Finerenone: Questions and Answers—The Four Fundamental Arguments on the New-Born Promising Non-Steroidal Mineralocorticoid Receptor Antagonist
Abstract
:1. Introduction
2. Finerenone: Why Another MRA?
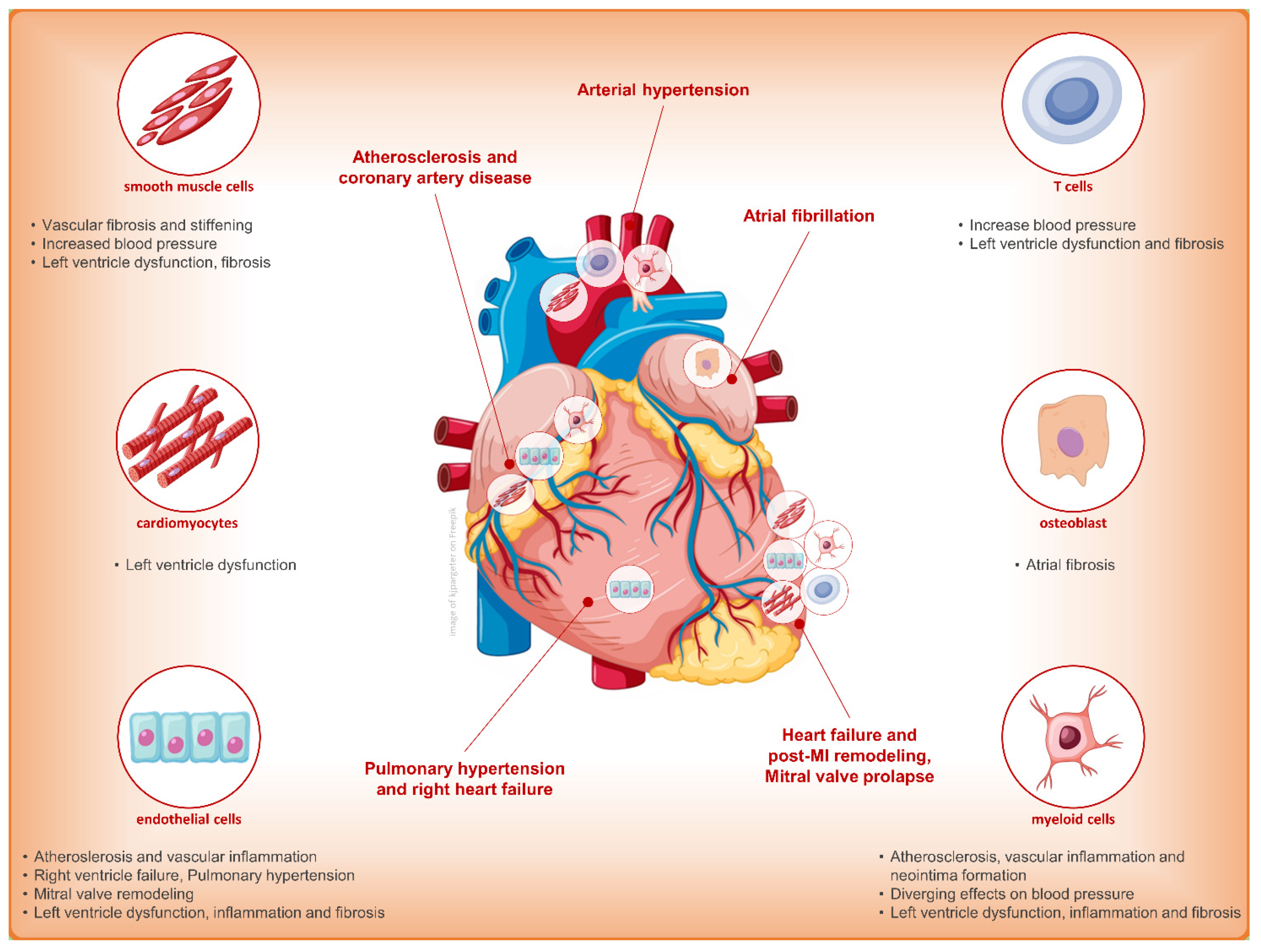
3. Finerenone and Cardiovascular Outcome: Only Diuretic or Something Else?
4. Finerenone and Diabetic Nephropathy: A New and Ultimate Cornerstone?
5. Finerenone and Perspectives: Stand Alone or in SGLT2i Joint Future for Renoprotection?
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Diabetes Association. 11. Micorvascular complications and foot care standards of medical care in diabetes-2020. Diabetes Care 2020, 43, S135–S151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, R.; Kolkhof, P.; Bakris, G.; Bauersachs, J.; Haller, H.; Wada, T.; Zannad, F. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur. Heart J. 2021, 42, 152–161. [Google Scholar] [CrossRef]
- Agarwal, R.; Anker, S.D.; Bakris, G.; Filippatos, G.; Pitt, B.; Rossing, P.; Ruilope, L.; Gebel, M.; Kolkhof, P.; Nowack, C.; et al. Investigating new treatment opportunities for patients with chronic kidney disease in type 2 diabetes: The role of finerenone. Nephrol. Dial. Transplantat. 2022, 37, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.; Ghanim, H.; Arora, P. Improving the residual risk of renal and cardiovascular outcomes in diabetic kidney disease: A review of pathophysiology, mechanisms, and evidence from recent trials. Diabetes Obes. Metab. 2022, 24, 365–376. [Google Scholar] [CrossRef]
- Lerma, E.; White, W.B.; Bakris, G. Effectiveness of nonsteroidal mineralocorticoid receptor antagonists in patients with diabetic kidney disease. Postgrad. Med. 2022, 135, 224–233. [Google Scholar] [CrossRef]
- Kintscher, U.; Bakris, G.L.; Kolkhof, P. Novel non-steroidal mineralocorticoid receptor antagonists in cardiorenal disease. Br. J. Pharmacol. 2022, 179, 3220–3234. [Google Scholar] [CrossRef]
- Barrera-Chimal, J.; Kolkhof, P.; Lima-Posada, I.; Joachim, A.; Rossignol, P.; Jaisser, F. Differentiation between emerging non-steroidal and established steroidal mineralocorticoid receptor antagonists: Head-to-head comparisons of pharmacological and clinical characteristics. Expert Opin. Investig. Drugs 2021, 30, 1141–1157. [Google Scholar] [CrossRef]
- Pitt, B.; Filippatos, G.; Gheorghiade, M.; Kober, L.; Krum, H.; Ponikowski, P.; Nowack, C.; Kolkhof, P.; Kim, S.Y.; Zannad, F. Rationale and design of ARTS: A randomized, double-blind study of BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease. Eur. J. Heart Fail. 2012, 14, 668–675. [Google Scholar] [CrossRef] [Green Version]
- Bramlage, P.; Swift, S.L.; Thoenes, M.; Minguet, J.; Ferrero, C.; Schmieder, R.E. Non-steroidal mineralocorticoid receptor antagonism for the treatment of cardiovascular and renal disease. Eur. J. Heart Fail. 2016, 18, 28–37. [Google Scholar] [CrossRef] [Green Version]
- Amazit, L.; Billan, F.L.; Kolkhof, P.; Lamribet, K.; Viengchareun, S.; Fay, M.R.; Khan, J.A.; Hillisch, A.; Lombès, M.; Rafestin-Oblin, M.E.; et al. Finerenone impedes aldosterone-dependent nuclear import of the mineralocorticoid receptor and prevents genomic recruitment of steroid receptor coactivator-1. J. Biol. Chem. 2015, 290, 21876–21889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, V.; Joharapurkar, A.; Jain, M. Role of mineralocorticoid receptor antagonists in kidney diseases. Drug Dev. Res. 2021, 82, 341–363. [Google Scholar] [CrossRef]
- Tesch, G.H.; Young, M.J. Mineralocorticoid receptor signaling as a therapeutic target for renal and cardiac fibrosis. Front. Pharmacol. 2017, 8, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Pandey, A.K.; Bhatt, D.L.; Cosentino, F.; Marx, N.; Rotstein, O.; Pitt, B.; Pandey, A.; Butler, J.; Verma, S. Non-steroidal mineralocorticoid receptor antagonists in cardiorenal disease. Eur. Heart J. 2022, 43, 2931–2945. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, A.; Yao, L.; Nagai, Y.; Miyata, K.; Yoshizumi, M.; Kagami, S.; Kondo, S.; Kiyomoto, H.; Shokoji, T.; Kimura, S.; et al. Possible Contributions of Reactive Oxygen Species and Mitogen-Activated Protein Kinase to Renal Injury in Aldosterone/Salt-Induced Hypertensive Rats. Hypertension 2004, 43, 841–848. [Google Scholar] [CrossRef] [Green Version]
- Mathew, J.T.; Patni, H.; Chaudhary, A.N.; Liang, W.; Gupta, A.; Chander, P.N.; Ding, G.; Singhal, P.C. Aldosterone induces mesangial cell apoptosis both in vivo and in vitro. Am. J. Physiol. Renal Physiol. 2008, 295, F73–F81. [Google Scholar] [CrossRef] [Green Version]
- Diah, S.; Zhang, G.X.; Nagai, Y.; Zhang, W.; Gang, L.; Kimura, S.; Hamid, M.R.A.; Tamiya, T.; Nishiyama, A.; Hitomi, H. Aldosterone induces myofibroblastic transdifferentiation and collagen gene expression through the Rho-kinase dependent signaling pathway in rat mesangial cells. Exp. Cell Res. 2008, 314, 3654–3662. [Google Scholar] [CrossRef]
- Shibata, S.; Ishizawa, K.; Uchida, S. Mineralocorticoid receptor as a therapeutic target in chronic kidney disease and hypertension. Hypertens. Res. 2017, 40, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Livingston, M.J.; Liu, Z.; Dong, Z. Autophagy in kidney homeostasis and disease. Nat. Rev. Nephrol. 2020, 16, 489–508. [Google Scholar] [CrossRef] [PubMed]
- Hashikabe, Y.; Suzuki, K.; Jojima, T.; Uchida, K.; Hattori, Y. Aldosterone impairs vascular endothelial cell function. J. Cardiovasc. Pharmacol. 2006, 47, 609–613. [Google Scholar] [CrossRef]
- Iwashima, F.; Yoshimoto, T.; Minami, I.; Sakurada, M.; Hirono, Y.; Hirata, Y. Aldosterone induces superoxide generation via Rac1 activation in endothelial cells. Endocrinology 2008, 149, 1009–1014. [Google Scholar] [CrossRef]
- Jaffe, I.Z.; Tintut, Y.; Newfell, B.G.; Demer, L.L.; Mendelsohn, M.E. Mineralocorticoid receptor activation promotes vascular cell calcification. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 799–805. [Google Scholar] [CrossRef] [Green Version]
- Barrera-Chimal, J.; Rocha, L.; Amador-Martínez, I.; Pérez-Villalva, R.; González, R.; Cortés-González, C.; Uribe, N.; Ramírez, V.; Berman, N.; Gamba, G.; et al. Delayed spironolactone administration prevents the transition from acute kidney injury to chronic kidney disease through improving renal inflammation. Nephrol. Dial. Transplant. 2019, 34, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Schierke, F.; Wyrwoll, M.J.; Wisdorf, M.; Niedzielski, L.; Maase, M.; Ruck, T.; Meuth, S.G.; Kusche-Vihrog, K. Nanomechanics of the endothelial glycocalyx contribute to Na+—Induced vascular inflammation. Sci. Rep. 2017, 7, 46476. [Google Scholar] [CrossRef] [Green Version]
- Banki, N.F.; Ver, A.; Wagner, L.J.; Vannay, A.; Degrell, P.; Prokai, A.; Gellai, R.; Lenart, L.; Szakal, D.N.; Kenesei, E.; et al. Aldosterone antagonists in monotherapy are protective against streptozotocin-induced diabetic nephropathy in rats. PLoS ONE 2012, 7, e39938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lian, M.; Hewitson, T.D.; Wigg, B.; Samuel, C.S.; Chow, F.; Becker, G.J. Long-term mineralocorticoid receptor blockade ameliorates progression of experimental diabetic renal disease. Nephrol. Dial. Transplant. 2012, 27, 906–912. [Google Scholar] [CrossRef] [Green Version]
- Lachaux, M.; Barrera-Chimal, J.; Nicol, L.; Rémy-Jouet, I.; Renet, S.; Dumesnil, A.; Wecker, D.; Richard, V.; Kolkhof, P.; Jaisser, F.; et al. Short- and long-term administration of the non-steroidal mineralocorticoid receptor antagonist finerenone opposes metabolic syndrome-related cardio-renal dysfunction. Diabetes Obes. Metab. 2018, 20, 2399–2407. [Google Scholar] [CrossRef]
- Kolkhof, P.; Bärfacker, L. Mineralocorticoid receptor antagonists: 60 years of research and development. J. Endocrinol. 2017, 234, T125–T140. [Google Scholar] [CrossRef] [Green Version]
- Pitt, B.; Zannad, F.; Remme, W.J.; Cody, R.; Castaigne, A.; Perez, A.; Palensky, J.; Wittes, J. The Effect of Spironolactone on Morbidity and Mortality in Patients with Severe Heart Failure. N. Engl. J. Med. 1999, 341, 709–717. [Google Scholar] [CrossRef] [Green Version]
- Pitt, B.; Remme, W.; Zannad, F.; Neaton, J.; Martinez, F.; Roniker, B.; Bittman, R.; Hurley, S.; Kleiman, J.; Gatlin, M. Eplerenone, a Selective Aldosterone Blocker, in Patients with Left Ventricular Dysfunction after Myocardial Infarction. N. Engl. J. Med. 2003, 348, 1309–1321. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [PubMed]
- Savarese, G.; Carrero, J.J.; Pitt, B.; Anker, S.D.; Rosano, G.M.C.; Dahlström, U.; Lund, L.H. Factors associated with underuse of mineralocorticoid receptor antagonists in heart failure with reduced ejection fraction: An analysis of 11 215 patients from the Swedish Heart Failure Registry. Eur. J. Heart Fail. 2018, 20, 1326–1334. [Google Scholar] [CrossRef]
- Carey, R.M.; Calhoun, D.A.; Bakris, G.L.; Brook, R.D.; Daugherty, S.L.; Dennison-Himmelfarb, C.R.; Egan, B.M.; Flack, J.M.; Gidding, S.S.; Judd, E.; et al. Resistant hypertension: Detection, evaluation, and management a scientific statement from the American Heart Association. Hypertension 2018, 72, e53–e90. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 Practice Guidelines for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens. 2018, 36, 2284–2309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakris, G.L.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Nowack, C.; Schloemer, P.; Joseph, A.; et al. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, R.W.; Nicolaisen, S.K.; Hasvold, P.; Sanchez, R.G.; Pedersen, L.; Adelborg, K.; Egstrup, K.; Egfjord, M.; Sørensen, H.T. Elevated potassium levels in patients with chronic kidney disease: Occurrence, risk factors and clinical outcomes—A Danish population-based cohort study. Nephrol. Dial. Transplant. 2018, 33, 1610–1620. [Google Scholar] [CrossRef] [Green Version]
- Barrera-Chimal, J.; Lima-Posada, I.; Bakris, G.L.; Jaisser, F. Mineralocorticoid receptor antagonists in diabetic kidney disease—Mechanistic and therapeutic effects. Nat. Rev. Nephrol. 2022, 18, 56–70. [Google Scholar] [CrossRef]
- Hene, R.J.; Boer, P.; Koomans, H.A.; Dorhout Mees, E.J. Plasma aldosterone concentrations in chronic renal disease. Kidney Int. 1982, 21, 98–101. [Google Scholar] [CrossRef] [Green Version]
- Reams, G.P.; Bauer, J.H. Effect of Enalapril in Subjects with Hypertension Associated with Moderate to Severe Renal Dysfunction. Arch. Intern. Med. 1986, 146, 2145–2148. [Google Scholar] [CrossRef]
- Bomback, A.S.; Klemmer, P.J. The incidence and implications of aldosterone breakthrough. Nat. Clin. Pract. Nephrol. 2007, 3, 486–492. [Google Scholar] [CrossRef]
- Hou, J.; Xiong, W.; Cao, L.; Wen, X.; Li, A. Spironolactone Add-on for Preventing or Slowing the Progression of Diabetic Nephropathy: A Meta-analysis. Clin. Ther. 2015, 37, 2086–2103. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.Y.M.; Ruospo, M.; Natale, P.; Bolignano, D.; Navaneethan, S.D.; Palmer, S.C.; Strippoli, G.F.M. Aldosterone antagonists in addition to renin angiotensin system antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst. Rev. 2020, 10, CD007004. [Google Scholar]
- Chen, Y.; Liu, P.; Chen, X.; Li, Y.; Zhang, F.; Wang, Y. Effects of Different Doses of Irbesartan Combined with Spironolactone on Urinary Albumin Excretion Rate in Elderly Patients with Early Type 2 Diabetic Nephropathy. Am. J. Med. Sci. 2018, 355, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Tofte, N.; Lindhardt, M.; Adamova, K.; Bakker, S.J.L.; Beige, J.; Beulens, J.W.J.; Birkenfeld, A.L.; Currie, G.; Delles, C.; Dimos, I.; et al. Early detection of diabetic kidney disease by urinary proteomics and subsequent intervention with spironolactone to delay progression (PRIORITY): A prospective observational study and embedded randomised placebo-controlled trial. Lancet Diabetes Endocrinol. 2020, 8, 301–312. [Google Scholar] [CrossRef]
- Epstein, M.; Buckalew, V.; Altamirano, J.; Roniker, B.; Krause, S.; Kleiman, J. Eplerenone reduces proteinuria in type II diabetes mellitus: Implications for aldosterone involvement in the pathogenesis of renal dysfunction. J. Am. Coll. Cardiol. 2002, 39, 249. [Google Scholar] [CrossRef] [Green Version]
- Le Billan, F.; Perrot, J.; Carceller, E.; Travers, S.; Viengchareun, S.; Kolkhof, P.; Lombès, M.; Fagart, J. Antagonistic effects of finerenone and spironolactone on the aldosterone-regulated transcriptome of human kidney cells. FASEB J. 2021, 35, e21314. [Google Scholar] [CrossRef]
- Grune, J.; Beyhoff, N.; Smeir, E.; Chudek, R.; Blumrich, A.; Ban, Z.; Brix, S.; Betz, I.R.; Schupp, M.; Foryst-Ludwig, A.; et al. Selective Mineralocorticoid Receptor Cofactor Modulation as Molecular Basis for Finerenone’s Antifibrotic Activity. Hypertension 2018, 71, 599–608. [Google Scholar] [CrossRef]
- Barrera-Chimal, J.; Estrela, G.R.; Lechner, S.M.; Giraud, S.; El Moghrabi, S.; Kaaki, S.; Kolkhof, P.; Hauet, T.; Jaisser, F. The myeloid mineralocorticoid receptor controls inflammatory and fibrotic responses after renal injury via macrophage interleukin-4 receptor signaling. Kidney Int. 2018, 93, 1344–1355. [Google Scholar] [CrossRef]
- Pitt, B.; Kober, L.; Ponikowski, P.; Gheorghiade, M.; Filippatos, G.; Krum, H.; Nowack, C.; Kolkhof, P.; Kim, S.Y.; Zannad, F. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: A randomized, double-blind trial. Eur. Heart J. 2013, 34, 2453–2463. [Google Scholar] [CrossRef] [PubMed]
- Bakris, G.L.; Agarwal, R.; Chan, J.C.; Cooper, M.E.; Gansevoort, R.T.; Haller, H.; Remuzzi, G.; Rossing, P.; Schmieder, R.E.; Nowack, C.; et al. Effect of finerenone on albuminuria in patients with diabetic nephropathy a randomized clinical trial. JAMA 2015, 314, 884–994. [Google Scholar] [CrossRef] [PubMed]
- Filippatos, G.; Anker, S.D.; Böhm, M.; Gheorghiade, M.; Køber, L.; Krum, H.; Maggioni, A.P.; Ponikowski, P.; Voors, A.A.; Zannad, F.; et al. A randomized controlled study of finerenone vs. eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease. Eur. Heart J. 2016, 37, 2105–2114. [Google Scholar] [CrossRef] [Green Version]
- Kolkhof, P.; Nowack, C.; Eitner, F. Nonsteroidal antagonists of the mineralocorticoid receptor. Curr. Opin. Nephrol. Hypertens. 2015, 24, 417–424. [Google Scholar] [CrossRef]
- Pitt, B.; Filippatos, G.; Agarwal, R.; Anker, S.D.; Bakris, G.L.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Schloemer, P.; et al. Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 2252–2263. [Google Scholar] [CrossRef]
- Filippatos, G.; Bakris, G.L.; Pitt, B.; Agarwal, R.; Rossing, P.; Ruilope, L.M.; Butler, J.; Lam, C.S.P.; Kolkhof, P.; Roberts, L.; et al. Finerenone Reduces New-Onset Atrial Fibrillation in Patients with Chronic Kidney Disease and Type 2 Diabetes. J. Am. Coll. Cardiol. 2021, 78, 142–152. [Google Scholar] [CrossRef]
- Brown, N.J. Contribution of aldosterone to cardiovascular and renal inflammation and fibrosis. Nat. Rev. Nephrol. 2013, 9, 459–469. [Google Scholar] [CrossRef]
- Bauersachs, J.; Lother, A. Mineralocorticoid receptor activation and antagonism in cardiovascular disease: Cellular and molecular mechanisms. Kidney Int. Suppl. 2022, 12, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Fraccarollo, D.; Thomas, S.; Scholz, C.J.; Hilfiker-Kleiner, D.; Galuppo, P.; Bauersachs, J. Macrophage Mineralocorticoid Receptor Is a Pleiotropic Modulator of Myocardial Infarct Healing. Hypertension 2019, 73, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Sun, X.N.; Zeng, M.R.; Zheng, X.J.; Zhang, Y.Y.; Wan, Q.; Zhang, W.C.; Shi, C.; Du, L.J.; Ai, T.J.; et al. Mineralocorticoid Receptor Deficiency in T Cells Attenuates Pressure Overload-Induced Cardiac Hypertrophy and Dysfunction through Modulating T-Cell Activation. Hypertension 2017, 70, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Biwer, L.A.; Elizabeth Moss, M.; Man, J.J.; Aronovitz, M.J.; Martin, G.L.; Carrillo-Salinas, F.J.; Salvador, A.M.; Alcaide, P.; Jaffe, I.Z. Mineralocorticoid Receptor in Smooth Muscle Contributes to Pressure Overload-Induced Heart Failure. Circ. Heart Fail. 2021, 14, e007279. [Google Scholar] [CrossRef]
- Calvier, L.; Martinez-Martinez, E.; Miana, M.; Cachofeiro, V.; Rousseau, E.; Sádaba, J.R.; Zannad, F.; Rossignol, P.; López-Andrés, N. The impact of galectin-3 inhibition on aldosterone-induced cardiac and renal injuries. JACC Heart Fail. 2015, 3, 59–67. [Google Scholar] [CrossRef]
- Moss, E.M.; Lu, Q.; Iyer, S.L.; Engelbertsen, D.; Marzolla, V.; Caprio, M.; Lichtman, A.H.; Jaffe, I.Z. Endothelial Mineralocorticoid Receptors Contribute to Vascular Inflammation in Atherosclerosis in a Sex-Specific Manner. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1588–1601. [Google Scholar] [CrossRef] [Green Version]
- McCurley, A.; Pires, P.W.; Bender, S.B.; Aronovitz, M.; Zhao, M.J.; Metzger, D.; Chambon, P.; Hill, M.A.; Dorrance, A.M.; Mendelsohn, M.E.; et al. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat. Med. 2012, 18, 1429–1433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowalski, J.; Deng, L.; Suennen, C.; Koca, D.; Meral, D.; Bode, C.; Hein, L.; Lother, A. Eplerenone Improves Pulmonary Vascular Remodeling and Hypertension by Inhibition of the Mineralocorticoid Receptor in Endothelial Cells. Hypertension 2021, 78, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Macdonald, T.M.; Morant, S.; Webb, D.J.; Sever, P.; McInnes, G.; Ford, I.; Cruickshank, J.K.; Caulfield, M.J.; Salsbury, J.; et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): A randomised, double-blind, crossover trial. Lancet 2015, 386, 2059–2068. [Google Scholar] [CrossRef] [Green Version]
- Pfeffer, M.A.; Claggett, B.; Assmann, S.F.; Boineau, R.; Anand, I.S.; Clausell, N.; Desai, A.S.; Diaz, R.; Fleg, J.L.; Gordeev, I.; et al. Regional variation in patients and outcomes in the treatment of preserved cardiac function heart failure with an aldosterone antagonist (TOPCAT) trial. Circulation 2015, 131, 34–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boccanelli, A.; Mureddu, G.F.; Cacciatore, G.; Clemenza, F.; Di Lenarda, A.; Gavazzi, A.; Porcu, M.; Latini, R.; Lucci, D.; Maggioni, A.; et al. Anti-remodelling effect of canrenone in patients with mild chronic heart failure (AREA IN-CHF study): Final results. Eur. J. Heart Fail. 2009, 11, 68–76. [Google Scholar] [CrossRef]
- Zannad, F.; McMurray, J.J.V.; Krum, H.; van Veldhuisen, D.J.; Swedberg, K.; Shi, H.; Vincent, J.; Pocock, S.J.; Pitt, B. Eplerenone in Patients with Systolic Heart Failure and Mild Symptoms. N. Engl. J. Med. 2011, 364, 11–21. [Google Scholar] [CrossRef] [Green Version]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Baumbach, A.; Böhm, M.; Burri, H.; Čelutkiene, J.; Chioncel, O.; Cleland, J.G.F.; Coats, A.J.S.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- de Groote, P.; Isnard, R.; Assyag, P.; Clerson, P.; Ducardonnet, A.; Galinier, M.; Jondeau, G.; Leurs, I.; Thébaut, J.F.; Komajda, M. Is the gap between guidelines and clinical practice in heart failure treatment being filled? Insights from the IMPACT RECO survey. Eur. J. Heart Fail. 2007, 9, 1205–1211. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Rossignol, P.; Machu, J.L.; Sharma, A.; Girerd, N.; Anker, S.D.; Cleland, J.G.; Dickstein, K.; Filippatos, G.; Hillege, H.L.; et al. Mineralocorticoid receptor antagonist pattern of use in heart failure with reduced ejection fraction: Findings from BIOSTAT-CHF. Eur. J. Heart Fail. 2017, 19, 1284–1293. [Google Scholar] [CrossRef] [Green Version]
- Noor, S.; Mohammad, T.; Ashraf, G.M.; Farhat, J.; Bilgrami, A.L.; Eapen, M.S.; Sohal, S.S.; Yadav, D.K.; Hassan, M.I. Mechanistic insights into the role of serum-glucocorticoid kinase 1 in diabetic nephropathy: A systematic review. Int. J. Biol. Macromol. 2021, 193, 562–573. [Google Scholar] [CrossRef]
- Gil-Ortega, M.; Vega-Martín, E.; Martín-Ramos, M.; González-Blázquez, R.; Pulido-Olmo, H.; Ruiz-Hurtado, G.; Schulz, A.; Ruilope, L.M.; Kolkhof, P.; Somoza, B.; et al. Finerenone Reduces Intrinsic Arterial Stiffness in Munich Wistar Frömter Rats, a Genetic Model of Chronic Kidney Disease. Am. J. Nephrol. 2020, 51, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Grune, J.; Benz, V.; Brix, S.; Salatzki, J.; Blumrich, A.; Höft, B.; Klopfleisch, R.; Foryst-Ludwig, A.; Kolkhof, P.; Kintscher, U. Steroidal and Nonsteroidal Mineralocorticoid Receptor Antagonists Cause Differential Cardiac Gene Expression in Pressure Overload-induced Cardiac Hypertrophy. J. Cardiovasc. Pharmacol. 2016, 67, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Kolkhof, P.; Delbeck, M.; Kretschmer, A.; Steinke, W.; Hartmann, E.; Bärfacker, L.; Eitner, F.; Albrecht-Küpper, B.; Schäfer, S. Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist protects from rat cardiorenal injury. J. Cardiovasc. Pharmacol. 2014, 64, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Filippatos, G.; Pitt, B.; Anker, S.D.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Gebel, M.; Ruilope, L.M.; et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: The FIDELITY pooled analysis. Eur. Heart J. 2022, 43, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Filippatos, G.; Pitt, B.; Agarwal, R.; Farmakis, D.; Ruilope, L.M.; Rossing, P.; Bauersachs, J.; Mentz, R.J.; Kolkhof, P.; Scott, C.; et al. Finerenone in patients with chronic kidney disease and type 2 diabetes with and without heart failure: A prespecified subgroup analysis of the FIDELIO-DKD trial. Eur. J. Heart Fail. 2022, 24, 996–1005. [Google Scholar] [CrossRef]
- Filippatos, G.; Anker, S.D.; Agarwal, R.; Ruilope, L.M.; Rossing, P.; Bakris, G.L.; Tasto, C.; Joseph, A.; Kolkhof, P.; Lage, A.; et al. Finerenone Reduces Risk of Incident Heart Failure in Patients with Chronic Kidney Disease and Type 2 Diabetes: Analyses from the FIGARO-DKD Trial. Circulation 2022, 145, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Rossing, P.; Filippatos, G.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Chan, J.C.N.; Kooy, A.; McCafferty, K.; Schernthaner, G.; et al. Finerenone in Predominantly Advanced CKD and Type 2 Diabetes with or without Sodium-Glucose Cotransporter-2 Inhibitor Therapy. Kidney Int. Rep. 2022, 7, 36–45. [Google Scholar] [CrossRef]
- Rossing, P.; Agarwal, R.; Anker, S.D.; Filippatos, G.; Pitt, B.; Ruilope, L.M.; Amod, A.; Marre, M.; Joseph, A.; Lage, A.; et al. Efficacy and safety of finerenone in patients with chronic kidney disease and type 2 diabetes by GLP-1RA treatment: A subgroup analysis from the FIDELIO-DKD trial. Diabetes Obes. Metab. 2022, 24, 125–134. [Google Scholar] [CrossRef]
- Jager, K.J.; Kovesdy, C.; Langham, R.; Rosenberg, M.; Jha, V.; Zoccali, C. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Nephrol. Dial. Transplant. 2019, 34, 1803–1805. [Google Scholar] [CrossRef] [Green Version]
- Brenner, B.M.; Cooper, M.E.; de Zeeuw, D.; Keane, W.F.; Mitch, W.E.; Parving, H.-H.; Remuzzi, G.; Snapinn, S.M.; Zhang, Z.; Shahinfar, S. Effects of Losartan on Renal and Cardiovascular Outcomes in Patients with Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2001, 345, 861–869. [Google Scholar] [CrossRef] [Green Version]
- Lewis, E.J.; Hunsicker, L.G.; Clarke, W.R.; Berl, T.; Pohl, M.A.; Lewis, J.B.; Ritz, E.; Atkins, R.C.; Rohde, R.; Raz, I. Renoprotective Effect of the Angiotensin-Receptor Antagonist Irbesartan in Patients with Nephropathy Due to Type 2 Diabetes. N. Engl. J. Med. 2001, 345, 851–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parving, H.-H.; Brenner, B.M.; McMurray, J.J.V.; de Zeeuw, D.; Haffner, S.M.; Solomon, S.D.; Chaturvedi, N.; Persson, F.; Desai, A.S.; Nicolaides, M.; et al. Cardiorenal End Points in a Trial of Aliskiren for Type 2 Diabetes. N. Engl. J. Med. 2012, 367, 2204–2213. [Google Scholar] [CrossRef] [Green Version]
- Sarafidis, P.; Ferro, C.J.; Morales, E.; Ortiz, A.; Malyszko, J.; Hojs, R.; Khazim, K.; Ekart, R.; Valdivielso, J.; Fouque, D.; et al. SGLT-2 inhibitors and GLP-1 receptor agonists for nephroprotection and cardioprotection in patients with diabetes mellitus and chronic kidney disease. A consensus statement by the EURECA-m and the DIABESITY working groups of the ERA-EDTA. Nephrol. Dial. Transplant. 2019, 34, 208–230. [Google Scholar] [CrossRef]
- Mehdi, U.F.; Adams-Huet, B.; Raskin, P.; Vega, G.L.; Toto, R.D. Addition of angiotensin receptor blockade or mineralocorticoid antagonism to maximal angiotensin-converting enzyme inhibition in diabetic nephropathy. J. Am. Soc. Nephrol. 2009, 20, 2641–2650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexandrou, M.E.; Papagianni, A.; Tsapas, A.; Loutradis, C.; Boutou, A.; Piperidou, A.; Papadopoulou, D.; Ruilope, L.; Bakris, G.; Sarafidis, P. Effects of mineralocorticoid receptor antagonists in proteinuric kidney disease: A systematic review and meta-Analysis of randomized controlled trials. J. Hypertens. 2019, 37, 2307–2324. [Google Scholar] [CrossRef] [PubMed]
- Bakris, G.L.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Nowack, C.; Kolkhof, P.; Ferreira, A.C.; Schloemer, P.; Filippatos, G. Design and Baseline Characteristics of the Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease Trial. Am. J. Nephrol. 2019, 50, 333–344. [Google Scholar] [CrossRef]
- Ruilope, L.M.; Agarwal, R.; Anker, S.D.; Bakris, G.L.; Filippatos, G.; Nowack, C.; Kolkhof, P.; Joseph, A.; Mentenich, N.; Pitt, B. Design and Baseline Characteristics of the Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease Trial. Am. J. Nephrol. 2019, 50, 345–356. [Google Scholar] [CrossRef]
- Oka, T.; Sakaguchi, Y.; Hattori, K.; Asahina, Y.; Kajimoto, S.; Doi, Y.; Kaimori, J.Y.; Isaka, Y. Mineralocorticoid Receptor Antagonist Use and Hard Renal Outcomes in Real-World Patients with Chronic Kidney Disease. Hypertension 2022, 79, 679–689. [Google Scholar] [CrossRef]
- Ferrannini, E.; Solini, A. SGLT2 inhibition in diabetes mellitus: Rationale and clinical prospects. Nat. Rev. Endocrinol. 2012, 8, 495–502. [Google Scholar] [CrossRef]
- Penno, G.; Orsi, E.; Solini, A.; Bonora, E.; Fondelli, C.; Trevisan, R.; Vedovato, M.; Cavalot, F.; Gruden, G.; Laviola, L.; et al. Renal hyperfiltration is independently associated with increased all-cause mortality in individuals with type 2 diabetes: A prospective cohort study. BMJ Open Diabetes Res. Care 2020, 8, e001481. [Google Scholar] [CrossRef]
- Kraus, B.J.; Weir, M.R.; Bakris, G.L.; Mattheus, M.; Cherney, D.Z.I.; Sattar, N.; Heerspink, H.J.L.; Ritter, I.; von Eynatten, M.; Zinman, B.; et al. Characterization and implications of the initial estimated glomerular filtration rate ‘dip’ upon sodium-glucose cotransporter-2 inhibition with empagliflozin in the EMPA-REG OUTCOME trial. Kidney Int. 2021, 99, 750–762. [Google Scholar] [CrossRef] [PubMed]
- Neuen, B.L.; Ohkuma, T.; Neal, B.; Matthews, D.R.; De Zeeuw, D.; Mahaffey, K.W.; Fulcher, G.; Li, Q.; Jardine, M.; Oh, R.; et al. Effect of canagliflozin on renal and cardiovascular outcomes across different levels of albuminuria: Data from the CANVAS program. J. Am. Soc. Nephrol. 2019, 30, 2229–2242. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.; Goto, S. Possible mechanism of hematocrit elevation by sodium glucose cotransporter 2 inhibitors and associated beneficial renal and cardiovascular effects. Circulation 2019, 139, 1985–1987. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. Mechanisms Leading to Differential Hypoxia-Inducible Factor Signaling in the Diabetic Kidney: Modulation by SGLT2 Inhibitors and Hypoxia Mimetics. Am. J. Kidney Dis. 2021, 77, 280–286. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Kosiborod, M.; Inzucchi, S.E.; Cherney, D.Z.I. Renoprotective effects of sodium-glucose cotransporter-2 inhibitors. Kidney Int. 2018, 94, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Zelniker, T.A.; Braunwald, E. Mechanisms of Cardiorenal Effects of Sodium-Glucose Cotransporter 2 Inhibitors: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 422–434. [Google Scholar] [CrossRef]
- Liu, H.; Sridhar, V.S.; Boulet, J.; Dharia, A.; Khan, A.; Lawler, P.R.; Cherney, D.Z.I. Cardiorenal protection with SGLT2 inhibitors in patients with diabetes mellitus: From biomarkers to clinical outcomes in heart failure and diabetic kidney disease. Metabolism 2022, 126, 154918. [Google Scholar] [CrossRef]
- Ruilope, L.M.; Agarwal, R.; Anker, S.D.; Filippatos, G.; Pitt, B.; Rossing, P.; Sarafidis, P.; Schmieder, R.E.; Joseph, A.; Rethemeier, N.; et al. Blood Pressure and Cardiorenal Outcomes with Finerenone in Chronic Kidney Disease in Type 2 Diabetes. Hypertension 2022, 79, 2685–2695. [Google Scholar] [CrossRef]
- Viengchareun, S.; Le Menuet, D.; Martinerie, L.; Munier, M.; Pascual-Le Tallec, L.; Lombès, M. The mineralocorticoid receptor: Insights into its molecular and (patho)physiological biology. Nucl. Recept. Signal. 2007, 5, e012. [Google Scholar] [CrossRef] [Green Version]
- Kolkhof, P.; Lawatscheck, R.; Filippatos, G.; Bakris, G.L. Nonsteroidal Mineralocorticoid Receptor Antagonism by Finerenone—Translational Aspects and Clinical Perspectives across Multiple Organ Systems. Int. J. Mol. Sci. 2022, 23, 9243. [Google Scholar] [CrossRef]
- Nakamura, T.; Girerd, S.; Jaisser, F.; Barrera-Chimal, J. Nonepithelial mineralocorticoid receptor activation as a determinant of kidney disease. Kidney Int. Suppl. 2022, 12, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, D.; Maiorino, M.I.; Bellastella, G.; Esposito, K. The residual cardiorenal risk in type 2 diabetes. Cardiovasc. Diabetol. 2021, 20, 36. [Google Scholar] [CrossRef]
- Kolkhof, P.; Hartmann, E.; Freyberger, A.; Pavkovic, M.; Mathar, I.; Sandner, P.; Droebner, K.; Joseph, A.; Hüser, J.; Eitner, F. Effects of Finerenone Combined with Empagliflozin in a Model of Hypertension-Induced End-Organ Damage. Am. J. Nephrol. 2021, 52, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, M.; Puchades, M.J.; Garofalo, C.; Jongs, N.; D’Marco, L.; Andreucci, M.; De Nicola, L.; Gorriz, J.L.; Heerspink, H.J.L. Albuminuria-Lowering Effect of Dapagliflozin, Eplerenone, and Their Combination in Patients with Chronic Kidney Disease: A Randomized Crossover Clinical Trial. J. Am. Soc. Nephrol. 2022, 33, 1569–1580. [Google Scholar] [CrossRef] [PubMed]
- Rossing, P.; Anker, S.D.; Filippatos, G.; Pitt, B.; Ruilope, L.M.; Birkenfeld, A.L.; McGill, J.B.; Rosas, S.E.; Joseph, A.; Gebel, M.; et al. Finerenone in Patients with Chronic Kidney Disease and Type 2 Diabetes by Sodium–Glucose Cotransporter 2 Inhibitor Treatment: The FIDELITY Analysis. Diabetes Care 2022, 45, 2991–2998. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, M.; Jongs, N.; Vart, P.; Stefánsson, B.V.; Chertow, G.M.; Langkilde, A.M.; McMurray, J.J.V.; Correa-Rotter, R.; Rossing, P.; Sjöström, C.D.; et al. The Kidney Protective Effects of the Sodium–Glucose Cotransporter-2 Inhibitor, Dapagliflozin, Are Present in Patients with CKD Treated with Mineralocorticoid Receptor Antagonists. Kidney Int. Rep. 2022, 7, 436–443. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Butler, J.; Zannad, F.; Filippatos, G.; Schueler, E.; Steubl, D.; Zeller, C.; Januzzi, J.L.; Pocock, S.; Packer, M.; et al. Mineralocorticoid Receptor Antagonists and Empagliflozin in Patients with Heart Failure and Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2022, 79, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.P.; Zannad, F.; Pocock, S.J.; Anker, S.D.; Butler, J.; Filippatos, G.; Brueckmann, M.; Jamal, W.; Steubl, D.; Schueler, E.; et al. Interplay of Mineralocorticoid Receptor Antagonists and Empagliflozin in Heart Failure: EMPEROR-Reduced. J. Am. Coll. Cardiol. 2021, 77, 1397–1407. [Google Scholar] [CrossRef]
- Green, J.B.; Mottl, A.K.; Bakris, G.; Heerspink, H.J.L.; Mann, J.F.E.; McGill, J.B.; Nangaku, M.; Rossing, P.; Scott, C.; Gay, A.; et al. Design of the COmbinatioN effect of FInerenone anD EmpaglifloziN in participants with chronic kidney disease and type 2 diabetes using a UACR Endpoint study (CONFIDENCE). Nephrol. Dial. Transplant. 2022, 38, 894–903. [Google Scholar] [CrossRef]
- ONTARGET Investigators; Yusuf, S.; Teo, K.K.; Pogue, J.; Dyal, L.; Copland, I.; Schumacher, H.; Dagenais, G.; Sleight, P.; Anderson, C. Telmisartan, Ramipril, or Both in Patients at High Risk for Vascular Events. N. Engl. J. Med. 2008, 358, 1547–1559. [Google Scholar] [CrossRef]
- Fried, L.F.; Emanuele, N.; Zhang, J.H.; Brophy, M.; Conner, T.A.; Duckworth, W.; Leehey, D.J.; McCullough, P.A.; O’Connor, T.; Palevsky, P.M.; et al. Combined Angiotensin Inhibition for the Treatment of Diabetic Nephropathy. N. Engl. J. Med. 2013, 369, 1892–1903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frimodt-Møller, M.; Persson, F.; Rossing, P. Mitigating risk of aldosterone in diabetic kidney disease. Curr. Opin. Nephrol. Hypertens. 2020, 29, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Meyers, K.; Cogan, D.A.; Burke, J.; Arenas, R.; Balestra, M.; Brown, N.F.; Chen, Z.; Cerny, M.A.; Clifford, H.E.; Colombo, F.; et al. Dihydrobenzisoxazole-4-one compounds are novel selective inhibitors of aldosterone synthase (CYP11B2) with in vivo activity. Bioorg. Med. Chem. Lett. 2018, 28, 979–984. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Lullo, L.; Lavalle, C.; Scatena, A.; Mariani, M.V.; Ronco, C.; Bellasi, A. Finerenone: Questions and Answers—The Four Fundamental Arguments on the New-Born Promising Non-Steroidal Mineralocorticoid Receptor Antagonist. J. Clin. Med. 2023, 12, 3992. https://doi.org/10.3390/jcm12123992
Di Lullo L, Lavalle C, Scatena A, Mariani MV, Ronco C, Bellasi A. Finerenone: Questions and Answers—The Four Fundamental Arguments on the New-Born Promising Non-Steroidal Mineralocorticoid Receptor Antagonist. Journal of Clinical Medicine. 2023; 12(12):3992. https://doi.org/10.3390/jcm12123992
Chicago/Turabian StyleDi Lullo, Luca, Carlo Lavalle, Alessia Scatena, Marco Valerio Mariani, Claudio Ronco, and Antonio Bellasi. 2023. "Finerenone: Questions and Answers—The Four Fundamental Arguments on the New-Born Promising Non-Steroidal Mineralocorticoid Receptor Antagonist" Journal of Clinical Medicine 12, no. 12: 3992. https://doi.org/10.3390/jcm12123992
APA StyleDi Lullo, L., Lavalle, C., Scatena, A., Mariani, M. V., Ronco, C., & Bellasi, A. (2023). Finerenone: Questions and Answers—The Four Fundamental Arguments on the New-Born Promising Non-Steroidal Mineralocorticoid Receptor Antagonist. Journal of Clinical Medicine, 12(12), 3992. https://doi.org/10.3390/jcm12123992






