General Critical Care, Temperature Control, and End-of-Life Decision Making in Patients Resuscitated from Cardiac Arrest
Abstract
1. Introduction
2. Pathophysiology
3. Immediate Management following Restoration of Cardiac Activity
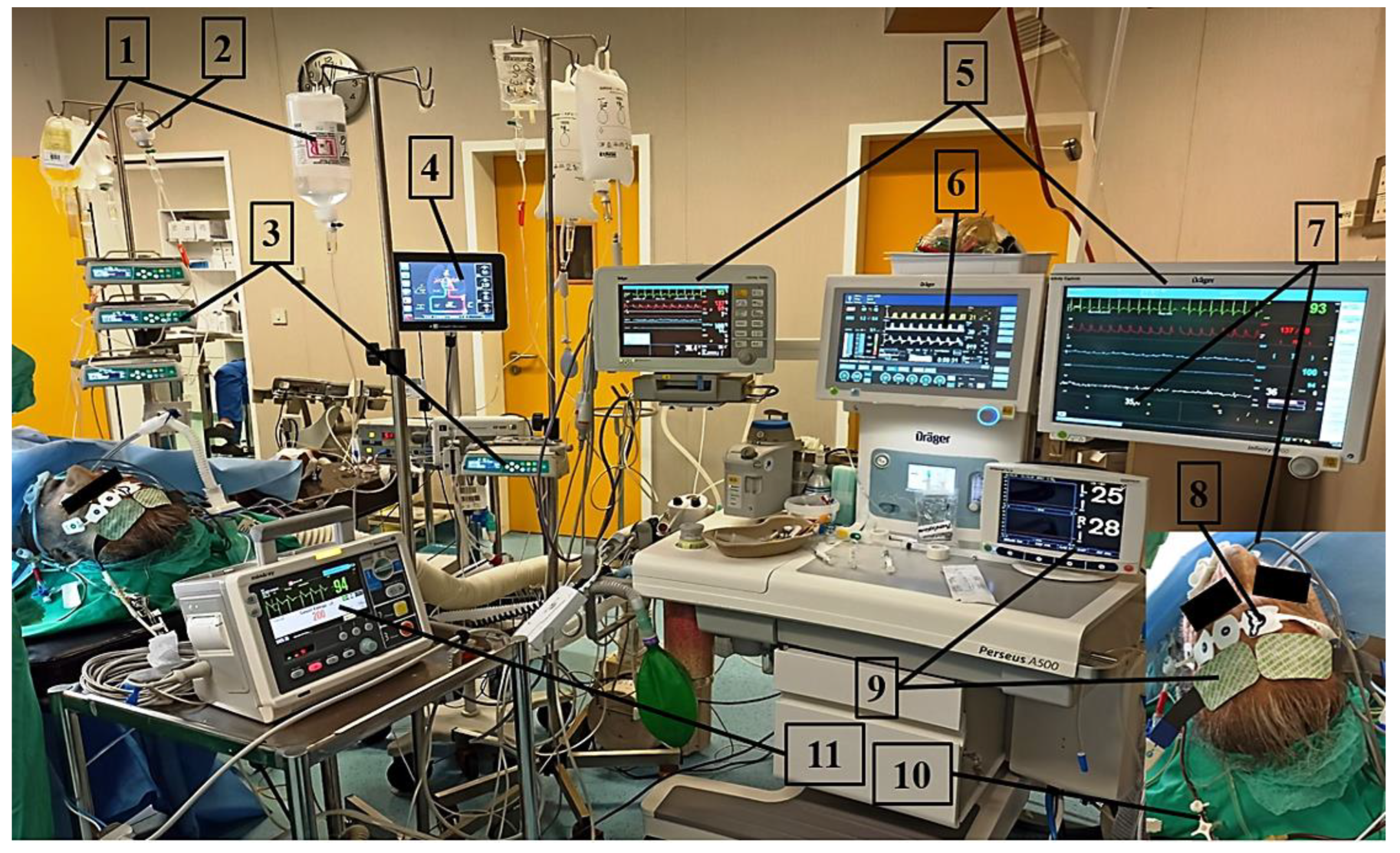
4. Airway and Anesthesia Management
5. Respiratory Management
6. Circulatory Management
7. Antibiotic Therapy
8. Active Temperature Control
9. Prognostication
10. Ethics of Critical Care and End-of-Life Decisions following Cardiac Arrest
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wissenberg, M.; Lippert, F.K.; Folke, F.; Weeke, P.; Hansen, C.M.; Christensen, E.F.; Jans, H.; Hansen, P.A.; Lang-Jensen, T.; Olesen, J.B.; et al. Association of national initiatives to improve cardiac arrest management with rates of bystander intervention and patient survival after out-of-hospital cardiac arrest. JAMA 2013, 310, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Girotra, S.; Nallamothu, B.K.; Spertus, J.A.; Li, Y.; Krumholz, H.M.; Chan, P.S.; American Heart Association Get with the Guidelines–Resuscitation Investigators. Trends in survival after in-hospital cardiac arrest. N. Engl. J. Med. 2012, 367, 1912–1920. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.P.; Soar, J.; Smith, G.B.; Gwinnutt, C.; Parrott, F.; Power, S.; Harrison, D.A.; Nixon, E.; Rowan, K.; National Cardiac Arrest Audit. Incidence and outcome of in-hospital cardiac arrest in the United Kingdom National Cardiac Arrest Audit. Resuscitation 2014, 85, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Oh, Y.M.; So, B.H.; Hong, T.Y.; Lee, W.J.; Choi, S.P.; Park, K.N. Systemic complications of comatose survivors following cardiopulmonary resuscitation. J. Korean Soc. Emerg. Med. 2008, 19, 88–93. [Google Scholar]
- Chalkias, A.; Xanthos, T. Pathophysiology and pathogenesis of post-resuscitation myocardial stunning. Heart Fail. Rev. 2012, 17, 117–128. [Google Scholar] [CrossRef]
- Ruiz-Bailén, M.; Aguayo de Hoyos, E.; Ruiz-Navarro, S.; Díaz-Castellanos, M.A.; Rucabado-Aguilar, L.; Gómez-Jiménez, F.J.; Martínez-Escobar, S.; Moreno, R.M.; Fierro-Rosón, J. Reversible myocardial dysfunction after cardiopulmonary resuscitation. Resuscitation 2005, 66, 175–181. [Google Scholar] [CrossRef]
- Kern, K.B.; Hilwig, R.W.; Berg, R.A.; Rhee, K.H.; Sanders, A.B.; Otto, C.W.; Ewy, G.A. Postresuscitation left ventricular systolic and diastolic dysfunction. Treatment with dobutamine. Circulation 1997, 95, 2610–2613. [Google Scholar] [CrossRef]
- Xu, T.; Tang, W.; Ristagno, G.; Wang, H.; Sun, S.; Weil, M.H. Postresuscitation myocardial diastolic dysfunction following prolonged ventricular fibrillation and cardiopulmonary resuscitation. Crit. Care Med. 2008, 36, 188–192. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Ussher, J.R.; Folmes, C.D.; Jaswal, J.S.; Stanley, W.C. Myocardial fatty acid metabolism in health and disease. Physiol. Rev. 2010, 90, 207–258. [Google Scholar] [CrossRef]
- Friberg, H.; Wieloch, T. Mitochondrial permeability transition in acute neurodegeneration. Biochimie 2002, 84, 241–250. [Google Scholar] [CrossRef]
- Lefer, A.M.; Lefer, D.J. The role of nitric oxide and cell adhesion molecules on the microcirculation in ischaemia–reperfusion. Cardiovasc. Res. 1996, 32, 743–751. [Google Scholar] [CrossRef]
- Goldhaber, J.I.; Qayyum, M.S. Oxygen free radicals and excitation–contraction coupling. Antioxid. Redox. Signal. 2000, 2, 55–64. [Google Scholar] [CrossRef]
- Sekhon, M.S.; Ainslie, P.N.; Griesdale, D.E. Clinical pathophysiology of hypoxic ischemic brain injury after cardiac arrest: A “two-hit” model. Crit. Care 2017, 21, 90. [Google Scholar] [CrossRef] [PubMed]
- Panchal, A.R.; Bartos, J.A.; Cabañas, J.G.; Donnino, M.W.; Drennan, I.R.; Hirsch, K.G.; Kudenchuk, P.J.; Kurz, M.C.; Lavonas, E.J.; Morley, P.T.; et al. Part 3: Adult Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2020, 142, S366–S468. [Google Scholar] [CrossRef] [PubMed]
- Hare, G.M.T.; Mazer, C.D.; Hutchison, J.S.; McLaren, A.T.; Liu, E.; Rassouli, A.; Ai, J.; Shaye, R.E.; Lockwood, J.A.; Hawkins, C.E.; et al. Severe hemodilutional anemia increases cerebral tissue injury following acute neurotrauma. J. Appl. Physiol. 2007, 103, 1021–1029. [Google Scholar] [CrossRef]
- van den Brule, J.M.D.; van der Hoeven, J.G.; Hoedemaekers, C.W.E. Cerebral Perfusion and Cerebral Autoregulation after Cardiac Arrest. Biomed. Res. Int. 2018, 2018, 4143636. [Google Scholar] [CrossRef]
- Traystman, R.J.; Kirsch, J.R.; Koehler, R.C. Oxygen radical mechanisms of brain injury following ischemia and reperfusion. J. Appl. Physiol. 1991, 71, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Lipton, P. Ischemic Cell Death in Brain Neurons. Physiol. Rev. 1999, 79, 1431–1568. [Google Scholar] [CrossRef]
- Morley, P.; Hogan, M.J.; Hakim, A.M. Calcium-Mediated Mechanisms of Ischemic Injury and Protection. Brain Pathol. 2008, 4, 37–47. [Google Scholar] [CrossRef]
- Adrie, C.; Laurent, I.; Monchi, M.; Cariou, A.; Dhainaou, J.F.; Spaulding, C. Postresuscitation disease after cardiac arrest: A sepsis-like syndrome? Curr. Opin. Crit. Care 2004, 10, 208–212. [Google Scholar] [CrossRef]
- Adams, J.A. Endothelium and cardiopulmonary resuscitation. Crit. Care Med. 2006, 34, S458–S465. [Google Scholar] [CrossRef] [PubMed]
- Eckle, T.; Faigle, M.; Grenz, A.; Laucher, S.; Thompson, L.F.; Eltzschig, H.K. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood 2008, 111, 2024–2035. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Strasser, A.; McDunn, J.E.; Swanson, P.E. Cell Death. N. Engl. J. Med. 2009, 361, 1570–1583. [Google Scholar] [CrossRef] [PubMed]
- Chalkias, A.; Scheetz, M.H.; Gulati, A.; Xanthos, T. Periarrest intestinal bacterial translocation and resuscitation outcome. J. Crit. Care 2016, 31, 217–220. [Google Scholar] [CrossRef]
- Tassopoulos, A.; Chalkias, A.; Papalois, A.; Iacovidou, N.; Xanthos, T. The effect of antioxidant supplementation on bacterial translocation after intestinal ischemia and reperfusion. Redox. Rep. 2017, 22, 1–9. [Google Scholar] [CrossRef]
- Hsing, C.H.; Lin, M.C.; Choi, P.C.; Huang, W.C.; Kai, J.I.; Tsai, C.C.; Cheng, Y.L.; Hsieh, C.Y.; Wang, C.Y.; Chang, Y.P.; et al. Anesthetic propofol reduces endotoxic inflammation by inhibiting reactive oxygen species-regulated Akt/IKKβ/NF-κB signaling. PLoS ONE 2011, 6, e17598. [Google Scholar] [CrossRef]
- Tassopoulos, A.; Chalkias, A.; Papalois, A.; Karlovasiti, P.; Zanda, J.S.A.; Chatzidakis, S.; Gazouli, M.; Iacovidou, N.; Fanni, D.; Xanthos, T. Assessment of Post-Resuscitation Intestinal Injury and Timing of Bacterial Translocation in Swine Anaesthetized with Propofol-Based Total Intravenous Anaesthesia. Cureus 2020, 12, e10362. [Google Scholar] [CrossRef]
- Asmussen, A.; Fink, K.; Busch, H.J.; Helbing, T.; Bourgeois, N.; Bode, C.; Grundmann, S. Inflammasome and toll-like receptor signaling in human monocytes after successful cardiopulmonary resuscitation. Crit. Care 2016, 20, 170. [Google Scholar] [CrossRef]
- Zhao, Q.; Shen, Y.; Li, R.; Wu, J.; Lyu, J.; Jiang, M.; Lu, L.; Zhu, M.; Wang, W.; Wang, Z.; et al. Cardiac arrest and resuscitation activates the hypothalamic-pituitary-adrenal axis and results in severe immunosuppression. J. Cereb. Blood Flow Metab. 2021, 41, 1091–1102. [Google Scholar] [CrossRef]
- Chalkias, A.; Xanthos, T. Post-cardiac arrest syndrome: Mechanisms and evaluation of adrenal insufficiency. World J. Crit. Care Med. 2012, 1, 4–9. [Google Scholar] [CrossRef]
- del Campo, R.; Martínez, E.; del Fresno, C.; Alenda, R.; Gómez-Piña, V.; Fernández-Ruíz, I.; Siliceo, M.; Jurado, T.; Toledano, V.; Arnalich, F.; et al. Translocated LPS might cause endotoxin tolerance in circulating monocytes of cystic fibrosis patients. PLoS ONE 2011, 6, e29577. [Google Scholar] [CrossRef] [PubMed]
- López-Collazo, E.; del Fresno, C. Pathophysiology of endotoxin tolerance: Mechanisms and clinical consequences. Crit. Care 2013, 17, 242. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.A.; Coppler, P.J.; Skolnik, A.B. The immunology of the post-cardiac arrest syndrome. Resuscitation 2022, 179, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Cavaillon, J.M.; Adib-Conquy, M. Bench-to-bedside review: Endotoxin tolerance as a model of leukocyte reprogramming in sepsis. Crit. Care 2006, 10, 233. [Google Scholar] [CrossRef]
- Qi, Z.; Liu, Q.; Zhang, Q.; Liu, B.; Li, C. Overexpression of programmed cell death-1 and human leucocyte antigen-DR on circulatory regulatory T cells in out-of-hospital cardiac arrest patients in the early period after return of spontaneous circulation. Resuscitation 2018, 130, 13–20. [Google Scholar] [CrossRef]
- Beurskens, C.J.; Horn, J.; de Boer, A.M.; Schultz, M.J.; van Leeuwen, E.M.; Vroom, M.B.; Juffermans, N.P. Cardiac arrest patients have an impaired immune response, which is not influenced by induced hypothermia. Crit. Care 2014, 18, R162. [Google Scholar] [CrossRef]
- Gelman, S. Venous Circulation: A Few Challenging Concepts in Goal-Directed Hemodynamic Therapy (GDHT). In Perioperative Fluid Management; Farag, E., Kurz, A., Troianos, C., Eds.; Springer Nature: Basel, Switzerland, 2020; pp. 365–385. [Google Scholar]
- Yen, R.T.; Fung, Y.C. Inversion of Fahraeus effect and effect of mainstream flow on capillary hematocrit. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1977, 42, 578–586. [Google Scholar] [CrossRef]
- Farina, A.; Fasano, A.; Rosso, F. A theoretical model for the Fåhræus effect in medium-large microvessels. J. Theor. Biol. 2023, 558, 111355. [Google Scholar] [CrossRef]
- Battaglini, D.; Pelosi, P.; Robba, C. Ten rules for optimizing ventilatory settings and targets in post-cardiac arrest patients. Crit. Care 2022, 26, 390. [Google Scholar] [CrossRef]
- Robba, C.; Badenes, R.; Battaglini, D.; Ball, L.; Brunetti, I.; Jakobsen, J.C.; Lilja, G.; Friberg, H.; Wendel-Garcia, P.D.; Young, P.J.; et al. Ventilatory settings in the initial 72 h and their association with outcome in out-of-hospital cardiac arrest patients: A preplanned secondary analysis of the targeted hypothermia versus targeted normothermia after out-of-hospital cardiac arrest (TTM2) trial. Intensive Care Med. 2022, 48, 1024–1038. [Google Scholar]
- Russotto, V.; Myatra, S.N.; Laffey, J.G.; Tassistro, E.; Antolini, L.; Bauer, P.; Lascarrou, J.B.; Szuldrzynski, K.; Camporota, L.; Pelosi, P.; et al. Intubation Practices and Adverse Peri-intubation Events in Critically Ill Patients From 29 Countries. JAMA 2021, 325, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Cook, T.M.; Woodall, N.; Harper, J.; Benger, J.; Fourth National Audit Project. Major complications of airway management in the UK: Results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 2: Intensive care and emergency departments. Br. J. Anaesth. 2011, 106, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Quintard, H.; l’Her, E.; Pottecher, J.; Adnet, F.; Constantin, J.M.; De Jong, A.; Diemunsch, P.; Fesseau, R.; Freynet, A.; Girault, C.; et al. Experts’ guidelines of intubation and extubation of the ICU patient of French Society of Anaesthesia and Intensive Care Medicine (SFAR) and French-speaking Intensive Care Society (SRLF): In collaboration with the pediatric Association of French-speaking Anaesthetists and Intensivists (ADARPEF), French-speaking Group of Intensive Care and Paediatric emergencies (GFRUP) and Intensive Care physiotherapy society (SKR). Ann. Intensive Care 2019, 9, 13. [Google Scholar] [PubMed]
- Myatra, S.N.; Divatia, J.V.; Brewster, D.J. The physiologically difficult airway: An emerging concept. Curr. Opin. Anaesthesiol. 2022, 35, 115–121. [Google Scholar] [CrossRef]
- Higgs, A.; McGrath, B.A.; Goddard, C.; Rangasami, J.; Suntharalingam, G.; Gale, R.; Cook, T.M., on behalf of the Difficult Airway Society, Intensive Care Society, Faculty of Intensive Care Medicine, Royal College of Anaesthetists. Guidelines for the management of tracheal intubation in critically ill adults. Br. J. Anaesth. 2018, 120, 323–352. [Google Scholar] [CrossRef]
- De Jong, A.; Rolle, A.; Molinari, N.; Paugam-Burtz, C.; Constantin, J.M.; Lefrant, J.Y.; Asehnoune, K.; Jung, B.; Futier, E.; Chanques, G.; et al. Cardiac Arrest and Mortality Related to Intubation Procedure in Critically Ill Adult Patients: A Multicenter Cohort Study. Crit. Care Med. 2018, 46, 532–539. [Google Scholar] [CrossRef]
- Mosier, J.M.; Hypes, C.D.; Sakles, J.C. Understanding preoxygenation and apneic oxygenation during intubation in the critically ill. Intensive Care Med. 2017, 43, 226–228. [Google Scholar] [CrossRef]
- Semler, M.W.; Janz, D.R.; Russell, D.W.; Casey, J.D.; Lentz, R.J.; Zouk, A.N.; deBoisblanc, B.P.; Santanilla, J.I.; Khan, Y.A.; Joffe, A.M.; et al. A Multicenter, Randomized Trial of Ramped Position vs Sniffing Position During Endotracheal Intubation of Critically Ill Adults. Chest 2017, 152, 712–722. [Google Scholar] [CrossRef]
- Frat, J.P.; Ricard, J.D.; Quenot, J.P.; Pichon, N.; Demoule, A.; Forel, J.M.; Mira, J.P.; Coudroy, R.; Berquier, G.; Voisin, B.; et al. Non-invasive ventilation versus high-flow nasal cannula oxygen therapy with apnoeic oxygenation for preoxygenation before intubation of patients with acute hypoxaemic respiratory failure: A randomised, multicentre, open-label trial. Lancet Respir. Med. 2019, 7, 303–312. [Google Scholar] [CrossRef]
- Guitton, C.; Ehrmann, S.; Volteau, C.; Colin, G.; Maamar, A.; Jean-Michel, V.; Mahe, P.J.; Landais, M.; Brule, N.; Bretonnière, C.; et al. Nasal high-flow preoxygenation for endotracheal intubation in the critically ill patient: A randomized clinical trial. Intensive Care Med. 2019, 45, 447–458. [Google Scholar] [CrossRef]
- Chalkias, A.; Pavlopoulos, F.; Papageorgiou, E.; Tountas, C.; Anania, A.; Panteli, M.; Beloukas, A.; Xanthos, T. Development and Testing of a Novel Anaesthesia Induction/Ventilation Protocol for Patients with Cardiogenic Shock Complicating Acute Myocardial Infarction. Can. J. Cardiol. 2018, 34, 1048–1058. [Google Scholar] [CrossRef] [PubMed]
- Natt, B.; Mosier, J. Airway Management in the Critically Ill Patient. Curr. Anesthesiol. Rep. 2021, 11, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Janz, D.R.; Casey, J.D.; Semler, M.W.; Russell, D.W.; Dargin, J.; Vonderhaar, D.J.; Dischert, K.M.; West, J.R.; Stempek, S.; Wozniak, J.; et al. Effect of a fluid bolus on cardiovascular collapse among critically ill adults undergoing tracheal intubation (PrePARE): A randomised controlled trial. Lancet Respir. Med. 2019, 7, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.W.; Casey, J.D.; Gibbs, K.W.; Ghamande, S.; Dargin, J.M.; Vonderhaar, D.J.; Joffe, A.M.; Khan, A.; Prekker, M.E.; Brewer, J.M.; et al. Effect of Fluid Bolus Administration on Cardiovascular Collapse Among Critically Ill Patients Undergoing Tracheal Intubation: A Randomized Clinical Trial. JAMA 2022, 328, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.B.; Peng, J.M.; Xu, B.; Liu, G.Y.; Du, B. Video Laryngoscopy for Endotracheal Intubation of Critically Ill Adults: A Systemic Review and Meta-Analysis. Chest 2017, 152, 510–517. [Google Scholar] [CrossRef]
- Jiang, J.; Ma, D.; Li, B.; Yue, Y.; Xue, F. Video laryngoscopy does not improve the intubation outcomes in emergency and critical patients—A systematic review and meta-analysis of randomized controlled trials. Crit. Care 2017, 21, 288. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, B.K.; Jeung, K.W.; Jung, Y.H.; Cho, Y.S.; Youn, C.S.; Min, Y.I. Neuromuscular blockade requirement is associated with good neurologic outcome in cardiac arrest survivors treated with targeted temperature management. J. Crit. Care 2017, 40, 218–224. [Google Scholar] [CrossRef] [PubMed]
- May, T.L.; Riker, R.R.; Fraser, G.L.; Hirsch, K.G.; Agarwal, S.; Duarte, C.; Friberg, H.; Søreide, E.; McPherson, J.; Hand, R.; et al. Variation in sedation and neuromuscular blockade regimens on outcome after cardiac arrest. Crit. Care Med. 2018, 46, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Bougouin, W.; Dumas, F.; Geri, G.; Champigneulle, B.; Guillemet, L.; Ben Hadj Salem, O.; Legriel, S.; Chiche, J.D.; Charpentier, J.; et al. Comparison of two sedation regimens during targeted temperature management after cardiac arrest. Resuscitation 2018, 128, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Bjelland, T.W.; Dale, O.; Kaisen, K.; Haugen, B.O.; Lydersen, S.; Strand, K.; Klepstad, P. Propofol and remifentanil versus midazolam and fentanyl for sedation during therapeutic hypothermia after cardiac arrest: A randomised trial. Intensive Care Med. 2012, 38, 959–967. [Google Scholar] [CrossRef]
- Dell’Anna, A.M.; Taccone, F.S.; Halenarova, K.; Citerio, G. Sedation after cardiac arrest and during therapeutic hypothermia. Minerva Anestesiol. 2014, 80, 954–962. [Google Scholar] [PubMed]
- Arpino, P.A.; Greer, D.M. Practical pharmacologic aspects of therapeutic hypothermia after cardiac arrest. Pharmacotherapy 2008, 28, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Bjelland, T.W.; Klepstad, P.; Haugen, B.O.; Nilsen, T.; Dale, O. Effects of hypothermia on the disposition of morphine, midazolam, fentanyl, and propofol in intensive care unit patients. Drug Metab. Dispos. 2013, 41, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Tortorici, M.A.; Kochanek, P.M.; Poloyac, S.M. Effects of hypothermia on drug disposition, metabolism, and response: A focus of hypothermia-mediated alterations on the cytochrome P450 enzyme system. Crit. Care Med. 2007, 35, 2196–2204. [Google Scholar] [CrossRef]
- Cronberg, T.; Horn, J.; Kuiper, M.A.; Friberg, H.; Nielsen, N. A structured approach to neurologic prognostication in clinical cardiac arrest trials. Scand. J. Trauma Resusc. Emerg. Med. 2013, 21, 45. [Google Scholar] [CrossRef]
- Amado-Rodríguez, L.; Rodríguez-Garcia, R.; Bellani, G.; Pham, T.; Fan, E.; Madotto, F.; Laffey, J.G.; Albaiceta, G.M.; LUNG SAFE investigators. Mechanical ventilation in patients with cardiogenic pulmonary edema: A sub-analysis of the LUNG SAFE study. J. Intensive Care 2022, 10, 55. [Google Scholar] [CrossRef]
- Serpa Neto, A.; Deliberato, R.O.; Johnson, A.E.W.; Bos, L.D.; Amorim, P.; Pereira, S.M.; Cazati, D.C.; Cordioli, R.L.; Correa, T.D.; Pollard, T.J.; et al. Mechanical power of ventilation is associated with mortality in critically ill patients: An analysis of patients in two observational cohorts. Intensive Care Med. 2018, 44, 1914–1922. [Google Scholar] [CrossRef]
- Nolan, J.P.; Sandroni, C.; Böttiger, B.W.; Cariou, A.; Cronberg, T.; Friberg, H.; Genbrugge, C.; Haywood, K.; Lilja, G.; Moulaert, V.R.M.; et al. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines 2021: Post-resuscitation care. Resuscitation 2021, 161, 220–269. [Google Scholar] [CrossRef]
- Awad, A.; Nordberg, P.; Jonsson, M.; Hofmann, R.; Ringh, M.; Hollenberg, J.; Olson, J.; Joelsson-Alm, E. Hyperoxemia after reperfusion in cardiac arrest patients: A potential dose-response association with 30-day survival. Crit. Care 2023, 27, 86. [Google Scholar] [CrossRef]
- Janz, D.R.; Hollenbeck, R.D.; Pollock, J.S.; McPherson, J.A.; Rice, T.W. Hyperoxia is associated with increased mortality in patients treated with mild therapeutic hypothermia after sudden cardiac arrest. Crit. Care Med. 2012, 40, 3135–3139. [Google Scholar] [CrossRef]
- Roberts, B.W.; Kilgannon, J.H.; Hunter, B.R.; Puskarich, M.A.; Pierce, L.; Donnino, M.; Leary, M.; Kline, J.A.; Jones, A.E.; Shapiro, N.I.; et al. Association Between Early Hyperoxia Exposure After Resuscitation from Cardiac Arrest and Neurological Disability: Prospective Multicenter Protocol-Directed Cohort Study. Circulation 2018, 137, 2114–2124. [Google Scholar] [CrossRef] [PubMed]
- Robba, C.; Badenes, R.; Battaglini, D.; Ball, L.; Sanfilippo, F.; Brunetti, I.; Jakobsen, J.C.; Lilja, G.; Friberg, H.; Wendel-Garcia, P.D.; et al. Oxygen targets and 6-month outcome after out of hospital cardiac arrest: A pre-planned sub-analysis of the targeted hypothermia versus targeted normothermia after Out-of-Hospital Cardiac Arrest (TTM2) trial. Crit. Care 2022, 26, 323. [Google Scholar] [CrossRef]
- Keeley, T.P.; Mann, G.E. Defining Physiological Normoxia for Improved Translation of Cell Physiology to Animal Models and Humans. Physiol. Rev. 2019, 99, 161–234. [Google Scholar] [CrossRef] [PubMed]
- Schödel, J.; Ratcliffe, P.J. Mechanisms of hypoxia signalling: New implications for nephrology. Nat. Rev. Nephrol. 2019, 15, 641–659. [Google Scholar] [CrossRef] [PubMed]
- Chalkias, A.; Xenos, M. Relationship of Effective Circulating Volume with Sublingual Red Blood Cell Velocity and Microvessel Pressure Difference: A Clinical Investigation and Computational Fluid Dynamics Modeling. J. Clin. Med. 2022, 11, 4885. [Google Scholar] [CrossRef] [PubMed]
- Chalkias, A.; Laou, E.; Mermiri, M.; Michou, A.; Ntalarizou, N.; Koutsona, S.; Chasiotis, G.; Garoufalis, G.; Agorogiannis, V.; Kyriakaki, A.; et al. Microcirculation-guided treatment improves tissue perfusion and hemodynamic coherence in surgical patients with septic shock. Eur. J. Trauma Emerg. Surg. 2022, 48, 4699–4711. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Kjaergaard, J.; Hassager, C.; Mølstrøm, S.; Grand, J.; Borregaard, B.; Roelsgaard Obling, L.E.; Venø, S.; Sarkisian, L.; Mamaev, D.; et al. Oxygen Targets in Comatose Survivors of Cardiac Arrest. N. Engl. J. Med. 2022, 387, 1467–1476. [Google Scholar] [CrossRef]
- McKenzie, N.; Williams, T.A.; Tohira, H.; Ho, K.M.; Finn, J. A systematic review and meta-analysis of the association between arterial carbon dioxide tension and outcomes after cardiac arrest. Resuscitation 2017, 111, 116–126. [Google Scholar] [CrossRef]
- Sitzwohl, C.; Kettner, S.C.; Reinprecht, A.; Dietrich, W.; Klimscha, W.; Fridrich, P.; Sladen, R.N.; Illievich, U.M. The arterial to end-tidal carbon dioxide gradient increases with uncorrected but not with temperature-corrected PaCO2 determination during mild to moderate hypothermia. Anesth. Analg. 1998, 86, 1131–1136. [Google Scholar]
- Lennox, W. The effect on epileptic seizures of varying the composition of the respired air. J. Clin. Investig. 1928, 6, 23–24. [Google Scholar]
- Shoja, M.M.; Tubbs, R.S.; Shokouhi, G.; Loukas, M.; Ghabili, K.; Ansarin, K. The potential role of carbon dioxide in the neuroimmunoendocrine changes following cerebral ischemia. Life Sci. 2008, 83, 381–387. [Google Scholar] [CrossRef]
- Schneider, A.G.; Eastwood, G.M.; Bellomo, R.; Bailey, M.; Lipcsey, M.; Pilcher, D.; Young, P.; Stow, P.; Santamaria, J.; Stachowski, E.; et al. Arterial carbon dioxide tension and outcome in patients admitted to the intensive care unit after cardiac arrest. Resuscitation 2013, 84, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Helmerhorst, H.J.; Roos-Blom, M.J.; van Westerloo, D.J.; Abu-Hanna, A.; de Keizer, N.F.; de Jonge, E. Associations of arterial carbon dioxide and arterial oxygen concentrations with hospital mortality after resuscitation from cardiac arrest. Crit. Care 2015, 19, 348. [Google Scholar] [CrossRef] [PubMed]
- Okada, N.; Matsuyama, T.; Okada, Y.; Okada, A.; Kandori, K.; Nakajima, S.; Kitamura, T.; Ohta, B. Post-Resuscitation Partial Pressure of Arterial Carbon Dioxide and Outcome in Patients with Out-of-Hospital Cardiac Arrest: A Multicenter Retrospective Cohort Study. J. Clin. Med. 2022, 11, 1523. [Google Scholar] [CrossRef] [PubMed]
- Chalkias, A.; Mongardon, N.; Boboshko, V.; Cerny, V.; Constant, A.L.; De Roux, Q.; Finco, G.; Fumagalli, F.; Gkamprela, E.; Legriel, S.; et al. Clinical practice recommendations on the management of perioperative cardiac arrest: A report from the PERIOPCA Consortium. Crit. Care 2021, 25, 265. [Google Scholar] [CrossRef]
- Bro-Jeppesen, J.; Hassager, C.; Wanscher, M.; Østergaard, M.; Nielsen, N.; Erlinge, D.; Friberg, H.; Køber, L.; Kjaergaard, J. Targeted temperature management at 33 °C versus 36 °C and impact on systemic vascular resistance and myocardial function after out-of-hospital cardiac arrest: A sub-study of the Target Temperature Management Trial. Circ. Cardiovasc. Interv. 2014, 7, 663–672. [Google Scholar] [CrossRef]
- Bro-Jeppesen, J.; Annborn, M.; Hassager, C.; Wise, M.P.; Pelosi, P.; Nielsen, N.; Erlinge, D.; Wanscher, M.; Friberg, H.; Kjaergaard, J.; et al. Hemodynamics and vasopressor support during targeted temperature management at 33 °C versus 36 °C after out-of-hospital cardiac arrest: A post hoc study of the target temperature management trial. Crit. Care Med. 2015, 43, 318–327. [Google Scholar] [CrossRef]
- Gaieski, D.F.; Band, R.A.; Abella, B.S.; Neumar, R.W.; Fuchs, B.D.; Kolansky, D.M.; Merchant, R.M.; Carr, B.G.; Becker, L.B.; Maguire, C.; et al. Early goal-directed hemodynamic optimization combined with therapeutic hypothermia in comatose survivors of out-of-hospital cardiac arrest. Resuscitation 2009, 80, 418–424. [Google Scholar] [CrossRef]
- Ko, J.I.; Kim, K.S.; Suh, G.J.; Kim, K.; Kwon, W.Y.; Shin, J.; Jo, Y.H.; Jung, Y.S.; Kim, T.; Shin, S.M. Relative tachycardia is associated with poor outcomes in post-cardiac arrest patients regardless of therapeutic hypothermia. Am. J. Emerg. Med. 2019, 37, 590–595. [Google Scholar] [CrossRef]
- Skrifvars, M.B.; Pettilä, V.; Rosenberg, P.H.; Castrén, M. A multiple logistic regression analysis of in-hospital factors related to survival at six months in patients resuscitated from out-of-hospital ventricular fibrillation. Resuscitation 2003, 59, 319–328. [Google Scholar] [CrossRef]
- Oh, V.M.; Kaye, C.M.; Warrington, S.J.; Taylor, E.A.; Wadsworth, J. Studies of cardioselectivity and partial agonist activity in beta-adrenoceptor blockade comparing effects on heart rate and peak expiratory flow rate during exercise. Br. J. Clin. Pharmacol. 1978, 5, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Lonjaret, L.; Lairez, O.; Minville, V.; Geeraerts, T. Optimal perioperative management of arterial blood pressure. Integr. Blood Press Control 2014, 7, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Bruning, R.; Dykes, H.; Jones, T.W.; Wayne, N.B.; Sikora Newsome, A. Beta-Adrenergic Blockade in Critical Illness. Front. Pharmacol. 2021, 12, 735841. [Google Scholar] [CrossRef]
- Oyama, Y.; Blaskowsky, J.; Eckle, T. Dose-dependent Effects of Esmolol-epinephrine Combination Therapy in Myocardial Ischemia and Reperfusion Injury. Curr. Pharm. Des. 2019, 25, 2199–2206. [Google Scholar] [CrossRef] [PubMed]
- Krumpl, G.; Ulč, I.; Trebs, M.; Kadlecová, P.; Hodisch, J.; Maurer, G.; Husch, B. Pharmacodynamic and -kinetic Behavior of Low-, Intermediate-, and High-Dose Landiolol During Long-Term Infusion in Whites. J. Cardiovasc. Pharmacol. 2017, 70, 42–51. [Google Scholar] [CrossRef]
- Krumpl, G.; Ulc, I.; Trebs, M.; Kadlecová, P.; Hodisch, J. Bolus application of landiolol and esmolol: Comparison of the pharmacokinetic and pharmacodynamic profiles in a healthy Caucasian group. Eur. J. Clin. Pharmacol. 2017, 73, 417–428. [Google Scholar] [CrossRef]
- Matsui, Y.; Suzuki, A.; Shiga, T.; Arai, K.; Hagiwara, N. Effects of Intravenous Landiolol on Heart Rate and Outcomes in Patients with Atrial Tachyarrhythmias and Acute Decompensated Heart Failure: A Single-Center Experience. Drugs Real World Outcomes 2019, 6, 19–26. [Google Scholar] [CrossRef]
- Morisaki, A.; Hosono, M.; Sasaki, Y.; Hirai, H.; Sakaguchi, M.; Nakahira, A.; Seo, H.; Suehiro, S. Very-low-dose continuous drip infusion of landiolol hydrochloride for postoperative atrial tachyarrhythmia in patients with poor left ventricular function. Gen Thorac. Cardiovasc. Surg. 2012, 60, 386–390. [Google Scholar] [CrossRef]
- Kobayashi, S.; Susa, T.; Tanaka, T.; Murakami, W.; Fukuta, S.; Okuda, S.; Doi, M.; Wada, Y.; Nao, T.; Yamada, J.; et al. Low-dose β-blocker in combination with milrinone safely improves cardiac function and eliminates pulsus alternans in patients with acute decompensated heart failure. Circ. J. 2012, 76, 1646–1653. [Google Scholar] [CrossRef]
- Hamaguchi, S.; Nagao, M.; Takahashi, Y.; Ikeda, T.; Yamaguchi, S. Low Dose Landiolol Combined with Catecholamine Can Decrease Heart Rate without Suppression of Cardiac Contraction after Cardiopulmonary Bypass. Dokkyo J. Med. Sci. 2014, 41, 27–33. [Google Scholar]
- Kobayashi, S.; Murakami, W.; Myoren, T.; Tateishi, H.; Okuda, S.; Doi, M.; Nao, T.; Wada, Y.; Matsuzaki, M.; Yano, M. A low-dose β1-blocker effectively and safely slows the heart rate in patients with acute decompensated heart failure and rapid atrial fibrillation. Cardiology 2014, 127, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Nitta, D.; Kinugawa, K.; Imamura, T.; Endo, M.; Amiya, E.; Inaba, T.; Maki, H.; Hatano, M.; Komuro, I. An Experience of Landiolol Use for an Advanced Heart Failure Patient with Severe Hypotension. Int. Heart J. 2015, 56, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Ditali, V.; Garatti, L.; Morici, N.; Villanova, L.; Colombo, C.; Oliva, F.; Sacco, A. Effect of landiolol in patients with tachyarrhythmias and acute decompensated heart failure (ADHF): A case series. ESC Heart Fail. 2022, 9, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar]
- Chalkias, A.; Arnaoutoglou, E.; Xanthos, T. Personalized physiology-guided resuscitation in highly monitored patients with cardiac arrest-the PERSEUS resuscitation protocol. Heart Fail. Rev. 2019, 24, 473–480. [Google Scholar] [CrossRef]
- Sakai, M.; Jujo, S.; Kobayashi, J.; Ohnishi, Y.; Kamei, M. Use of low-dose β1-blocker for sinus tachycardia in patients with catecholamine support following cardiovascular surgery: A retrospective study. J. Cardiothorac. Surg. 2019, 14, 145. [Google Scholar] [CrossRef]
- Imabayashi, T.; Murayama, H.; Kuroki, C.; Kiyonaga, N.; Oryouji, T.; Tashiro, S.; Yasuda, T.; Kakihana, Y.; Matunaga, A.; Kanmura, Y. Study of hemodynamics in patients treated with landiolol in the ICU. Crit. Care 2009, 13, P173. [Google Scholar] [CrossRef]
- Sakaguchi, M.; Sasaki, Y.; Hirai, H.; Hosono, M.; Nakahira, A.; Seo, H.; Suehiro, S. Efficacy of landiolol hydrochloride for prevention of atrial fibrillation after heart valve surgery. Int. Heart J. 2012, 53, 359–363. [Google Scholar] [CrossRef]
- Yoshida, Y.; Terajima, K.; Sato, C.; Akada, S.; Miyagi, Y.; Hongo, T.; Takeda, S.; Tanaka, K.; Sakamoto, A. Clinical role and efficacy of landiolol in the intensive care unit. J. Anesth. 2008, 22, 64–69. [Google Scholar] [CrossRef]
- Dabrowski, W.; Siwicka-Gieroba, D.; Piasek, E.; Schlegel, T.T.; Jaroszynski, A. Successful Combination of Landiolol and Levosimendan in Patients with Decompensated Heart Failure. Int. Heart J. 2020, 61, 384–389. [Google Scholar] [CrossRef]
- Anifanti, M.; Iona, I.; Tsikritsaki, K.; Chrysikos, S.; Kalogeromitros, A.; Koukoulitsios, G. Landiolol vs Esmolol on hemodynamic response during weaning of post-operative ICU patients with heart failure. Eur. Respir. J. 2021, 58, PA3320. [Google Scholar]
- Levijoki, J.; Pollesello, P.; Kaivola, J.; Tilgmann, C.; Sorsa, T.; Annila, A.; Kilpeläinen, I.; Haikala, H. Further evidence for the cardiac troponin C mediated calcium sensitization by levosimendan: Structure-response and binding analysis with analogs of levosimendan. J. Mol. Cell Cardiol. 2000, 32, 479–491. [Google Scholar] [CrossRef]
- Huang, L.; Weil, M.H.; Tang, W.; Sun, S.; Wang, J. Comparison between dobutamine and levosimendan for management of postresuscitation myocardial dysfunction. Crit. Care Med. 2005, 33, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Kakavas, S.; Chalkias, A.; Xanthos, T. Vasoactive support in the optimization of post-cardiac arrest hemodynamic status: From pharmacology to clinical practice. Eur. J. Pharmacol. 2011, 667, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Kjaergaard, J.; Møller, J.E.; Schmidt, H.; Grand, J.; Mølstrøm, S.; Borregaard, B.; Venø, S.; Sarkisian, L.; Mamaev, D.; Jensen, L.O.; et al. Blood-Pressure Targets in Comatose Survivors of Cardiac Arrest. N. Engl. J. Med. 2022, 387, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Mølstrøm, S.; Nielsen, T.H.; Nordstrøm, C.H.; Forsse, A.; Møller, S.; Venø, S.; Mamaev, D.; Tencer, T.; Theódórsdóttir, Á.; Krøigård, T.; et al. A randomized, double-blind trial comparing the effect of two blood pressure targets on global brain metabolism after out-of-hospital cardiac arrest. Crit. Care 2023, 27, 73. [Google Scholar] [CrossRef]
- McGuigan, P.J.; Giallongo, E.; Blackwood, B.; Doidge, J.; Harrison, D.A.; Nichol, A.D.; Rowan, K.M.; Shankar-Hari, M.; Skrifvars, M.B.; Thomas, K.; et al. The effect of blood pressure on mortality following out-of-hospital cardiac arrest: A retrospective cohort study of the United Kingdom Intensive Care National Audit and Research Centre database. Crit. Care 2023, 27, 4. [Google Scholar] [CrossRef] [PubMed]
- Dupont, V.; Bonnet-Lebrun, A.S.; Boileve, A.; Charpentier, J.; Mira, J.P.; Geri, G.; Cariou, A.; Jozwiak, M. Impact of early mean arterial pressure level on severe acute kidney injury occurrence after out-of-hospital cardiac arrest. Ann. Intensive Care 2022, 12, 69. [Google Scholar] [CrossRef] [PubMed]
- Russo, J.J.; Di Santo, P.; Simard, T.; James, T.E.; Hibbert, B.; Couture, E.; Marbach, J.; Osborne, C.; Ramirez, F.D.; Wells, G.A.; et al. Optimal mean arterial pressure in comatose survivors of out-of-hospital cardiac arrest: An analysis of area below blood pressure thresholds. Resuscitation 2018, 128, 175–180. [Google Scholar] [CrossRef]
- Donadello, K.; Su, F.; Annoni, F.; Scolletta, S.; He, X.; Peluso, L.; Gottin, L.; Polati, E.; Creteur, J.; De Witte, O.; et al. The Effects of Temperature Management on Brain Microcirculation, Oxygenation and Metabolism. Brain Sci. 2022, 12, 1422. [Google Scholar] [CrossRef] [PubMed]
- Chalkias, A. Increasing stress volume vs. increasing tissue perfusion in septic patients. Eur. J. Anaesthesiol. 2022, 39, 390–391. [Google Scholar] [CrossRef]
- Koopmans, M.; Kuiper, M.A.; Endeman, H.; Veenstra, G.; Vellinga, N.A.; de Vos, R.; Boerma, E.C. Microcirculatory perfusion and vascular reactivity are altered in post cardiac arrest patients, irrespective of target temperature management to 33 °C vs. 36 °C. Resuscitation 2015, 86, 14–18. [Google Scholar] [CrossRef]
- Han, C.; Lee, J.H.; Korean Hypothermia Network Investigators. Heart rate and diastolic arterial pressure in cardiac arrest patients: A nationwide, multicenter prospective registry. PLoS ONE 2022, 17, e0274130. [Google Scholar] [CrossRef]
- Chalkias, A.; Laou, E.; Papagiannakis, N.; Spyropoulos, V.; Kouskouni, E.; Theodoraki, K.; Xanthos, T. Assessment of Dynamic Changes in Stressed Volume and Venous Return during Hyperdynamic Septic Shock. J. Pers. Med. 2022, 12, 724. [Google Scholar] [CrossRef] [PubMed]
- Joffre, J.; Lloyd, E.; Wong, E.; Chung-Yeh, C.; Nguyen, N.; Xu, F.; Legrand, M.; Hellman, J. Catecholaminergic Vasopressors Reduce Toll-Like Receptor Agonist-Induced Microvascular Endothelial Cell Permeability But Not Cytokine Production. Crit. Care Med. 2021, 49, e315–e326. [Google Scholar] [CrossRef] [PubMed]
- Demiselle, J.; Fage, N.; Radermacher, P.; Asfar, P. Vasopressin and its analogues in shock states: A review. Ann. Intensive Care 2020, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Landry, D.W.; Levin, H.R.; Gallant, E.M.; Ashton, R.C., Jr.; Seo, S.; D’Alessandro, D.; Oz, M.C.; Oliver, J.A. Vasopressin deficiency contributes to the vasodilation of septic shock. Circulation 1997, 95, 1122–1125. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, W.F.; Um, K.J.; Alhazzani, W.; Lengyel, A.P.; Hajjar, L.; Gordon, A.C.; Lamontagne, F.; Healey, J.S.; Whitlock, R.P.; Belley-Côté, E.P. Association of Vasopressin Plus Catecholamine Vasopressors vs Catecholamines Alone with Atrial Fibrillation in Patients With Distributive Shock: A Systematic Review and Meta-analysis. JAMA 2018, 319, 1889–1900. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, R.; Forni, L.G.; Busse, L.W.; McCurdy, M.T.; Ham, K.R.; Boldt, D.W.; Hästbacka, J.; Khanna, A.K.; Albertson, T.E.; Tumlin, J.; et al. Renin and Survival in Patients Given Angiotensin II for Catecholamine-Resistant Vasodilatory Shock. A Clinical Trial. Am. J. Respir. Crit. Care Med. 2020, 202, 1253–1261. [Google Scholar] [CrossRef]
- Leisman, D.E.; Fernandes, T.D.; Bijol, V.; Abraham, M.N.; Lehman, J.R.; Taylor, M.D.; Capone, C.; Yaipan, O.; Bellomo, R.; Deutschman, C.S. Impaired angiotensin II type 1 receptor signaling contributes to sepsis-induced acute kidney injury. Kidney Int. 2021, 99, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Chalkias, A.; Laou, E.; Papagiannakis, N.; Varvarousi, G.; Ragias, D.; Koutsovasilis, A.; Makris, D.; Varvarousis, D.; Iacovidou, N.; Pantazopoulos, I.; et al. Determinants of venous return in steady-state physiology and asphyxia-induced circulatory shock and arrest: An experimental study. Intensive Care Med. Exp. 2022, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Grübler, M.R.; Wigger, O.; Berger, D.; Blöchlinger, S. Basic concepts of heart-lung interactions during mechanical ventilation. Swiss Med. Wkly. 2017, 147, w14491. [Google Scholar] [CrossRef] [PubMed]
- Rothe, C.F.; Maass-Moreno, R.; Flanagan, A.D. Effects of hypercapnia and hypoxia on the cardiovascular system: Vascular capacitance and aortic chemoreceptors. Am. J. Physiol. 1990, 259, H932–H939. [Google Scholar] [CrossRef] [PubMed]
- Stegman, B.; Aggarwal, B.; Senapati, A.; Shao, M.; Menon, V. Serial hemodynamic measurements in post-cardiac arrest cardiogenic shock treated with therapeutic hypothermia. Eur. Heart J. Acute Cardiovasc. Care 2015, 4, 263–269. [Google Scholar] [CrossRef]
- Mentzelopoulos, S.D.; Mongardon, N.; Xanthos, T.; Zakynthinos, S.G. Possible significance of hemodynamic and immunomodulatory effects of early stress-dose steroids in cardiac arrest. Crit. Care 2016, 20, 211. [Google Scholar] [CrossRef]
- Mentzelopoulos, S.D.; Malachias, S.; Chamos, C.; Konstantopoulos, D.; Ntaidou, T.; Papastylianou, A.; Kolliantzaki, I.; Theodoridi, M.; Ischaki, H.; Makris, D.; et al. Vasopressin, steroids, and epinephrine and neurologically favorable survival after in-hospital cardiac arrest: A randomized clinical trial. JAMA 2013, 310, 270–279. [Google Scholar] [CrossRef]
- Mentzelopoulos, S.D.; Koliantzaki, I.; Karvouniaris, M.; Vrettou, C.; Mongardon, N.; Karlis, G.; Makris, D.; Zakynthinos, E.; Sourlas, S.; Aloizos, S.; et al. Exposure to Stress-Dose Steroids and Lethal Septic Shock After In-Hospital Cardiac Arrest: Individual Patient Data Reanalysis of Two Prior Randomized Clinical Trials that Evaluated the Vasopressin-Steroids-Epinephrine Combination Versus Epinephrine Alone. Cardiovasc. Drugs Ther. 2018, 32, 339–351. [Google Scholar] [CrossRef]
- Mentzelopoulos, S.D.; Pappa, E.; Malachias, S.; Vrettou, C.S.; Giannopoulos, A.; Karlis, G.; Adamos, G.; Pantazopoulos, I.; Megalou, A.; Louvaris, Z.; et al. Physiologic effects of stress dose corticosteroids in in-hospital cardiac arrest (CORTICA): A randomized clinical trial. Resusc. Plus 2022, 10, 100252. [Google Scholar] [CrossRef]
- Chalkias, A. Letter to the editor: “The emerging concept of fluid tolerance: A position paper”. J. Crit. Care 2023, 74, 154235. [Google Scholar] [CrossRef]
- Kattan, E.; Castro, R.; Miralles-Aguiar, F.; Hernández, G.; Rola, P. The emerging concept of fluid tolerance: A position paper. J. Crit. Care 2022, 71, 154070. [Google Scholar] [CrossRef]
- Beaubien-Souligny, W.; Rola, P.; Haycock, K.; Bouchard, J.; Lamarche, Y.; Spiegel, R.; Denault, A.Y. Quantifying systemic congestion with Point-Of-Care ultrasound: Development of the venous excess ultrasound grading system. Ultrasound J. 2020, 12, 16. [Google Scholar] [CrossRef]
- Thiele, H.; Zeymer, U.; Neumann, F.J.; Ferenc, M.; Olbrich, H.G.; Hausleiter, J.; Richardt, G.; Hennersdorf, M.; Empen, K.; Fuernau, G.; et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N. Engl. J. Med. 2012, 367, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Deballon, I.; Hornby, L.; Shemie, S.D.; Bhanji, F.; Guadagno, E. Extracorporeal resuscitation for refractory out-of-hospital cardiac arrest in adults: A systematic review of international practices and outcomes. Resuscitation 2016, 101, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Fjølner, J.; Greisen, J.; Jørgensen, M.R.; Terkelsen, C.J.; Ilkjaer, L.B.; Hansen, T.M.; Eiskjaer, H.; Christensen, S.; Gjedsted, J. Extracorporeal cardiopulmonary resuscitation after out-of-hospital cardiac arrest in a Danish health region. Acta Anaesthesiol. Scand. 2017, 61, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Ouweneel, D.M.; Eriksen, E.; Sjauw, K.D.; van Dongen, I.M.; Hirsch, A.; Packer, E.J.; Vis, M.M.; Wykrzykowska, J.J.; Koch, K.T.; Baan, J.; et al. Percutaneous mechanical circulatory support versus intra-aortic balloon pump in cardiogenic shock after acute myocardial infarction. J. Am. Coll. Cardiol. 2017, 69, 278–287. [Google Scholar] [CrossRef]
- Coba, V.; Jaehne, A.K.; Suarez, A.; Dagher, G.A.; Brown, S.C.; Yang, J.J.; Manteuffel, J.; Rivers, E.P. The incidence and significance of bacteremia in out of hospital cardiac arrest. Resuscitation 2014, 85, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Polderman, K.H.; Herold, I. Therapeutic hypothermia and controlled normothermia in the intensive care unit: Practical considerations, side effects, and cooling methods. Crit. Care Med. 2009, 37, 1101–1120. [Google Scholar] [CrossRef]
- Cueni-Villoz, N.; Devigili, A.; Delodder, F.; Cianferoni, S.; Feihl, F.; Rossetti, A.O.; Eggimann, P.; Vincent, J.L.; Taccone, F.S.; Oddo, M. Increased blood glucose variability during therapeutic hypothermia and outcome after cardiac arrest. Crit. Care Med. 2011, 39, 2225–2231. [Google Scholar] [CrossRef]
- Booth, G.; Stalker, T.J.; Lefer, A.M.; Scalia, R. Elevated ambient glucose induces acute inflammatory events in the microvasculature: Effects of insulin. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E848–E856. [Google Scholar] [CrossRef] [PubMed]
- Efrati, S.; Berman, S.; Hamad, R.A.; Siman-Tov, Y.; Chanimov, M.; Weissgarten, J. Hyperglycaemia emerging during general anaesthesia induces rat acute kidney injury via impaired microcirculation, augmented apoptosis and inhibited cell proliferation. Nephrology (Carlton) 2012, 17, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.B.; Affonso, F.S.; Cailleaux, S.; Almeida, A.L.; Pinto, L.F.; Tibiriçá, E. Glucose levels observed in daily clinical practice induce endothelial dysfunction in the rabbit macro- and microcirculation. Fundam. Clin. Pharmacol. 2004, 18, 339–346. [Google Scholar] [CrossRef] [PubMed]
- See, K.C. Glycemic targets in critically ill adults: A mini-review. World J. Diabetes 2021, 12, 1719–1730. [Google Scholar] [CrossRef] [PubMed]
- Couper, K.; Laloo, R.; Field, R.; Perkins, G.D.; Thomas, M.; Yeung, J. Prophylactic antibiotic use following cardiac arrest: A systematic review and meta-analysis. Resuscitation 2019, 141, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Bernard, S.A.; Gray, T.W.; Buist, M.D.; Jones, B.M.; Silvester, W.; Gutteridge, G.; Smith, K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N. Engl. J. Med. 2002, 346, 557–563. [Google Scholar] [CrossRef]
- Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N. Engl. J. Med. 2002, 346, 549–556. [Google Scholar] [CrossRef]
- Dankiewicz, J.; Cronberg, T.; Lilja, G.; Jakobsen, J.C.; Levin, H.; Ullén, S.; Rylander, C.; Wise, M.P.; Oddo, M.; Cariou, A.; et al. Hypothermia versus Normothermia after Out-of-Hospital Cardiac Arrest. N. Engl. J. Med. 2021, 384, 2283–2294. [Google Scholar] [CrossRef]
- Le May, M.; Osborne, C.; Russo, J.; So, D.; Chong, A.Y.; Dick, A.; Froeschl, M.; Glover, C.; Hibbert, B.; Marquis, J.F.; et al. Effect of Moderate vs Mild Therapeutic Hypothermia on Mortality and Neurologic Outcomes in Comatose Survivors of Out-of-Hospital Cardiac Arrest: The CAPITAL CHILL Randomized Clinical Trial. JAMA 2021, 326, 1494–1503. [Google Scholar] [CrossRef]
- Schwab, S.; Schwarz, S.; Spranger, M.; Keller, E.; Bertram, M.; Hacke, W. Moderate hypothermia in the treatment of patients with severe middle cerebral artery infarction. Stroke 1998, 29, 2461–2466. [Google Scholar] [CrossRef]
- Schwab, S.; Georgiadis, D.; Berrouschot, J.; Schellinger, P.D.; Graffagnino, C.; Mayer, S.A. Feasibility and safety of moderate hypothermia after massive hemispheric infarction. Stroke 2001, 32, 2033–2035. [Google Scholar] [CrossRef]
- Nutma, S.; Tjepkema-Cloostermans, M.C.; Ruijter, B.J.; Tromp, S.C.; van den Bergh, W.M.; Foudraine, N.A.; Kornips, F.H.M.; Drost, G.; Scholten, E.; Strang, A.; et al. Effects of targeted temperature management at 33 °C vs. 36 °C on comatose patients after cardiac arrest stratified by the severity of encephalopathy. Resuscitation 2022, 173, 147–153. [Google Scholar] [CrossRef]
- Sadaka, F.; Veremakis, C. Therapeutic hypothermia for the management of intracranial hypertension in severe traumatic brain injury: A systematic review. Brain Inj. 2012, 26, 899. [Google Scholar] [CrossRef] [PubMed]
- Dunkley, S.; McLeod, A. Therapeutic hypothermia in patients following traumatic brain injury: A systematic review. Nurs. Crit. Care 2017, 22, 150. [Google Scholar] [CrossRef] [PubMed]
- Seule, M.A.; Muroi, C.; Mink, S.; Yonekawa, Y.; Keller, E. Therapeutic hypothermia in patients with aneurysmal subarachnoid hemorrhage, refractory intracranial hypertension, or cerebral vasospasm. Neurosurgery 2009, 64, 86–93. [Google Scholar] [CrossRef]
- Stravitz, R.T.; Larsen, F.S. Therapeutic hypothermia for acute liver failure. Crit. Care Med. 2009, 37, S258. [Google Scholar] [CrossRef] [PubMed]
- Dmello, D.; Cruz-Flores, S.; Matuschak, G.M. Moderate hypothermia with intracranial pressure monitoring as a therapeutic paradigm for the management of acute liver failure: A systematic review. Intensive Care Med. 2010, 36, 210. [Google Scholar] [CrossRef] [PubMed]
- Lascarrou, J.B.; Merdji, H.; Le Gouge, A.; Colin, G.; Grillet, G.; Girardie, P.; Coupez, E.; Dequin, P.F.; Cariou, A.; Boulain, T.; et al. Targeted Temperature Management for Cardiac Arrest with Nonshockable Rhythm. N. Engl. J. Med. 2019, 381, 2327–2337. [Google Scholar] [CrossRef] [PubMed]
- Hassager, C.; Schmidt, H.; Møller, J.E.; Grand, J.; Mølstrøm, S.; Beske, R.P.; Boesgaard, S.; Borregaard, B.; Bekker-Jensen, D.; Dahl, J.S.; et al. Duration of Device-Based Fever Prevention after Cardiac Arrest. N. Engl. J. Med. 2023, 388, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Hachimi-Idrissi, S.; Zizi, M.; Nguyen, D.N.; Schiettecate, J.; Ebinger, G.; Michotte, Y.; Huyghens, L. The evolution of serum astroglial S-100 beta protein in patients with cardiac arrest treated with mild hypothermia. Resuscitation 2005, 64, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Laurent, I.; Adrie, C.; Vinsonneau, C.; Cariou, A.; Chiche, J.D.; Ohanessian, A.; Spaulding, C.; Carli, P.; Dhainaut, J.F.; Monchi, M. High-volume hemofiltration after out-of-hospital cardiac arrest: A randomized study. J. Am. Coll. Cardiol. 2005, 46, 432–437. [Google Scholar] [CrossRef]
- Castrén, M.; Nordberg, P.; Svensson, L.; Taccone, F.; Vincent, J.L.; Desruelles, D.; Eichwede, F.; Mols, P.; Schwab, T.; Vergnion, M.; et al. Intra-arrest transnasal evaporative cooling: A randomized, prehospital, multicenter study (PRINCE: Pre-ROSC IntraNasal Cooling Effectiveness). Circulation 2010, 122, 729–736. [Google Scholar] [CrossRef]
- Bernard, S.A.; Smith, K.; Cameron, P.; Masci, K.; Taylor, D.M.; Cooper, D.J.; Kelly, A.M.; Silvester, W.; Rapid Infusion of Cold Hartmanns (RICH) Investigators. Induction of therapeutic hypothermia by paramedics after resuscitation from out-of-hospital ventricular fibrillation cardiac arrest: A randomized controlled trial. Circulation 2010, 122, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, N.; Wetterslev, J.; Cronberg, T.; Erlinge, D.; Gasche, Y.; Hassager, C.; Horn, J.; Hovdenes, J.; Kjaergaard, J.; Kuiper, M.; et al. Targeted temperature management at 33 °C versus 36 °C after cardiac arrest. N. Engl. J. Med. 2013, 369, 2197–2206. [Google Scholar] [CrossRef] [PubMed]
- Lilja, G.; Nielsen, N.; Friberg, H.; Horn, J.; Kjaergaard, J.; Nilsson, F.; Pellis, T.; Wetterslev, J.; Wise, M.P.; Bosch, F.; et al. Cognitive function in survivors of out-of-hospital cardiac arrest after target temperature management at 33 °C versus 36 °C. Circulation 2015, 131, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Kim, F.; Nichol, G.; Maynard, C.; Hallstrom, A.; Kudenchuk, P.J.; Rea, T.; Copass, M.K.; Carlbom, D.; Deem, S.; Longstreth, W.T., Jr.; et al. Effect of prehospital induction of mild hypothermia on survival and neurological status among adults with cardiac arrest: A randomized clinical trial. JAMA 2014, 311, 45–52. [Google Scholar] [CrossRef]
- Debaty, G.; Maignan, M.; Savary, D.; Koch, F.X.; Ruckly, S.; Durand, M.; Picard, J.; Escallier, C.; Chouquer, R.; Santre, C.; et al. Impact of intra-arrest therapeutic hypothermia in outcomes of prehospital cardiac arrest: A randomized controlled trial. Intensive Care Med. 2014, 40, 1832–1842. [Google Scholar] [CrossRef]
- Maynard, C.; Longstreth, W.T., Jr.; Nichol, G.; Hallstrom, A.; Kudenchuk, P.J.; Rea, T.; Copass, M.K.; Carlbom, D.; Deem, S.; Olsufka, M.; et al. Effect of prehospital induction of mild hypothermia on 3-month neurological status and 1-year survival among adults with cardiac arrest: Long-term follow-up of a randomized, clinical trial. J. Am. Heart Assoc. 2015, 4, e001693. [Google Scholar] [CrossRef]
- Deye, N.; Cariou, A.; Girardie, P.; Pichon, N.; Megarbane, B.; Midez, P.; Tonnelier, J.M.; Boulain, T.; Outin, H.; Delahaye, A.; et al. Clinical and Economical Impact of Endovascular Cooling in the Management of Cardiac Arrest (ICEREA) Study Group. Endovascular Versus External Targeted Temperature Management for Patients with Out-of-Hospital Cardiac Arrest: A Randomized, Controlled Study. Circulation 2015, 132, 182–193. [Google Scholar] [CrossRef]
- Cronberg, T.; Lilja, G.; Horn, J.; Kjaergaard, J.; Wise, M.P.; Pellis, T.; Hovdenes, J.; Gasche, Y.; Åneman, A.; Stammet, P.; et al. Neurologic Function and Health-Related Quality of Life in Patients Following Targeted Temperature Management at 33 °C vs. 36 °C After Out-of-Hospital Cardiac Arrest: A Randomized Clinical Trial. JAMA Neurol. 2015, 72, 634–641. [Google Scholar] [CrossRef]
- Pang, P.Y.; Wee, G.H.; Hoo, A.E.; Sheriff, I.M.; Lim, S.L.; Tan, T.E.; Loh, Y.J.; Kerk, K.L.; Sin, Y.K.; Lim, C.H. Therapeutic hypothermia in adult patients receiving extracorporeal life support: Early results of a randomized controlled study. J. Cardiothorac. Surg. 2016, 11, 43. [Google Scholar] [CrossRef]
- Bernard, S.A.; Smith, K.; Finn, J.; Hein, C.; Grantham, H.; Bray, J.E.; Deasy, C.; Stephenson, M.; Williams, T.A.; Straney, L.D.; et al. Induction of Therapeutic Hypothermia During Out-of-Hospital Cardiac Arrest Using a Rapid Infusion of Cold Saline: The RINSE Trial (Rapid Infusion of Cold Normal Saline). Circulation 2016, 134, 797–805. [Google Scholar] [CrossRef]
- Scales, D.C.; Cheskes, S.; Verbeek, P.R.; Pinto, R.; Austin, D.; Brooks, S.C.; Dainty, K.N.; Goncharenko, K.; Mamdani, M.; Thorpe, K.E.; et al. Prehospital cooling to improve successful targeted temperature management after cardiac arrest: A randomized controlled trial. Resuscitation 2017, 121, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Look, X.; Li, H.; Ng, M.; Lim, E.T.S.; Pothiawala, S.; Tan, K.B.K.; Sewa, D.W.; Shahidah, N.; Pek, P.P.; Ong, M.E.H. Randomized controlled trial of internal and external targeted temperature management methods in post-cardiac arrest patients. Am. J. Emerg. Med. 2018, 36, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Kirkegaard, H.; Søreide, E.; de Haas, I.; Pettilä, V.; Taccone, F.S.; Arus, U.; Storm, C.; Hassager, C.; Nielsen, J.F.; Sørensen, C.A.; et al. Targeted Temperature Management for 48 vs. 24 Hours and Neurologic Outcome After Out-of-Hospital Cardiac Arrest: A Randomized Clinical Trial. JAMA 2017, 318, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Lopez-de-Sa, E.; Juarez, M.; Armada, E.; Sanchez-Salado, J.C.; Sanchez, P.L.; Loma-Osorio, P.; Sionis, A.; Monedero, M.C.; Martinez-Sellés, M.; Martín-Benitez, J.C.; et al. A multicentre randomized pilot trial on the effectiveness of different levels of cooling in comatose survivors of out-of-hospital cardiac arrest: The FROST-I trial. Intensive Care Med. 2018, 44, 1807–1815. [Google Scholar] [CrossRef]
- Nordberg, P.; Taccone, F.S.; Truhlar, A.; Forsberg, S.; Hollenberg, J.; Jonsson, M.; Cuny, J.; Goldstein, P.; Vermeersch, N.; Higuet, A.; et al. Effect of Trans-Nasal Evaporative Intra-arrest Cooling on Functional Neurologic Outcome in Out-of-Hospital Cardiac Arrest: The PRINCESS Randomized Clinical Trial. JAMA 2019, 321, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
- Glover, G.W.; Thomas, R.M.; Vamvakas, G.; Al-Subaie, N.; Cranshaw, J.; Walden, A.; Wise, M.P.; Ostermann, M.; Thomas-Jones, E.; Cronberg, T.; et al. Intravascular versus surface cooling for targeted temperature management after out-of-hospital cardiac arrest: An analysis of the TTM trial data. Crit. Care 2016, 20, 381. [Google Scholar] [CrossRef]
- Kim, Y.J.; Park, J.M.; Kim, M.S.; Ryoo, S.M.; Park, Y.S.; Kim, S.S.; Kim, Y.O.; Kim, W.Y. Independent risk factors for the shivering occurrence during induction period in out-of-hospital cardiac arrest survivors treated with targeted temperature management. Ther. Hypothermia Temp. Manag. 2019, 9, 70–75. [Google Scholar] [CrossRef]
- Bouwes, A.; Robillard, L.B.; Binnekade, J.M.; de Pont, A.C.; Wieske, L.; Hartog, A.W.; Schultz, M.J.; Horn, J. The influence of rewarming after therapeutic hypothermia on outcome after cardiac arrest. Resuscitation 2012, 83, 996–1000. [Google Scholar] [CrossRef]
- Bro-Jeppesen, J.; Hassager, C.; Wanscher, M.; Søholm, H.; Thomsen, J.H.; Lippert, F.K.; Møller, J.E.; Køber, L.; Kjaergaard, J. Post-hypothermia fever is associated with increased mortality after out-of-hospital cardiac arrest. Resuscitation 2013, 84, 1734–1740. [Google Scholar] [CrossRef] [PubMed]
- Elbadawi, A.; Sedhom, R.; Baig, B.; Mahana, I.; Thakker, R.; Gad, M.; Eid, M.; Nair, A.; Kayani, W.; Denktas, A.; et al. Targeted Hypothermia vs Targeted Normothermia in Survivors of Cardiac Arrest: A Systematic Review and Meta-Analysis of Randomized Trials. Am. J. Med. 2022, 135, 626–633.e4. [Google Scholar] [CrossRef]
- Fernando, S.M.; Di Santo, P.; Sadeghirad, B.; Lascarrou, J.B.; Rochwerg, B.; Mathew, R.; Sekhon, M.S.; Munshi, L.; Fan, E.; Brodie, D.; et al. Targeted temperature management following out-of-hospital cardiac arrest: A systematic review and network meta-analysis of temperature targets. Intensive Care Med. 2021, 47, 1078–1088. [Google Scholar] [CrossRef] [PubMed]
- Granfeldt, A.; Holmberg, M.J.; Nolan, J.P.; Soar, J.; Andersen, L.W.; International Liaison Committee on Resuscitation (ILCOR) Advanced Life Support Task Force. Targeted temperature management in adult cardiac arrest: Systematic review and meta-analysis. Resuscitation 2021, 167, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Aneman, A.; Frost, S.; Parr, M.; Skrifvars, M.B. Target temperature management following cardiac arrest: A systematic review and Bayesian meta-analysis. Crit. Care 2022, 26, 58. [Google Scholar] [CrossRef] [PubMed]
- Koren, O.; Rozner, E.; Yosefia, S.; Turgeman, Y. Therapeutic hypothermia after out of hospital cardiac arrest improve 1-year survival rate for selective patients. PLoS ONE 2020, 15, e0226956. [Google Scholar] [CrossRef]
- Paul, M.; Bougouin, W.; Geri, G.; Dumas, F.; Champigneulle, B.; Legriel, S.; Charpentier, J.; Mira, J.P.; Sandroni, C.; Cariou, A. Delayed awakening after cardiac arrest: Prevalence and risk factors in the Parisian registry. Intensive Care Med. 2016, 42, 1128–1136. [Google Scholar] [CrossRef]
- Rey, A.; Rossetti, A.O.; Miroz, J.P.; Eckert, P.; Oddo, M. Late Awakening in Survivors of Postanoxic Coma: Early Neurophysiologic Predictors and Association with ICU and Long-Term Neurologic Recovery. Crit. Care Med. 2019, 47, 85–92. [Google Scholar] [CrossRef]
- Crombez, T.; Hachimi-Idrissi, S. The influence of targeted temperature management on the pharmacokinetics of drugs administered during and after cardiac arrest: A systematic review. Acta Clin. Belg. 2017, 72, 116–122. [Google Scholar] [CrossRef]
- Lemiale, V.; Dumas, F.; Mongardon, N.; Giovanetti, O.; Charpentier, J.; Chiche, J.D.; Carli, P.; Mira, J.P.; Nolan, J.; Cariou, A. Intensive care unit mortality after cardiac arrest: The relative contribution of shock and brain injury in a large cohort. Intensive Care Med. 2013, 39, 1972–1980. [Google Scholar] [CrossRef]
- Dragancea, I.; Wise, M.P.; Al-Subaie, N.; Cranshaw, J.; Friberg, H.; Glover, G.; Pellis, T.; Rylance, R.; Walden, A.; Nielsen, N.; et al. Protocol-driven neurological prognostication and withdrawal of life-sustaining therapy after cardiac arrest and targeted temperature management. Resuscitation 2017, 117, 50–57. [Google Scholar] [CrossRef]
- Sandroni, C.; Dell’anna, A.M.; Tujjar, O.; Geri, G.; Cariou, A.; Taccone, F.S. Acute kidney injury after cardiac arrest: A systematic review and meta-analysis of clinical studies. Minerva Anestesiol. 2021, 82, 989–999. [Google Scholar]
- Taccone, F.; Cronberg, T.; Friberg, H.; Greer, D.; Horn, J.; Oddo, M.; Scolletta, S.; Vincent, J.L. How to assess prognosis after cardiac arrest and therapeutic hypothermia. Crit. Care 2014, 18, 202. [Google Scholar] [CrossRef] [PubMed]
- Stammet, P.; Collignon, O.; Hassager, C.; Wise, M.P.; Hovdenes, J.; Åneman, A.; Horn, J.; Devaux, Y.; Erlinge, D.; Kjaergaard, J.; et al. Neuron-specific enolase as a predictor of death or poor neurological outcome after out-of-hospital cardiac arrest and targeted temperature management at 33 °C and 36 °C. J. Am. Coll. Cardiol. 2015, 65, 2104–2114. [Google Scholar] [CrossRef] [PubMed]
- Streitberger, K.J.; Leithner, C.; Wattenberg, M.; Tonner, P.H.; Hasslacher, J.; Joannidis, M.; Pellis, T.; Di Luca, E.; Födisch, M.; Krannich, A.; et al. Neuronspecific enolase predicts poor outcome after cardiac arrest and targeted temperature management: A multicenter study on 1,053 patients. Crit. Care Med. 2017, 45, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Lamartine Monteiro, M.; Taccone, F.S.; Depondt, C.; Lamanna, I.; Gaspard, N.; Ligot, N.; Mavroudakis, N.; Naeije, G.; Vincent, J.L.; Legros, B. The prognostic value of 48-h continuous EEG during therapeutic hypothermia after cardiac arrest. Neurocrit. Care 2016, 24, 153–162. [Google Scholar] [CrossRef]
- Eertmans, W.; Genbrugge, C.; Haesen, J.; Drieskens, C.; Demeestere, J.; Vander Laenen, M.; Boer, W.; Mesotten, D.; Dens, J.; Ernon, L.; et al. The Prognostic Value of Simplified EEG in Out-of-Hospital Cardiac Arrest Patients. Neurocrit. Care 2019, 30, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Fernández, J.A.; Pérez-Quintero, R. Use of transcranial Doppler ultrasound in the management of post-cardiac arrest syndrome. Resuscitation 2009, 80, 1321–1322. [Google Scholar] [CrossRef]
- Lin, J.J.; Hsia, S.H.; Wang, H.S.; Chiang, M.C.; Lin, K.L. Transcranial Doppler ultrasound in therapeutic hypothermia for children after resuscitation. Resuscitation 2015, 89, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Aaslid, R.; Lindegaard, K.F.; Sorteberg, W.; Nornes, H. Cerebral autoregulation dynamics in humans. Stroke 1989, 20, 45–52. [Google Scholar] [CrossRef]
- Lassen, N.A. Cerebral blood flow and oxygen consumption in man. Physiol. Rev. 1959, 39, 183–238. [Google Scholar] [CrossRef]
- Tan, C.O. Defining the characteristic relationship between arterial pressure and cerebral flow. J. Appl. Physiol. (1985) 2012, 113, 1194–1200. [Google Scholar] [CrossRef]
- Buunk, G.; van der Hoeven, J.G.; Frölich, M.; Meinders, A.E. Cerebral vasoconstriction in comatose patients resuscitated from a cardiac arrest? Intensive Care Med. 1996, 22, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Bisschops, L.L.; Hoedemaekers, C.W.; Simons, K.S.; van der Hoeven, J.G. Preserved metabolic coupling and cerebrovascular reactivity during mild hypothermia after cardiac arrest. Crit. Care Med. 2010, 38, 1542–1547. [Google Scholar] [CrossRef] [PubMed]
- Sundgreen, C.; Larsen, F.S.; Herzog, T.M.; Knudsen, G.M.; Boesgaard, S.; Aldershvile, J. Autoregulation of cerebral blood flow in patients resuscitated from cardiac arrest. Stroke 2001, 32, 128–132. [Google Scholar] [CrossRef]
- Jha, R.M.; Elmer, J. Transcranial dopplers after cardiac arrest: Should we ride this wave? Resuscitation 2019, 141, 204–206. [Google Scholar] [CrossRef]
- Castro, P.; Azevedo, E.; Sorond, F. Cerebral Autoregulation in Stroke. Curr. Atheroscler. Rep. 2018, 20, 37. [Google Scholar] [CrossRef]
- Wessels, T.; Harrer, J.U.; Jacke, C.; Janssens, U.; Klötzsch, C. The prognostic value of early transcranial Doppler ultrasound following cardiopulmonary resuscitation. Ultrasound Med. Biol. 2006, 32, 1845–1851. [Google Scholar] [CrossRef] [PubMed]
- Lemiale, V.; Huet, O.; Vigué, B.; Mathonnet, A.; Spaulding, C.; Mira, J.P.; Carli, P.; Duranteau, J.; Cariou, A. Changes in cerebral blood flow and oxygen extraction during post-resuscitation syndrome. Resuscitation 2008, 76, 17–24. [Google Scholar] [CrossRef]
- Pollock, J.M.; Whitlow, C.T.; Deibler, A.R.; Tan, H.; Burdette, J.H.; Kraft, R.A.; Maldjian, J.A. Anoxic injury-associated cerebral hyperperfusion identified with arterial spin-labeled MR imaging. AJNR Am. J. Neuroradiol. 2008, 29, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Iida, K.; Satoh, H.; Arita, K.; Nakahara, T.; Kurisu, K.; Ohtani, M. Delayed hyperemia causing intracranial hypertension after cardiopulmonary resuscitation. Crit. Care Med. 1997, 25, 971–976. [Google Scholar] [CrossRef]
- Rafi, S.; Tadie, J.M.; Gacouin, A.; Leurent, G.; Bedossa, M.; Le Tulzo, Y.; Maamar, A. Doppler sonography of cerebral blood flow for early prognostication after out-of-hospital cardiac arrest: DOTAC study. Resuscitation 2019, 141, 188–194. [Google Scholar] [CrossRef]
- Iordanova, B.; Li, L.; Clark, R.S.B.; Manole, M.D. Alterations in Cerebral Blood Flow after Resuscitation from Cardiac Arrest. Front. Pediatr. 2017, 5, 174. [Google Scholar] [CrossRef] [PubMed]
- de Riva, N.; Budohoski, K.P.; Smielewski, P.; Kasprowicz, M.; Zweifel, C.; Steiner, L.A.; Reinhard, M.; Fábregas, N.; Pickard, J.D.; Czosnyka, M. Transcranial Doppler pulsatility index: What it is and what it isn’t. Neurocrit. Care 2012, 17, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Ameloot, K.; Genbrugge, C.; Meex, I.; Jans, F.; Boer, W.; Vander Laenen, M.; Ferdinande, B.; Mullens, W.; Dupont, M.; Dens, J.; et al. An observational near-infrared spectroscopy study on cerebral autoregulation in post-cardiac arrest patients: Time to drop ‘one-size-fits-all’ hemodynamic targets? Resuscitation 2015, 90, 121–126. [Google Scholar] [CrossRef]
- Balu, R.; Baghshomali, S.; Abella, B.S.; Kofke, W.A. Abstract 12: Cerebrovascular Pressure Reactivity Predicts Outcome in Diffuse Hypoxic-Ischemic Brain Injury. Circulation 2018, 138, A12. [Google Scholar] [CrossRef]
- Caldwell, M.; Scholkmann, F.; Wolf, U.; Wolf, M.; Elwell, C.; Tachtsidis, I. Modelling confounding effects from extracerebral contamination and systemic factors on functional near-infrared spectroscopy. Neuroimage 2016, 143, 91–105. [Google Scholar] [CrossRef]
- Gonella, S.; Basso, I.; Dimonte, V.; Martin, B.; Berchialla, P.; Campagna, S.; Di Giulio, P. Association Between End-of-Life Conversations in Nursing Homes and End-of-Life Care Outcomes: A Systematic Review and Meta-analysis. J. Am. Med. Dir. Assoc. 2019, 20, 249–261. [Google Scholar] [CrossRef]
- Davidson, J.E.; Aslakson, R.A.; Long, A.C.; Puntillo, K.A.; Kross, E.K.; Hart, J.; Cox, C.E.; Wunsch, H.; Wickline, M.A.; Nunnally, M.E.; et al. Guidelines for Family-Centered Care in the Neonatal, Pediatric, and Adult ICU. Crit. Care Med. 2017, 45, 103–128. [Google Scholar] [CrossRef]
- Adelman, R.D.; Tmanova, L.L.; Delgado, D.; Dion, S.; Lachs, M.S. Caregiver burden: A clinical review. JAMA 2014, 311, 1052–1060. [Google Scholar] [CrossRef]
- Chen, C.; Michaels, J.; Meeker, M.A. Family Outcomes and Perceptions of End-of-Life Care in the Intensive Care Unit: A Mixed-Methods Review. J. Palliat. Care 2020, 35, 143–153. [Google Scholar] [CrossRef]
- Lee, H.W.; Park, Y.; Jang, E.J.; Lee, Y.J. Intensive care unit length of stay is reduced by protocolized family support intervention: A systematic review and meta-analysis. Intensive Care Med. 2019, 45, 1072–1081. [Google Scholar] [CrossRef]
- Walczak, A.; Butow, P.N.; Bu, S.; Clayton, J.M. A systematic review of evidence for end-of-life communication interventions: Who do they target, how are they structured and do they work? Patient Educ. Couns. 2016, 99, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Oczkowski, S.J.; Chung, H.O.; Hanvey, L.; Mbuagbaw, L.; You, J.J. Communication tools for end-of-life decision-making in the intensive care unit: A systematic review and meta-analysis. Crit. Care 2016, 20, 97. [Google Scholar] [CrossRef] [PubMed]
- DeSanto-Madeya, S.; Safizadeh, P. Family Satisfaction with End-of-Life Care in the Intensive Care Unit: A Systematic Review of the Literature. Dimens. Crit. Care Nurs. 2017, 36, 278–283. [Google Scholar] [CrossRef]
- You, J.J.; Jayaraman, D.; Swinton, M.; Jiang, X.; Heyland, D.K. Supporting shared decision-making about cardiopulmonary resuscitation using a video-based decision-support intervention in a hospital setting: A multisite before-after pilot study. CMAJ Open 2019, 7, E630–E637. [Google Scholar] [CrossRef] [PubMed]
- Sahgal, S.; Yande, A.; Thompson, B.B.; Chen, E.P.; Fagerlin, A.; Morgenstern, L.B.; Zahuranec, D.B. Surrogate Satisfaction with Decision Making After Intracerebral Hemorrhage. Neurocrit. Care 2021, 34, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.E.; Ng, R.W.; Cheng, N.C.; Cigolini, M.; Kwok, C.; Brennan, F. Advance care planning for haemodialysis patients. Cochrane Database Syst. Rev. 2016, 7, CD010737. [Google Scholar] [CrossRef]
- Kavalieratos, D.; Corbelli, J.; Zhang, D.; Dionne-Odom, J.N.; Ernecoff, N.C.; Hanmer, J.; Hoydich, Z.P.; Ikejiani, D.Z.; Klein-Fedyshin, M.; Zimmermann, C.; et al. Association Between Palliative Care and Patient and Caregiver Outcomes: A Systematic Review and Meta-analysis. JAMA 2016, 316, 2104–2114. [Google Scholar] [CrossRef]
- Huber, M.T.; Highland, J.D.; Krishnamoorthi, V.R.; Tang, J.W. Utilizing the Electronic Health Record to Improve Advance Care Planning: A Systematic Review. Am. J. Hosp. Palliat. Care 2018, 35, 532–541. [Google Scholar] [CrossRef]
- Mentzelopoulos, S.D.; Couper, K.; Voorde, P.V.; Druwé, P.; Blom, M.; Perkins, G.D.; Lulic, I.; Djakow, J.; Raffay, V.; Lilja, G.; et al. European Resuscitation Council Guidelines 2021: Ethics of resuscitation and end of life decisions. Resuscitation 2021, 161, 408–432. [Google Scholar] [CrossRef]
- Brinkman-Stoppelenburg, A.; Rietjens, J.A.; van der Heide, A. The effects of advance care planning on end-of-life care: A systematic review. Palliat. Med. 2014, 28, 1000–1025. [Google Scholar] [CrossRef]
- MacKenzie, M.A.; Smith-Howell, E.; Bomba, P.A.; Meghani, S.H. Respecting Choices and Related Models of Advance Care Planning: A Systematic Review of Published Evidence. Am. J. Hosp. Palliat. Care 2018, 35, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, C.; Sim, D.; Jaufeerally, F.R.; Hu, M.; Nadkarni, N.; Ng, C.S.H.; Wong, G.; Tan, B.C.; Lim, J.F.; Chuang, C.Y.; et al. Impact of a Formal Advance Care Planning Program on End-of-Life Care for Patients with Heart Failure: Results From a Randomized Controlled Trial. J. Card. Fail. 2020, 26, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Kernick, L.A.; Hogg, K.J.; Millerick, Y.; Murtagh, F.E.M.; Djahit, A.; Johnson, M. Does advance care planning in addition to usual care reduce hospitalisation for patients with advanced heart failure: A systematic review and narrative synthesis. Palliat. Med. 2018, 32, 1539–1551. [Google Scholar] [CrossRef]
- Scarpi, E.; Dall’Agata, M.; Zagonel, V.; Gamucci, T.; Bertè, R.; Sansoni, E.; Amaducci, E.; Broglia, C.M.; Alquati, S.; Garetto, F.; et al. Early Palliative Care Italian Study Group (EPCISG). Systematic vs. on-demand early palliative care in gastric cancer patients: A randomized clinical trial assessing patient and healthcare service outcomes. Support Care Cancer 2019, 27, 2425–2434. [Google Scholar] [CrossRef] [PubMed]
- Mentzelopoulos, S.D.; Slowther, A.M.; Fritz, Z.; Sandroni, C.; Xanthos, T.; Callaway, C.; Perkins, G.D.; Newgard, C.; Ischaki, E.; Greif, R.; et al. Ethical challenges in resuscitation. Intensive Care Med. 2018, 44, 703–716. [Google Scholar] [CrossRef] [PubMed]
- e-End of Life Care for All (e-ELCA) eLearning Programme. Available online: https://www.skillsforcare.org.uk/Developing-your-workforce/Care-topics/End-of-life-care/e-End-of-Life-Care-for-All-e-ELCA-elearning-programme.aspx (accessed on 26 May 2023).
| Cause | Management |
|---|---|
| Electrolyte derangements | Urgent correction; medical treatment; continuous renal replacement therapy |
| Acidosis | Urgent correction; mechanical ventilation; maintain plasma pH > 7.20 and avoid pH normalization (mild acidosis facilitates tissue oxygenation); avoid normal saline (hyperchloremia); use of the anion gap corrected for albumin; initiation of renal replacement therapy when pH < 7.15 in the absence of severe respiratory acidosis and despite other medical treatment interventions |
| Acute coronary syndrome | Percutaneous coronary intervention; coronary artery bypass graft; optimization of myocardial perfusion; anticoagulation; thrombolysis |
| Heart failure | Advanced hemodynamic monitoring; deresuscitation/fluid removal; early point-of-care (POCUS) and venous excess ultrasound (VexUS); optimization of intravascular volume; preload, afterload, and heart-lung interactions; medical treatment; mechanical circulatory support; cardiac transplantation |
| Arrhythmia | Early rate control; correction of electrolyte disorders, acidosis, and other metabolic processes; diagnosis and treatment of abnormal conduction syndromes; medical treatment; cardioversion; pacing |
| Myocardial trauma | Resuscitative thoracotomy; surgical intervention |
| Pericardial tamponade | Emergency pericardiocentesis; resuscitative thoracotomy; surgical pericardiectomy or pericardial window |
| Tension pneumothorax | Emergency decompression; surgical intervention |
| Pulmonary embolus | Thrombolysis; embolus aspiration; mechanical circulatory support; prevention and treatment of pulmonary hypertension and acute right ventricular failure; surgical intervention |
| Airway obstruction | Removal of obstacle (e.g., mucus plug); endotracheal intubation; endotracheal/tracheostomy tube exchange; cricothyroidotomy |
| Asthma/COPD exacerbation | Medical therapies; non-invasive ventilation; high flow oxygen therapy; mechanical ventilation (aiming at improving gas exchange abnormality and avoiding auto-positive end-expiratory pressure); bronchial thermoplasty (within the context of a clinical trial or registry) |
| Hemorrhage/hypovolemia | Advanced hemodynamic monitoring; fluid resuscitation (patients with maximal vasoconstriction and increased endogenous vasopressin levels may need less fluid); massive transfusion; avoid overload, hemostatic resuscitation, vasopressor use targeted at maintaining perfusion of vital organs *; surgical intervention |
| Poisoning | Antidote administration; medical treatment; extracorporeal blood purification interventions (e.g., continuous veno-venous hemodiafiltration or hemoadsorption); volume expansion; vasopressor therapy; correction of electrolyte and acid-base disturbances; mechanical circulatory support |
| Sepsis | 30 mL kg−1 of crystalloid within 3 h; assess for fluid responsiveness/tolerance; early norepinephrine use; vasopressin when norepinephrine > 0.15 μg−1 kg−1 min−1; use of point-of-care (POCUS) and venous excess ultrasound (VexUS); medical management; source control; early antibiotics; lung-protective ventilation # |
| Author, Year | Intervention | Inclusion Criteria | Exclusion Criteria | No of Patients | Primary Outcome | Adverse Events | Net Effect of TTM |
|---|---|---|---|---|---|---|---|
| Bernard et al., 2002 [155]. | 33 °C vs. 37 °C | Age > 18 y (>50 y for women), OHCA with the initial cardiac rhythm of VF, persistent coma after ROSC | Cardiogenic shock, drug overdose, trauma, cerebrovascular accident, pregnancy | 77 | Discharge with good neurologic outcome: 49% (hypothermia), 26% (normothermia) (p = 0.046); for each 2-year increase in age, 9% decrease in likelihood of good outcome (OR, 0.91; p = 0.014); for each 1.5 min in time from collapse to ROSC, 14% decrease in likelihood of good outcome (OR, 0.86; p = 0.001); multivariate log regression analysis for good outcome: OR, 5.25 in hypothermia group (p = 0.011) | No clinically significant arrythmias in the hypothermia group, no statistically significant differences in platelet and white cell count | Positive |
| Hachimi-Idrissi et al., 2005 [169]. | 33 °C vs. 37 °C | Short study period: asystole or in pulseless electrical activity, >18 y, tympanic temperature > 30 °C, GCS < 7; Long study period: age 18–75 y, witnessed cardiac arrest, VF or non-perfusing VT, estimated interval of 5–15 min from collapse to first attempt at CPR, interval of <60 min from collapse to ROSC | Short study period: history of central nervous system depressant drug prior to cardiac arrest, pregnancy, coagulopathy; Long study period: cardiac arrest resulting from intoxication or trauma, responding to verbal command after ROSC, with tympanic temperature <30 °C at admission, evidence of hypotension (mean arterial pressure < 60 mmHg for more than 30 min on admission), terminal illness, pre-existing coagulopathy, pregnancy, unavailability for follow up | 28 | TTM 33 °C: survival until six months (57%), favorable neurological outcome (43%); Controls: survival until six months controls (43%); favorable neurological outcome (21%) | N/A | Positive |
| Laurent et al., 2005 [170]. | 32–33 °C vs. 37 °C | OHCA apparently related to heart disease, age between 18 and 75 years, initial ventricular fibrillation or asystole, estimated interval of <10 min from cardiac arrest to initiation of CPR, and interval of <50 min from initiation of CPR to ROSC | Pregnancy, response to verbal commands after ROSC, or a terminal illness present before the cardiac arrest | 42 | TTM 33°C: survival until six months (32%), favorable neurological outcome (32%); Controls: survival until six months controls (45%); favorable neurological outcome (45%) | Hypokalemia (45%) Hypophosphatemia (<0.70 mmol L−1) occurred in 20 patients (9 in the HF group and 12 in the HF + HT group) and was corrected by intravenous infusion of disodium phosphate, 8 mmol h−1, during HF. During the first 24 h in ICU, ventricular tachycardia occurred in 6 patients in the HF + HT group, in 2 patients in HF group, and 3 patients in the control group (p = 0.31) | Positive |
| Hypothermia After Cardiac Arrest Study Group, 2002 [156]. | 32–34 °C vs. 37–37.5 °C | Age 18–75 y, witnessed cardiac arrest with shockable initial rhythm (VT, VF), arrest of presumed cardiac cause, estimated interval of 5–15 min from collapse to first resuscitation attempt, <60 min from collapse to ROSC | Spontaneous hypothermia < 30 °C, pregnancy, known coagulopathy, terminal disease, comatose state before cardiac arrest, response to verbal commands after ROSC, hypotension for >30 min after ROSC, hypoxemia for >15 min after ROSC | 247 | Favorable neurologic outcome (CPC 1–2) at 6 mo: 55% (hypothermia), 39% (normothermia) (p = 0.009) | Number of patients with any complication: 73% (hypothermia), 70% (normothermia) (p = 0.7); sepsis: 13% (hypothermia), 7% (normothermia); lethal or long-lasting arrhythmia: 36% (hypothermia), 32% (normothermia) | Positive |
| Castrén et al., 2010 [171]. | 34 °C vs. 35.8 °C | Age > 18 y, OHCA irrespective of initial rhythm, witnessed arrest, CPR initiated within 20 min of collapse | Trauma, drug overdose, cerebrovascular accident, known coagulopathy, asphyxia, or known requirement for supplemental oxygen, electrocution, spontaneous hypothermia, ROSC before randomization, DNR order, transnasal obstruction | 194 | Median interval from collapse to transnasal cooling: 26 min; median interval from collapse to systemic cooling: 113 min; median time to target core temperature (34 °C): 115 min (transnasal cooling), 284 min (control); mean core temperature on hospital arrival: 35.1 °C (transnasal cooling), 35.8 °C (control) (p = 0.01) | Nasal whitening: 14% (resolved in all survivors); epistaxis: 3.2% (serious bleeding in 1 patient with underlying coagulopathy); periorbital emphysema: 1% (resolved within 24 h); total serious adverse events: n = 7 (transnasal cooling), n = 14 (control) (p = 0.23) | Neutral |
| Bernard et al., 2010 [172]. | Prehospital TTM 33 °C vs. in-hospital TTM 33 °C | Age > 15 y, OHCA with initial cardiac rhythm of VF, ROSC, systolic blood pressure > 90 mm Hg, cardiac arrest time > 10 min, established IV access | Patient not intubated, dependent on others for activities of daily living before cardiac arrest, spontaneous hypothermia < 34 °C, pregnancy | 234 | Rate of favorable outcome (discharge home or to rehabilitation facility): 47.5% (early hypothermia), 52.6% (hospital hypothermia) (p = 0.43) | No adverse events related to early hypothermia reported | Neutral |
| Nielsen et al., 2013 [173]. | 33 °C vs. 36 °C | Age > 18 y, OHCA of presumed cardiac cause irrespective of initial rhythm, >20 min of ROSC | >240 min from ROSC to screening, unwitnessed arrest with asystole as initial rhythm, intracranial hemorrhage or stroke, spontaneous hypothermia < 30 °C | 939 | All-cause mortality: 50% (33 °C), 48% (36 °C) (p = 0.51) | Number of patients with 1 or more complications: 93% (33 °C), 90% (36 °C) (p = 0.09); hypokalemia: 19% (33 °C), 13% (36 °C) (p = 0.02) | Neutral |
| Lilja et al., 2014 [174]. | 33 °C vs. 36 °C | Age > 18 y, OHCA of presumed cardiac cause irrespective of initial rhythm, >20 min of spontaneous circulation after resuscitation | >240 min from ROSC to screening, unwitnessed arrest with asystole as initial rhythm, intracranial hemorrhage or stroke, spontaneous hypothermia < 30 °C | 652 | Cognitive function assessed by memory, executive function, and attention/mental speed test did not differ between 33 °C and 36 °C group; attention/mental speed was more affected in all patients with cardiac arrest compared with STEMI controls | N/A | Neutral |
| Kim et al., 2014 [175]. | Prehospital TTM <34 °C vs. in-hospital <34 °C | Age > 18 y, ROSC after OHCA irrespective of initial rhythm, endotracheal intubation, established i.v. access, successful placement of esophageal temperature probe, unconsciousness | Trauma, spontaneous hypothermia < 34 °C | 1359 | Survival to hospital discharge: 62.7% (prehospital hypothermia), 64.3% (control) (p = 0.69) (VF); 19.2% (prehospital hypothermia), 16.3% (control) (p = 0.30) (non-VF); full recovery or mild neurological impairment at discharge: 57.5% (prehospital hypothermia), 61.9% (control) (p = 0.69) (VF); 14.4% (prehospital hypothermia), 13.4% (control) (p = 0.30) (non-VF) | Rearrest in the field: 26% (prehospital hypothermia), 21% (control) (p = 0.008); use of diuretics within 12 h of hospital admission: 18% (prehospital hypothermia), 13% (control) (p = 0.009); PaO2 on first arterial blood gas: 189 mm Hg (prehospital hypothermia), 218 mm Hg (control) (p < 0.001); evidence of pulmonary edema on first chest x-ray: 41% (prehospital hypothermia), 30% (control) (p < 0.001) | Neutral |
| Debaty et al., 2014 [176]. | Intra-arrest TTM 32–34 °C vs. in-hospital TTM 32–34 °C | Age > 18 y, patients with OHCA eligible for advanced life support irrespective of initial rhythm | Trauma, hemorrhage, asphyxia, spontaneous hypothermia < 34 °C, ROSC before randomization, DNR order, pregnancy | 245 | Median NSE at 24 h: 96.7 μg L−1 (intra-arrest hypothermia), 97.6 μg L−1 (hospital hypothermia) (p = 0.64) | Pulmonary edema: n = 7 (intra-arrest), n = 8 (hospital) (p = 0.59); pneumonia: n = 7 (intra-arrest), n = 3 (hospital) (p = 0.24); hyperthermia: n = 9 (intra-arrest), n = 5 (hospital) (p = 0.36); bacteremia: n = 1 (intra-arrest), n = 0 (hospital) (p = 1); hemorrhage: n = 3 (intra-arrest), n = 3 (hospital) (p = 0.88); arrhythmia: n = 5 (intra-arrest), n = 7 (hospital) (p = 0.39); convulsion: n = 8 (intra-arrest), n = 2 (hospital) (p = 0.06) | Neutral |
| Maynard et al., 2015 [177]. | Kim et al. 2014 substudy (Prehospital TTM <34 °C vs. in-hospital <34 °C) | Age > 18 y, ROSC after OHCA irrespective of initial rhythm, endotracheal intubation, established i.v. access, successful placement of esophageal temperature probe, unconsciousness | Trauma, spontaneous hypothermia <34 °C | 508 | No difference between CPC or mRS scores 3 mo after randomization (p = 0.70 and p = 0.49, respectively) | N/A | Neutral |
| Deye et al., 2015 [178]. | Advanced endovascular cooling system (34 °C) vs. basic external group (34 °C) | Age 18–79 y, OHCA of presumed cardiac cause, estimated interval of <60 min from collapse to ROSC, <4 h from ROSC to cooling initiation, unconscious patient, availability of endovascular cooling device | Terminal disease, DNR order, pregnancy, uncontrolled bleeding, known coagulopathy, spontaneous hypothermia < 30 °C, OHCA of extracardiac cause, in-hospital cardiac arrest, contraindication to endovascular device, immediate need for ECLS or renal replacement therapy | 400 | Survival without major neurological damage (CPC 1–2) at day 28: 36.0% (endovascular), 28.4% (external) (p = 0.107) | Minor bleeding, hematoma, or Arteriovenous fistula: 43.9% (endovascular), 29.4% (external); microbiological colonization of central venous catheters: 38.5% (endovascular), 26.4% (external); patients experiencing at least 1 cooling-related side effect: 24.6% (endovascular), 14.2% (external) (p = 0.0086); 3 patients experienced deep accidental hypothermia (all in external group) | Neutral |
| Cronberg et al., 2015 [179]. | 33 °C vs. 36 °C | Age > 18 y, OHCA of presumed cardiac cause irrespective of initial rhythm, >20 min of ROSC | >240 min from ROSC to screening, unwitnessed arrest with asystole as initial rhythm, intracranial hemorrhage or stroke, spontaneous hypothermia < 30 °C | 939 | Median Mini-Mental State Examination score for all patients, including non-survivors: 14 (33 °C), 17 (36 °C) (p = 0.77); median IQCODE score for all patients Including non-survivors: 115 (33 °C), 115 (36 °C) (p = 0.57) | N/A | Neutral |
| Pang et al., 2016 [180]. | ECLS TTM 34 °C vs. ECLS TTM 37 °C | Age > 21 y, cardiac arrest irrespective of initial rhythm, interval < 45 min from onset of arrest to ACLS initiation, comatose state, and unresponsiveness after ROSC, intubated with mechanical ventilation, total ACLS time < 60 min | CPR duration > 45 min, severe coagulopathy, drug overdose, head trauma, stroke, pregnancy, terminal illness, spontaneous hypothermia < 30 °C | 21 | Survival to hospital discharge: 33.3% (hypothermia), 18.2% (normothermia) (p = 0.44); survival with good neurologic function (CPC 1–2): 22.2% (hypothermia), 8.3% (normothermia) (p = 0.37) | N/A | Neutral |
| Bernard et al., 2016 [181]. | 33 °C vs. 36 °C | Age > 18 y, OHCA, established i.v. access, cardiac arrest sustained after initial resuscitation treatment | Trauma, suspected intracranial bleeding, pregnancy, spontaneous hypothermia < 34.5 °C, DNR order | 1198 | Number of patients transported with ROSC: 33.5% (hypothermia), 39.1% (standard) (p = 0.04); overall survival to discharge: 10.2% (hypothermia), 11.4% (standard) (p = 0.51) | Acute pulmonary edema: 10.0% (hypothermia), 4.5% (standard) (p < 0.001) | Neutral—may cause harm |
| Scales et al., 2017 [182]. | Prehospital cooling at 32–34 °C vs. no prehospital cooling | Age > 18 y, OHCA with sustained ROSC > 5 min, unresponsive to verbal stimuli or requiring intubation | Trauma, burn, spontaneous hypothermia, severe bleeding, severe sepsis, known coagulopathy, DNR order, pregnancy, prisoner status | 585 | Rate of TT 32 °C–34 °C reached: 30% (prehospital cooling), 25% (standard care) (p = 0.22); first temperature measured at hospital admission: 35.1 °C (prehospital cooling), 35.2 °C (standard care) (p = 0.53) | Pulmonary edema: 12% (prehospital cooling), 18% (standard care) (p = 0.04); use of vasopressors during first 24 h: 54% (prehospital cooling), 62% (standard care) (p = 0.04) | Neutral—may increase the application of TTM in hospital |
| Look et al., 2017 [183]. | Internal vs. external cooling | Age 18–80 y, OHCA or IHCA irrespective of initial rhythm, ROSC for >30 min, unresponsiveness after ROSC | Trauma, intracranial hemorrhage, women <50 y, pregnancy, terminal illness, hemodynamic instability | 45 | Survival to hospital discharge: OR, 3.36 (1.13–10.41) (internal cooling vs. control); OR, 1.96 (0.59–6.86) (internal vs. external); OR, 2.44 (0.95–6.30) (intervention vs. control) | Any cardiac arrhythmias: OR, 0.18 (0.04–0.63) (internal cooling vs. control); OR, 0.26 (0.10–0.70) (intervention vs. control) | Internal cooling method of providing TTM resulted in better survival outcomes compared to controls No significant difference in outcomes for external cooling compared to internal cooling |
| Kirkegaard et al., 2017 [184]. | 33 °C for 48 h vs. 33 °C for 24 h | Age 17–80 y, OHCA of presumed cardiac cause irrespective of initial rhythm, sustained ROSC for >20 min, GCS < 8 | Unwitnessed cardiac arrest, asystole as initial rhythm | 355 | Favorable neurologic outcome (CPC 1–2) at 6 mo: 69% (48 h), 64% (24 h) (p = 0.33) | Number of patients with 1 or more complications: 97% (48 h), 91% (24 h) (p = 0.03); hypotension: 62% (48 h), 49% (24 h) (p = 0.013); pneumonia: 49% (48 h), 43% (24 h) (p = 0.24); severe bleeding: 4% (48 h), 1% (24 h) (p = 0.03) | Neutral |
| Lopez-de-Sa et al., 2018 [185]. | 32 °C vs. 33 °C vs. 34 °C | Age 18–80 y, witnessed OHCA of presumed cardiac cause, shockable initial rhythm, interval < 20 min from collapse to CPR initiation, interval < 60 min from collapse to ROSC | Trauma, toxicological cause, pregnancy, DNR order, interval > 240 min from ROSC to randomization, Spontaneous hypothermia < 34 °C, intracranial bleeding, stroke, neurological disability before event, terminal illness | 150 | Favorable neurologic outcome (mRS ≤ 3) at 90 d: 63.3% (32 °C), 68.2% (33 °C), 65.1% (34 °C) (ns) | Number of patients with 1 or more complications: 84.6% (32 °C), 79.6% (33 °C), 87.8% (34 °C) (ns); respiratory tract infections: 21.2% (32 °C), 49.0% (33 °C), 36.7% (34 °C) (p = 0.012) | Neutral |
| Nordberg et al., 2019 [186]. | Prehospital trans-nasal evaporative intra-arrest cooling vs. prehospital standard care Patients admitted to the hospital in both groups received therapeutic hypothermia at 32–34 °C for 24 h | Age 18–80 y, witnessed cardiac arrest irrespective of initial rhythm | Trauma, severe bleeding, drug overdose, cerebrovascular accident, drowning, smoke inhalation, electrocution, hanging, spontaneous hypothermia, anatomical contraindications for nasal catheter, DNR order, terminal illness, pregnancy, known coagulopathy, ROSC before randomization, interval > 15 min from collapse to EMS arrival, need for supplemental oxygen | 677 | 90-day survival with good Neurologic outcome (CPC 1–2): 16.6% (transnasal cooling), 13.5% (control) (p = 0.25); 90-d survival with good neurologic outcome (CPC 1–2) in patients with initial shockable rhythm: 34.8% (transnasal cooling), 25.9% (control) (p = 0.11); overall 90-day survival rate: 17.8% (transnasal cooling), 15.6% (control) (p = 0.44) | Severe cooling-related nose bleeding in 4 patients, pneumocephalus in 1 patient (resolved, patient survived with good neurologic outcome), adverse event rate within 7 d after randomization: 50.4% (transnasal cooling), 48.8% (control) | Neutral |
| Lascarrou et al., 2019 [167]. | 32 °C vs. 33 °C Cardiac arrest with non-shockable rhythm | Age >18 y, OHCA or IHCA with non-shockable initial rhythm (asystole or pulseless electrical activity), GCS ≤ 8 | Interval > 10 min from collapse to CPR initiation, interval > 60 min from CPR to ROSC, hemodynamic instability, interval of >300 min from cardiac arrest to screening, terminal illness, severe hepatic dysfunction, pregnancy/breast-feeding, lack of insurance | 581 | Favorable neurologic Outcome (CPC 1–2) at 90 d: 10.2% (hypothermia), 5.7% (normothermia) (p = 0.04) | Severe cardiac arrhythmia between 0 and 7 d: 12.3% (hypothermia), 10.4% (normothermia) (p = 0.48); seizures between 0 and 7 d: 23.6% (hypothermia), 24.2% (normothermia) (p = 0.73); acute pulmonary edema: 6.7% (hypothermia), 8.7% (normothermia) (p = 0.33) | Positive |
| Le May et al., 2021 [158]. | 31 °C vs. 34 °C | Age ≥ 18 y, OHCA, all cardiac arrest rhythms | Inability to perform activities of daily living, cardiac arrest secondary to intracranial bleeding, severe coagulopathy with clinical evidence of major bleeding, coma not attributable to the cardiac arrest, life expectancy of <1 y due to reasons unrelated to the cardiac arrest | 366 | All-cause mortality or poor neurologic outcome at 180 d: 89 (48.4%) patients in 31 °C group vs. 83 (45.4%) patients in 34 °C group (risk difference, 3.0% [95% CI, −7.2% to 13.2%]; RR, 1.07 [95% CI, 0.86–1.33]; p = 0.56) | Deep vein thrombosis: 21 (11.4%) patients in 31 °C group vs. 20 (10.9%) patients in 34 °C group (risk difference, 0.5% [95% CI, −6.0% to 6.9%]; RR, 1.04 [95% CI, 0.59–1.86]; p = 0.88); Thrombus in the inferior vena cava: 7 (3.8%) patients in 31 °C group vs. 14 (7.7%) patients in 34 °C group (risk difference, −3.9% [95% CI, −8.6% to 0.9%]; RR, 0.50 [95% CI, 0.21–1.20]; p = 0.11). | Neutral |
| Dankiewicz et al., 2021 [157]. | 33 °C vs. 36.5–37.7 °C | Age > 18 y, OHCA of presumed cardiac or unknown cause irrespective of initial rhythm, unconsciousness without response to verbal commands or pain, >20 min of spontaneous circulation after resuscitation | Interval of >180 min from ROSC to screening, unwitnessed cardiac arrest, asystole as initial rhythm, limitations in care, spontaneous hypothermia < 30 °C, ECMO initiation before ROSC, pregnancy, intracranial hemorrhage, severe COPD | 1861 | 6-mo mortality rate: 50% (hypothermia), 48% (normothermia) (p = 0.37) | Arrhythmias resulting in Hemodynamic compromise: 24% (hypothermia), 17% (normothermia) (p < 0.001); bleeding: 5% (hypothermia), 5% (normothermia) (p = 0.81); skin complication: 1% (hypothermia), <1% (normothermia) (p = 0.21); pneumonia: 36% (hypothermia), 35% (normothermia) (p = 0.75); sepsis: 11% (hypothermia), 9% (normothermia) (p = 0.23) | Neutral |
| Hassager et al., 2023 [168]. | Device-based temperature control targeting 36 °C for 24 h followed by targeting of 37 °C for either 12 or 48 h (for total intervention times of 36 and 72 h, respectively) or until the patient regained consciousness | Age ≥ 18 years, OHCA of presumed cardiac cause, Sustained ROSC, unconsciousness (GCS < 8) (patients not able to obey verbal commands) after sustained ROSC | Conscious patients (obeying verbal commands), Females of childbearing potential (unless a negative HCG test can rule out pregnancy within the inclusion window), IHCA, OHCA of presumed non-cardiac cause, e.g., after trauma or dissection/rupture of major artery or cardiac arrest caused by initial hypoxia (i.e., drowning, suffocation, hanging), known bleeding diathesis (medically induced coagulopathy (e.g., warfarin, NOAC, clopidogrel) does not exclude the patient), Suspected or confirmed acute intracranial bleeding, Suspected or confirmed acute stroke, Unwitnessed asystole, known limitations in therapy and Do Not Resuscitate-order, known disease making 180 days survival unlikely, Known pre-arrest CPC 3 or 4, >4 h (240 min) from ROSC to screening, Systolic blood pressure < 80 mm Hg in spite of fluid loading/vasopressor and/or inotropic medication/intra-aortic balloon pump/axial flow device, Temperature on admission < 30 °C | 789 | Death from any cause or CPC of 3 or 4 at discharge within 90 days (TTM 36 h 32.3%–TTM 72 h 33.6%, p = 0.70) | Infection in ICU (TTM 36 h 26.0%–TTM 72 h 27.8%, p = 0.56); Arrhythmia in ICU (TTM 36 h 15.8%–TTM 72 h 11.9%, p = 0.11); Any bleeding (TTM 36 h 21.4%–TTM 72 h 22.7%, p = 0.65); Uncontrolled bleeding (TTM 36 h 4.3%–TTM 72 h 5.3%, p = 0.52); Acute kidney injury with renal-replacement therapy (TTM 36 h 9.9%–TTM 72 h 10.6%, p = 75; Electrolyte disorder (TTM 36 h 7.6%–TTM 72 h 6.8, p = 0.66); Metabolic disorder (TTM 36 h 8.7%–TTM 72 h 7.1%, p = 0.41); Seizure (TTM 36 h 21.4%–TTM 72 h 20.2%, p = 0.69) | Neutral |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chalkias, A.; Adamos, G.; Mentzelopoulos, S.D. General Critical Care, Temperature Control, and End-of-Life Decision Making in Patients Resuscitated from Cardiac Arrest. J. Clin. Med. 2023, 12, 4118. https://doi.org/10.3390/jcm12124118
Chalkias A, Adamos G, Mentzelopoulos SD. General Critical Care, Temperature Control, and End-of-Life Decision Making in Patients Resuscitated from Cardiac Arrest. Journal of Clinical Medicine. 2023; 12(12):4118. https://doi.org/10.3390/jcm12124118
Chicago/Turabian StyleChalkias, Athanasios, Georgios Adamos, and Spyros D. Mentzelopoulos. 2023. "General Critical Care, Temperature Control, and End-of-Life Decision Making in Patients Resuscitated from Cardiac Arrest" Journal of Clinical Medicine 12, no. 12: 4118. https://doi.org/10.3390/jcm12124118
APA StyleChalkias, A., Adamos, G., & Mentzelopoulos, S. D. (2023). General Critical Care, Temperature Control, and End-of-Life Decision Making in Patients Resuscitated from Cardiac Arrest. Journal of Clinical Medicine, 12(12), 4118. https://doi.org/10.3390/jcm12124118










