Anterior Cervical Discectomy and Fusion Performed Using a CaO-SiO2-P2O5-B2O3 Bioactive Glass Ceramic or Polyetheretherketone Cage Filled with Hydroxyapatite/β-Tricalcium Phosphate: A Prospective Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Randomization and Follow-Ups
2.3. Interventions: Anterior Cervical Discectomy and Fusion
2.4. Outcomes and Measurements
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Primary Outcome at 12-Month Follow-Up
3.3. Secondary Outcomes and Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fischer, C.R.; Cassilly, R.; Cantor, W.; Edusei, E.; Hammouri, Q.; Errico, T. A systematic review of comparative studies on bone graft alternatives for common spine fusion procedures. Eur. Spine J. 2013, 22, 1423–1435. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; He, W.; Mahaney, K.; Noeller, J.; Mhanna, N.; Viljoen, S.; Torner, J.; Hitchon, P. Alternative grafts in anterior cervical fusion. Clin. Neurol. Neurosurg. 2013, 115, 2049–2055. [Google Scholar] [CrossRef] [PubMed]
- Tuchman, A.; Brodke, D.S.; Youssef, J.A.; Meisel, H.J.; Dettori, J.R.; Park, J.B.; Yoon, S.T.; Wang, J.C. Autograft versus Allograft for Cervical Spinal Fusion: A Systematic Review. Global Spine J. 2017, 7, 59–70. [Google Scholar] [CrossRef] [PubMed]
- D’Antonio, N.; Lambrechts, M.J.; Heard, J.; Bertiaume, E.; Toci, G.; Karamian, B.; Breyer, G.; Bodnar, J.; Canseco, J.; Hilibrand, A. Effect of Interbody Composition on the Development of Pseudarthrosis Following Anterior Cervical Discectomy and Fusion. Asian Spine J. 2023. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Jang, H.D.; Ahn, J.; Choi, S.W.; Kang, D.; Shin, B.J. Comparison of Cortical Ring Allograft and Plate Fixation with Autologous Iliac Bone Graft for Anterior Cervical Discectomy and Fusion. Asian Spine J. 2019, 13, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Chau, A.M.; Xu, L.L.; Wong, J.H.; Mobbs, R.J. Current status of bone graft options for anterior interbody fusion of the cervical and lumbar spine. Neurosurg. Rev. 2014, 37, 23–37. [Google Scholar] [CrossRef]
- Yi, J.; Lee, G.W.; Nam, W.D.; Han, K.Y.; Kim, M.H.; Kang, J.W.; Won, J.; Kim, S.W.; Noh, W.; Yeom, J.S. A Prospective Randomized Clinical Trial Comparing Bone Union Rate Following Anterior Cervical Discectomy and Fusion Using a Polyetheretherketone Cage: Hydroxyapatite/B-Tricalcium Phosphate Mixture versus Hydroxyapatite/Demineralized Bone Matrix Mixture. Asian Spine J. 2015, 9, 30–38. [Google Scholar] [CrossRef]
- Ohtsuki, C.; Kamitakahara, M.; Miyazaki, T. Bioactive ceramic-based materials with designed reactivity for bone tissue regeneration. J. R. Soc. Interface 2009, 6, S349–S360. [Google Scholar] [CrossRef]
- Kim, Y.; Lim, J.Y.; Yang, G.H.; Seo, J.H.; Ryu, H.S.; Kim, G. 3D-printed PCL/bioglass (BGS-7) composite scaffolds with high toughness and cell-responses for bone tissue regeneration. J. Ind. Eng. Chem. 2019, 79, 163–171. [Google Scholar] [CrossRef]
- Koo, K.H.; Hwang, C.J.; Lee, J.H.; Chang, B.-S.; Lee, C.-K. Treatment of bone defects in rabbit tibiae using CaO-SiO2-P2O5-B2O3 bioactive ceramics: Radiological, biomechanical, and histological evaluation. Tissue Eng. Regen. Med. 2009, 6, 811. [Google Scholar]
- Lee, J.H.; Jeung, U.-O.; Jeon, D.-H.; Chang, B.-S.; Lee, C.-K. Quantitative comparison of novel CaO-SiO2-P2O5-B2O3 glass-ceramics (bgs-7) with hydroxyapatite as bone graft extender in rabbit ilium. Tissue Eng. Regen. Med. 2010, 7, 540. [Google Scholar]
- Lee, J.H.; Nam, H.; Ryu, H.S.; Seo, J.H.; Chang, B.S.; Lee, C.K. Bioactive ceramic coating of cancellous screws improves the osseointegration in the cancellous bone. J. Orthop. Sci. 2011, 16, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Ryu, H.S.; Seo, J.H.; Chang, B.S.; Lee, C.K. A 90-day intravenous administration toxicity study of CaO-SiO2-P2O5-B2O3 glass-ceramics (BGS-7) in rat. Drug Chem. Toxicol. 2010, 33, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, S.K.; Kang, S.S.; Han, S.J.; Lee, C.K.; Chang, B.S. A Long-Term Follow-up, Multicenter, Comparative Study of the Radiologic, and Clinical Results Between a CaO-SiO2-P2O5-B2O3 Bioactive Glass Ceramics (BGS-7) Intervertebral Spacer and Titanium Cage in 1-Level Posterior Lumbar Interbody Fusion. Clin. Spine Surg. 2020, 33, E322. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kong, C.B.; Yang, J.J.; Shim, H.J.; Koo, K.H.; Kim, J.; Lee, C.K.; Chang, B.S. Comparison of fusion rate and clinical results between CaO-SiO2-P2O5-B2O3 bioactive glass ceramics spacer with titanium cages in posterior lumbar interbody fusion. Spine J. 2016, 16, 1367–1376. [Google Scholar] [CrossRef]
- Ryu, S.; Ryu, D.S.; Kim, K.S. Long-term results comparison after anterior cervical discectomy with BGS-7 spacer (NOVOMAX®-C) and allograft spacer: A prospective observational study. Front. Bioeng. Biotechnol. 2023, 11, 1100462. [Google Scholar] [CrossRef]
- Rhee, J.M.; Chapman, J.R.; Norvell, D.C.; Smith, J.; Sherry, N.A.; Riew, K.D. Radiological Determination of Postoperative Cervical Fusion: A Systematic Review. Spine 2015, 40, 974–991. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Song, K.J.; Choi, B.Y. Current concepts of anterior cervical discectomy and fusion: A review of literature. Asian Spine J. 2014, 8, 531–539. [Google Scholar] [CrossRef]
- Gu, Y.T.; Jia, L.S.; Chen, T.Y. Biomechanical study of a hat type cervical intervertebral fusion cage. Int. Orthop. 2007, 31, 101–105. [Google Scholar] [CrossRef]
- Kersten, R.F.; van Gaalen, S.M.; de Gast, A.; Öner, F.C. Polyetheretherketone (PEEK) cages in cervical applications: A systematic review. Spine J. 2015, 15, 1446–1460. [Google Scholar] [CrossRef] [PubMed]
- Villavicencio, A.T.; Nelson, E.L.; Rajpal, S.; Beasley, K.; Burneikiene, S. Prospective, Randomized, Blinded Clinical Trial Comparing PEEK and Allograft Spacers in Patients Undergoing Anterior Cervical Discectomy and Fusion Surgeries. Spine 2022, 47, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Ryu, H.S.; Seo, J.H.; Lee, D.Y.; Chang, B.S.; Lee, C.K. Negative effect of rapidly resorbing properties of bioactive glass-ceramics as bone graft substitute in a rabbit lumbar fusion model. Clin. Orthop. Surg. 2014, 6, 87–95. [Google Scholar] [CrossRef]
- Kumar, N.; Judith, M.R.; Kumar, A.; Mishra, V.; Robert, M.C. Analysis of stress distribution in lumbar interbody fusion. Spine 2005, 30, 1731–1735. [Google Scholar] [CrossRef]
- Faizan, A.; Kiapour, A.; Kiapour, A.M.; Goel, V.K. Biomechanical analysis of various footprints of transforaminal lumbar interbody fusion devices. J. Spinal. Disord. Tech. 2014, 27, E118–E127. [Google Scholar] [CrossRef]
- Shen, Y.W.; Yang, Y.; Liu, H.; Qiu, Y.; Li, M.; Ma, L.T.; Gan, F.J. Biomechanical Evaluation of Intervertebral Fusion Process After Anterior Cervical Discectomy and Fusion: A Finite Element Study. Front. Bioeng. Biotechnol. 2022, 10, 842382. [Google Scholar] [CrossRef] [PubMed]
- McCaffrey, K.; McCaffrey, M.H.; Pelletier, M.H.; Lovric, V.; Mobbs, R.J.; Walsh, W.R. Load Sharing and Endplate Pressure Distribution in Anterior Interbody Fusion Influenced by Graft Choice. World Neurosurg. 2021, 146, e336–e340. [Google Scholar] [CrossRef] [PubMed]
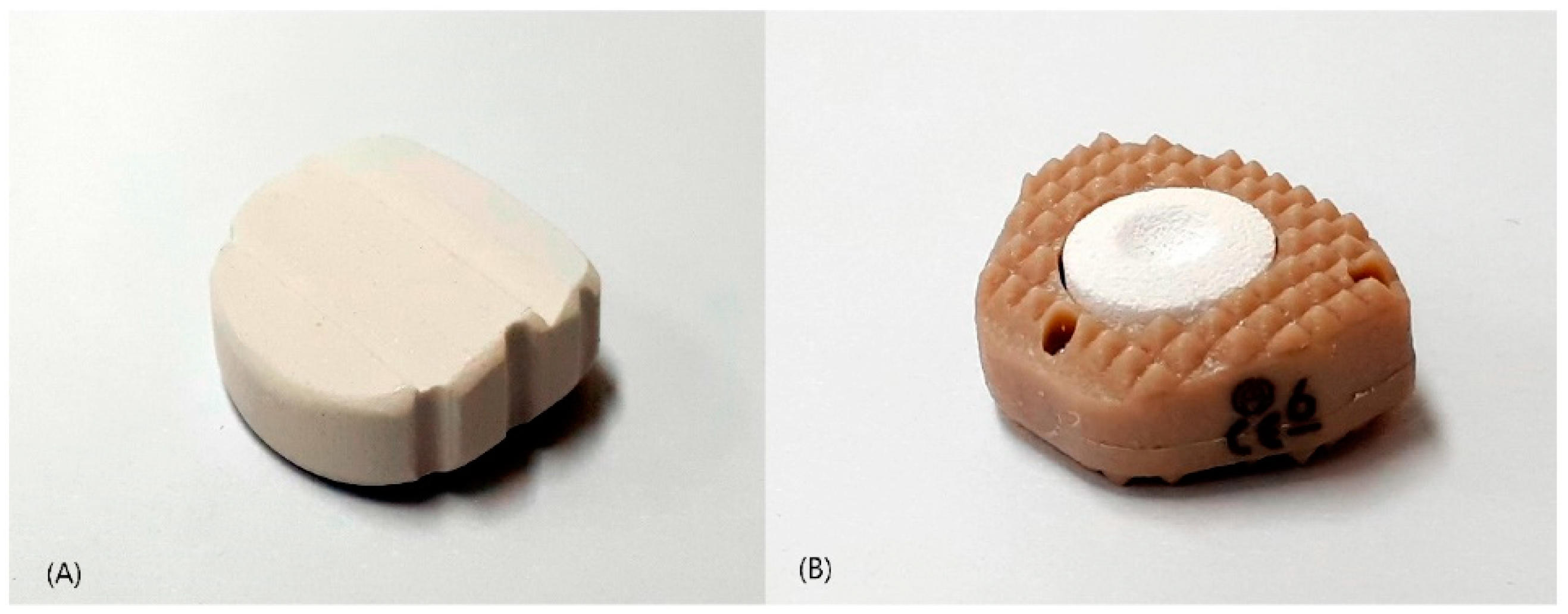
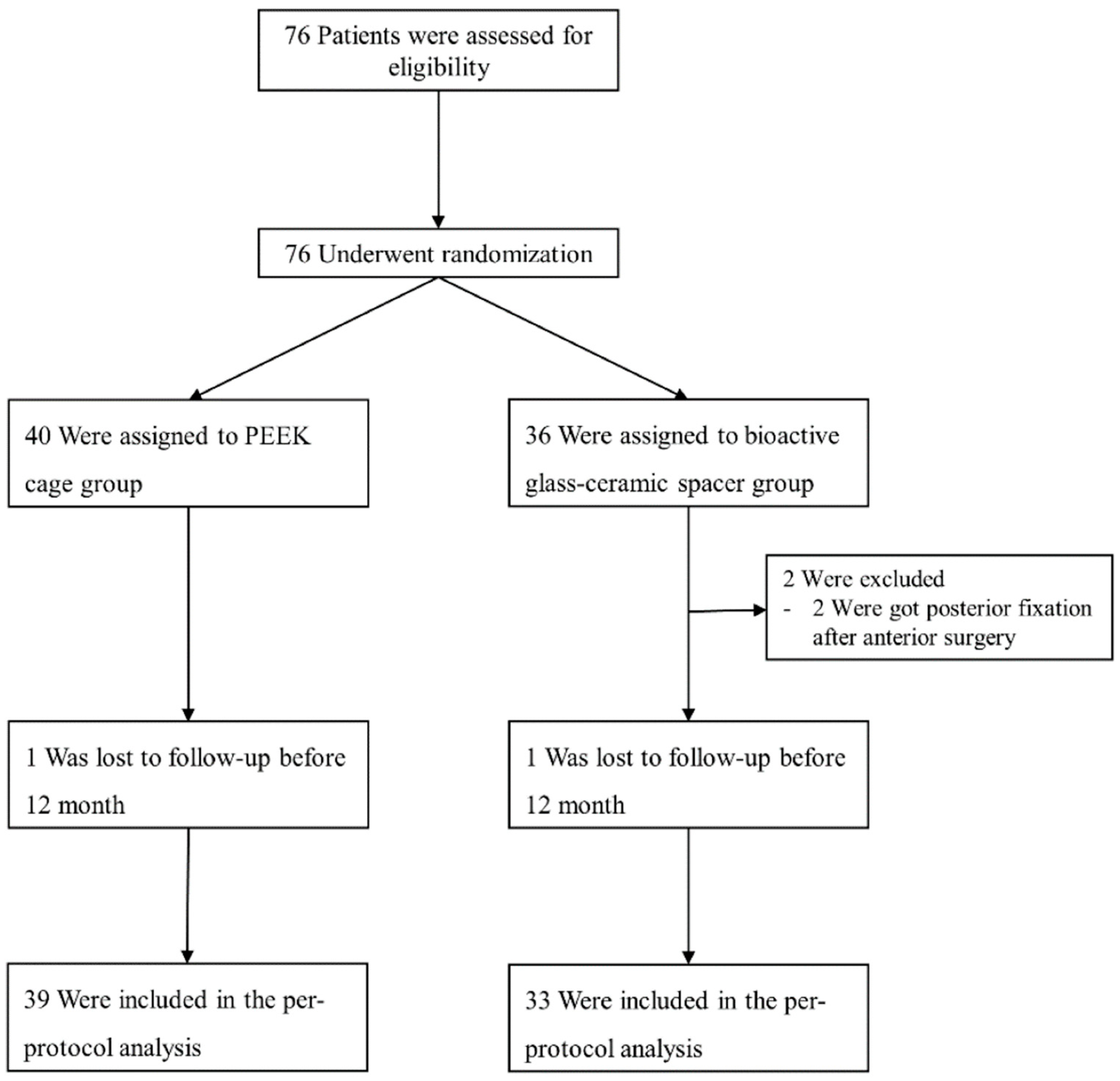
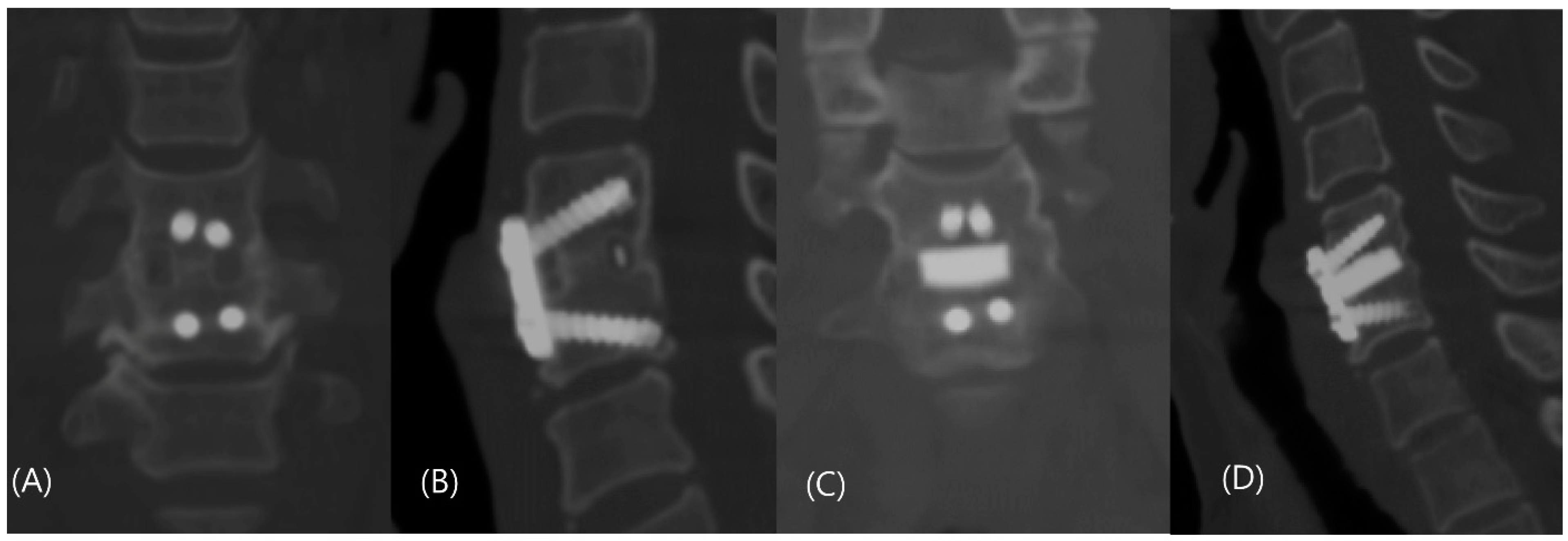
| Characteristic | Control Group (n = 40) | Study Group (n = 36) |
|---|---|---|
| Age (years) | 55.0 ± 11.3 | 54.4 ± 10.6 |
| Male/Female * | 27/13 | 25/11 |
| BMI (kg/m2) | 24.7 ± 2.3 | 24.4 ± 2.3 |
| CCI score | 1.7 ± 1.6 | 1.6 ± 1.5 |
| ASA score | 1.5 ± 0.6 | 1.4 ± 0.5 |
| Smoking status, n (%) * | ||
| Non/Ex-smoker | 21 (52%) | 19 (53%) |
| Current smoker | 19 (48%) | 17 (47%) |
| Alcohol consumption, n (%) * | ||
| None | 10 (25%) | 12 (33%) |
| ≥1 drink/month | 30 (75%) | 24 (67%) |
| VAS for neck pain | 2.8 ± 2.9 | 3.0 ± 2.9 |
| VAS for arm pain | 4.6 ± 2.7 | 4.9 ± 2.3 |
| NDI | 21.0 ± 22.8 | 25.1 ± 21.9 |
| EQ-5D | 0.649 ± 0.189 | 0.695 ± 0.139 |
| SF-12 | ||
| PCS | 39.7 ± 9.3 | 38.9 ± 8.7 |
| MCS | 45.0 ± 12.1 | 43.9 ± 12.3 |
| Diagnosis, n (%) * | ||
| Myelopathy | 22 (55%) | 17 (47%) |
| Radiculopathy | 17 (43%) | 17 (47%) |
| Myeloradiculopathy | 1 (2%) | 2 (6%) |
| Approach side, n (%) * | ||
| Right | 22 (55%) | 19 (53%) |
| Left | 18 (45%) | 17 (47%) |
| Operation level, n (%) * | ||
| C3-4 | 5 (10%) | 4 (9%) |
| C4-5 | 10 (20%) | 9 (19%) |
| C5-6 | 20 (40%) | 19 (40%) |
| C6-7 | 12 (24%) | 12 (26%) |
| C7-T1 | 4 (6%) | 3 (6%) |
| Group C | Group N | p-Value * | Risk Difference (95% CI) | |
|---|---|---|---|---|
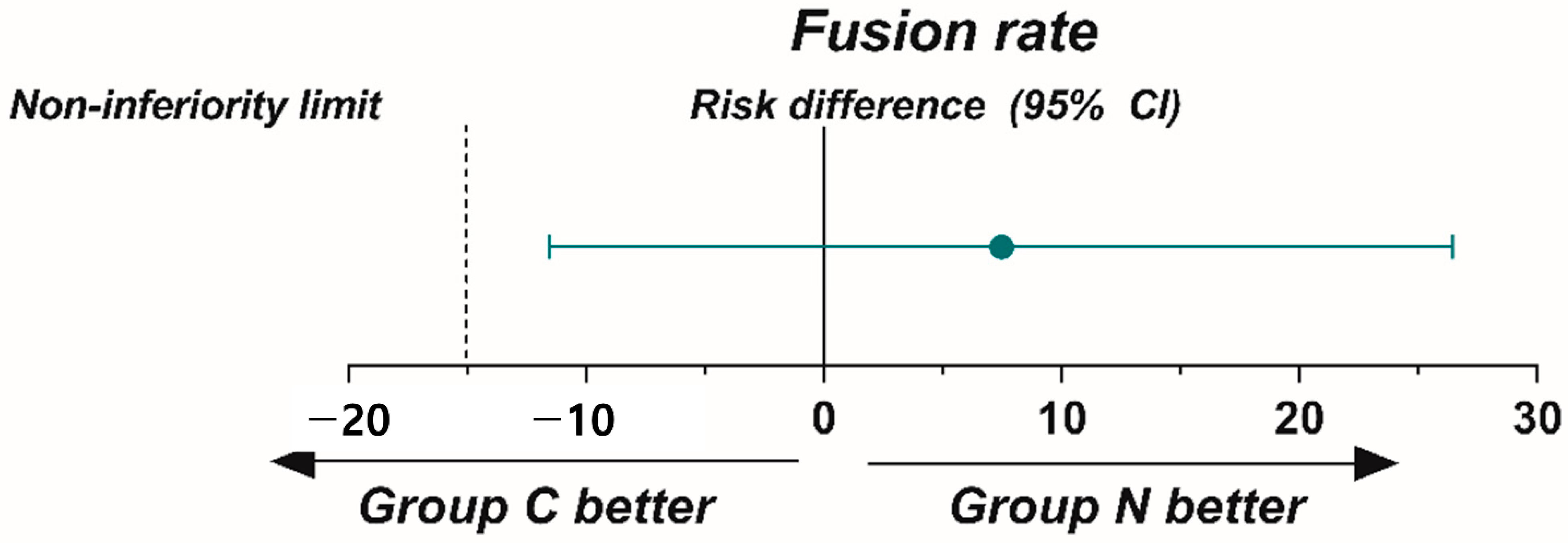
| Fusion rate on CT † | 74.4% | 81.8% | 7.4% (−11.5% to 26.5%) | |
| Fusion rate on dynamic radiographs (ISM < 1 mm) ‡ | 73.7% | 78.1% | 0.666 |
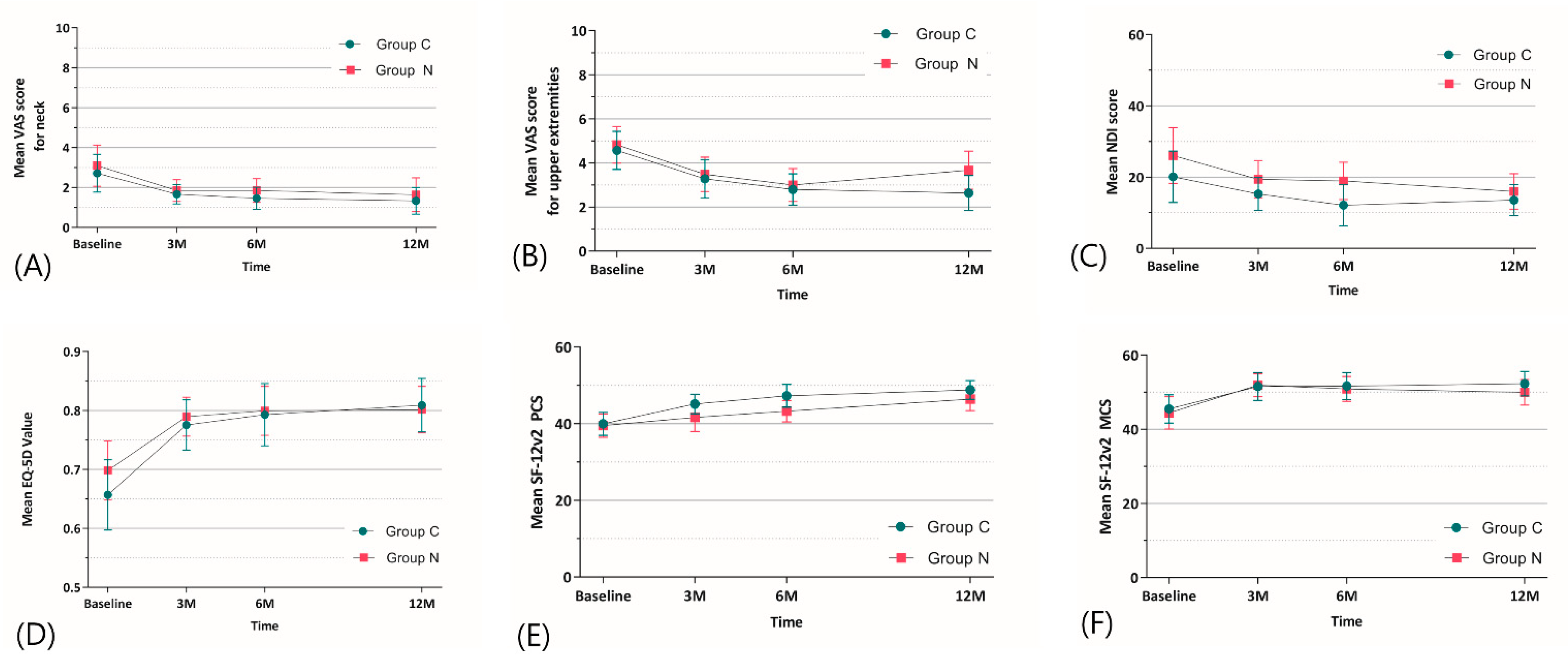
| Variables | Group C | Group N | Mean Difference (95% CI) | p-Value |
|---|---|---|---|---|
| VAS neck | ||||
| 3 months | 1.67 ± 1.51 | 1.85 ± 1.52 | −0.18 (−0.90–0.53) | 0.722 |
| 6 months | 1.46 ± 1.70 | 1.85 ± 1.68 | −0.39 (−1.18–0.41) | 0.449 |
| 12 months | 1.33 ± 2.06 | 1.64 ± 2.40 | −0.30 (−1.35–0.74) | 0.554 |
| Overall intervention effect * | NA | NA | NA | 0.988 |
| VAS upper extremities | ||||
| 3 months | 3.28 ± 2.69 | 3.48 ± 2.21 | −0.20 (−1.37–0.97) | 0.723 |
| 6 months | 2.79 ± 2.21 | 3.00 ± 2.11 | −0.21 (−1.23–0.82) | 0.720 |
| 12 months | 2.64 ± 2.48 | 3.67 ± 2.45 | −1.03 (−2.19–0.14) | 0.073 |
| Overall intervention effect * | NA | NA | NA | 0.535 |
| NDI | ||||
| 3 months | 15.26 ± 14.36 | 19.39 ± 14.83 | −4.13 (−11.01–2.74) | 0.307 |
| 6 months | 12.14 ± 17.92 | 18.90 ± 14.74 | −6.76 (−14.56–1.05) | 0.095 |
| 12 months | 13.57 ± 13.53 | 15.97 ± 14.11 | −2.40 (−8.91–4.11) | 0.553 |
| Overall intervention effect * | NA | NA | NA | 0.782 |
| EQ-5D | ||||
| 3 months | 0.775 ± 0.132 | 0.790 ± 0.093 | −0.014 (−0.069–0.040) | 0.665 |
| 6 months | 0.793 ± 0.163 | 0.799 ± 0.117 | −0.007 (−0.074–0.061) | 0.842 |
| 12 months | 0.809 ± 0.140 | 0.801 ± 0.111 | 0.008 (−0.053–0.068) | 0.816 |
| Overall intervention effect * | NA | NA | NA | 0.591 |
| SF12-PCS | ||||
| 3 months | 45.16 ± 7.92 | 41.64 ± 10.41 | 3.52 (−0.79–7.83) | 0.089 |
| 6 months | 47.28 ± 9.29 | 43.28 ± 7.98 | 4.01 (−0.11–8.12) | 0.053 |
| 12 months | 48.80 ± 7.54 | 46.41 ± 8.47 | 2.38 (−1.38–6.14) | 0.259 |
| Overall intervention effect * | NA | NA | NA | 0.444 |
| SF12-MCS | ||||
| 3 months | 51.52 ± 11.71 | 51.89 ± 8.84 | −0.36 (−5.31–4.59) | 0.887 |
| 6 months | 51.62 ± 44.45 | 50.88 ± 9.45 | 0.74 (−4.23–5.71) | 0.773 |
| 12 months | 52.32 ± 10.24 | 49.96 ± 9.74 | 2.35 (−2.37–7.08) | 0.357 |
| Overall intervention effect * | NA | NA | NA | 0.879 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Park, S.-M.; Ham, D.-W.; Hong, J.-Y.; Kim, H.-J.; Yeom, J.S. Anterior Cervical Discectomy and Fusion Performed Using a CaO-SiO2-P2O5-B2O3 Bioactive Glass Ceramic or Polyetheretherketone Cage Filled with Hydroxyapatite/β-Tricalcium Phosphate: A Prospective Randomized Controlled Trial. J. Clin. Med. 2023, 12, 4069. https://doi.org/10.3390/jcm12124069
Park J, Park S-M, Ham D-W, Hong J-Y, Kim H-J, Yeom JS. Anterior Cervical Discectomy and Fusion Performed Using a CaO-SiO2-P2O5-B2O3 Bioactive Glass Ceramic or Polyetheretherketone Cage Filled with Hydroxyapatite/β-Tricalcium Phosphate: A Prospective Randomized Controlled Trial. Journal of Clinical Medicine. 2023; 12(12):4069. https://doi.org/10.3390/jcm12124069
Chicago/Turabian StylePark, Jiwon, Sang-Min Park, Dae-Woong Ham, Jae-Young Hong, Ho-Joong Kim, and Jin S. Yeom. 2023. "Anterior Cervical Discectomy and Fusion Performed Using a CaO-SiO2-P2O5-B2O3 Bioactive Glass Ceramic or Polyetheretherketone Cage Filled with Hydroxyapatite/β-Tricalcium Phosphate: A Prospective Randomized Controlled Trial" Journal of Clinical Medicine 12, no. 12: 4069. https://doi.org/10.3390/jcm12124069





