 2021 J Clin Med Kaz [45] | Bell, J. et al., 2000 Am J Clin Nutr. [8] Nigdikar, S.V. et al., 1998 Am J Clin Nutr. [13] Chiva-Blanch, G. et al., 2011 Am. J. Clin. Nutr. [18] Torres, A. et al., 2015 Revista Clínica Española [27] Nova, E. et al., 2019. Nutr Res. [28] Kaya, C. et al., 2006 Int J Impot Res. [46] Mondaini, N. et al., 2009 J Sex Med. [47] Chew, K.K. et al., 2009 J Sex Med. 2009 [48] Anil, K.B. et al., 2017 Asian J Psychiatr. [49] Chiva-Blanch, G. et al., 2012 Circulation Research [50] |
| Participants | Dose/Methods | Effects | |
|---|---|---|---|
| ell, J. et al., 2000 Am J Clin Nutr. [8] | 9 (5 men, 4 women) | 120 mL/d of dealcoholized red wine reconstituted with either water or water and alcohol. | Moderate consumption of both red wine reconstituted with either water or water and alcohol induces an increase in the blood of (+)-catechin levels. |
| Nigdikar, S.V. et al., 1998 Am J Clin Nutr. [13] | 30 men | 375 mL/d of red wine or white wine; 1 g/d of red wine polyphenols in capsules, 1 g/d red wine polyphenols dissolved in white wine, or 400 mL/d alcoholic drink as vodka and lemonade (containing no polyphenols). | Red wine consumption provides beneficial effect on LDL oxidation. |
| Chiva-Blanch, G. et al., 2011 Am. J. Clin. Nutr. [18] | 67 men | 272 mL/d of red wine or dealcoholized red wine or 100 mL/d of gin. | Phenolic compounds in red wine interfere with leukocyte adhesion molecules. Both portions of ethanol and polyphenols in red wine modulate soluble inflammatory mediators. |
| Torres, A. et al., 2015 Revista Clínica Española [27] | 16 (8 men, 8 women) | 16 g/m2 of alcohol or different beverages (red wine, vodka, brandy, or rum). | Moderate red wine consumption improves both pro-inflammatory factors and serum antioxidant capacity (decreased in mean concentrations of hsCRP, TNFα, and IL-6) after a pro-atherogenic meal. |
| Nova, E. et al., 2019. Nutr Res. [28] | 143 (56 men, 87 women) | Less than 4 alcoholic drinks per month (abstainers and occasional consumers); beer more than 80% of total alcohol intake (beer consumers); and wine, beer, and liquor (mixed beverage consumers). Total alcohol intake (g/d) was calculated as average grams of alcohol content per 100 mL of each alcoholic beverage. | Moderate red wine intake decreases pro-inflammatory factors and increased total antioxidant capacity (higher levels of HDL-c and adiponectin). |
| Kaya, C. et al., 2006 Int J Impot Res. [46] | 57 men | Administration of the Sexual Health Inventory for Men (SHIM) 5-item questionnaire based on the International Index of Erectile Function (IIEF) questionnaire. Physical examination and analysis of fasting serum glucose and triglyceride, HDL cholesterol, and LDL cholesterol levels. | Reduction of NO bioavailability in patients with ED is related to structural and functional endothelial changes in small vessels of the penis. |
| Mondaini, N. et al., 2009 J Sex Med. [47] | 798 women | One to two glasses of red wine (wine consumers) or less than one glass per day (occasional drinkers of wine or other alcoholic beverages). | Regular, moderate red wine improves sexual functioning in women (sexual desire and lubrication with an overall improvement in sexual function). |
| Chew, K.K. et al., 2009 J Sex Med. 2009 [48] | 1580 men | Less than 1, 1 to 20, or more than 20 standard drinks a week, or less than 1, 1–5, or more than 5 days each week (current drinkers). Five or more standard drinks on one, or more days each week (binge drinkers). More than 20 standard drinks a week, or more than 4 standard drinks a day on 1 day, or more a week (high-risk drinkers). A standard drink consists of 375 mL of beer (3–4%), 100 mL of glass of wine (10–14%), or 30 mL nip of spirits (37–43%). | Moderate consumption of alcohol has a protective effect on ED. |
| Anil, K.B. et al., 2017 Asian J Psychiatr. [49] | 80 women | 90 to 359 mL/d of alcohol or 360 mL/d and above. | Alcohol dependence causes sexual dysfunction (low sex desire and orgasm-related problems.). |
| Chiva-Blanch, G. et al., 2012 Circulation Research [50] | 67 men | 100 mL/d of gin (30 g ethanol/day), 272 mL/d of red wine (30 g ethanol/day), or 272 mL/d of dealcoholized red wine. | Dealcoholized red wine decreases systolic and diastolic blood pressure via a NO-dependent mechanism. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basile, L.; Condorelli, R.A.; Calogero, A.E.; Cannarella, R.; Barbagallo, F.; Crafa, A.; Aversa, A.; La Vignera, S. Red Wine and Sexual Function in Men: An Original Point of View. J. Clin. Med. 2023, 12, 3883. https://doi.org/10.3390/jcm12123883
Basile L, Condorelli RA, Calogero AE, Cannarella R, Barbagallo F, Crafa A, Aversa A, La Vignera S. Red Wine and Sexual Function in Men: An Original Point of View. Journal of Clinical Medicine. 2023; 12(12):3883. https://doi.org/10.3390/jcm12123883
Chicago/Turabian StyleBasile, Livia, Rosita A. Condorelli, Aldo E. Calogero, Rossella Cannarella, Federica Barbagallo, Andrea Crafa, Antonio Aversa, and Sandro La Vignera. 2023. "Red Wine and Sexual Function in Men: An Original Point of View" Journal of Clinical Medicine 12, no. 12: 3883. https://doi.org/10.3390/jcm12123883
APA StyleBasile, L., Condorelli, R. A., Calogero, A. E., Cannarella, R., Barbagallo, F., Crafa, A., Aversa, A., & La Vignera, S. (2023). Red Wine and Sexual Function in Men: An Original Point of View. Journal of Clinical Medicine, 12(12), 3883. https://doi.org/10.3390/jcm12123883









