Trabecular Bone Score Significantly Influences Treatment Decisions in Secondary Osteoporosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Involvement
2.2. Eligibility Criteria
2.3. Data Collection
2.4. Bone Densitometry
2.5. Statistical Analysis
3. Results
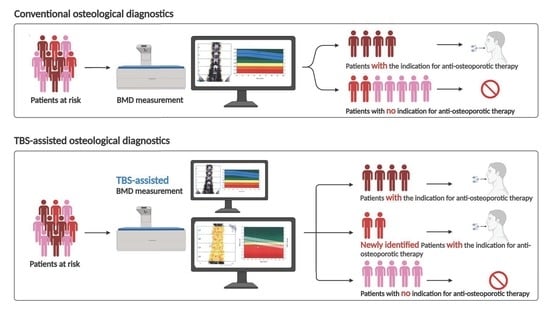
3.1. High Patient Acceptance for TBS Measurement and the Strong Influence of TBS on Therapy Decision
3.2. Demographic and Laboratory Data
- Patients with ongoing glucocorticoid therapy (due to inflammatory rheumatic disease, endocrinological disease, or other indications such as COPD),
- Patients with only inflammatory rheumatic diseases (e.g., rheumatoid arthritis),
- Patients with only endocrine disease (e.g., primary hyperparathyroidisms), and
- Patients with the indication for bone examination: higher age or an osteoporotic fracture that had occurred.
3.3. Comparison of DXA and TBS Measurements in Different Patient Groups
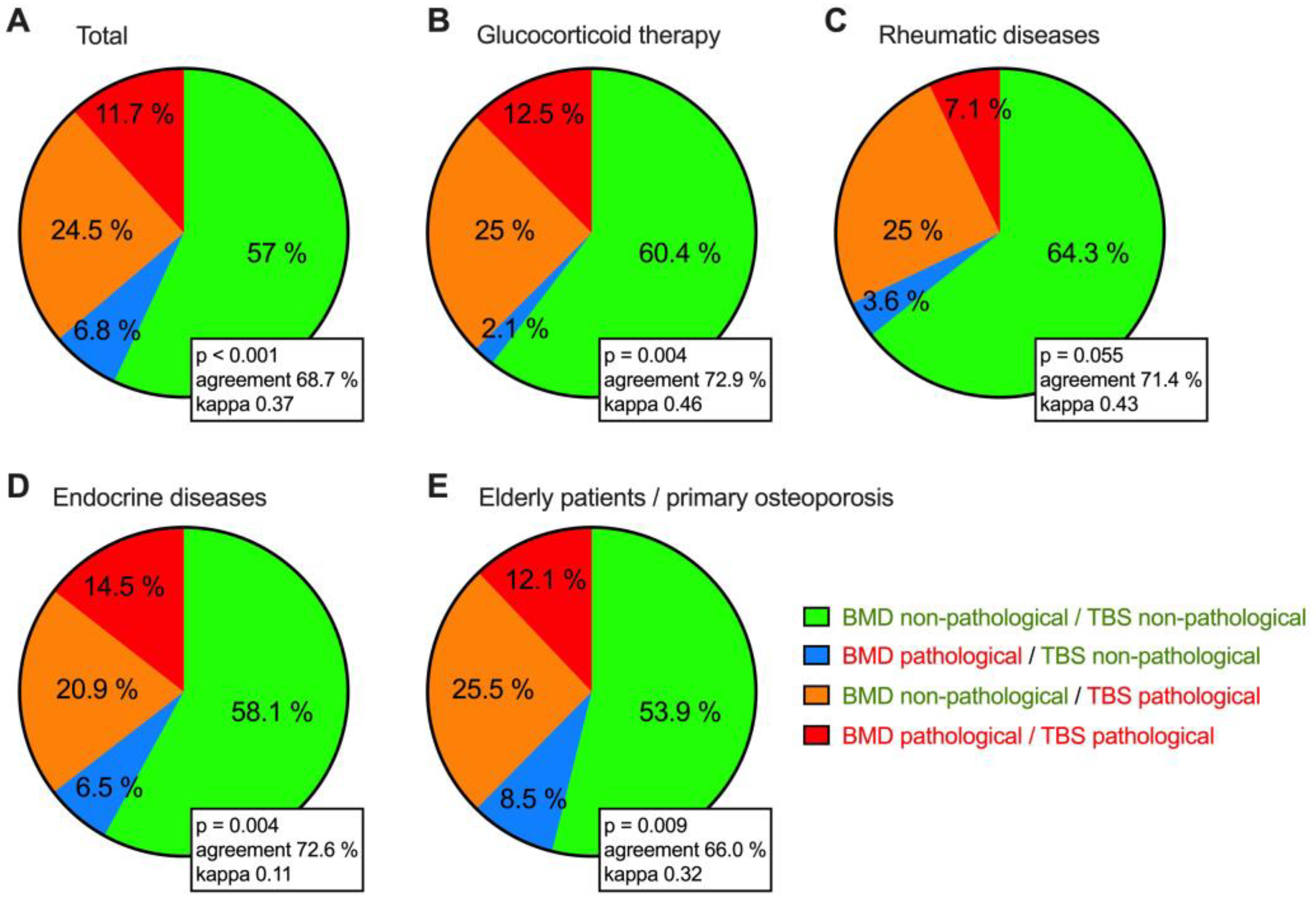
3.4. Do DXA and TBS Match in the Classification of Bone Status?
3.5. Association Analysis of DXA T-Scores, TBS, Vitamin D, and the Bone Remodeling Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Konnopka, A.; Jerusel, N.; König, H.H. The health and economic consequences of osteopenia- and osteoporosis-attributable hip fractures in Germany: Estimation for 2002 and projection until 2050. Osteoporos. Int. 2009, 20, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.J.; Chan, D.D.; Lee, J.K.; Tabu, I.; Alpuerto, B.B. The global burden of fragility fractures—What are the differences, and where are the gaps. Best Pract. Res. Clin. Rheumatol. 2022, 36, 101777. [Google Scholar] [CrossRef] [PubMed]
- Caffarelli, C.; Mondanelli, N.; Crainz, E.; Giannotti, S.; Frediani, B.; Gonnelli, S. The Phenotype of Bone Turnover in Patients with Fragility Hip Fracture: Experience in a Fracture Liaison Service Population. Int. J. Environ. Res. Public Health 2022, 19, 7362. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.; Narla, R.; Baker, J.F.; Wysham, K.D. Risk factors for osteoporosis and fractures in rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 2022, 36, 101773. [Google Scholar] [CrossRef] [PubMed]
- Tanski, W.; Kosiorowska, J.; Szymanska-Chabowska, A. Osteoporosis—Risk factors, pharmaceutical and non-pharmaceutical treatment. Eur. Rev. Med. Pharm. Sci. 2021, 25, 3557–3566. [Google Scholar] [CrossRef]
- Shevroja, E.; Cafarelli, F.P.; Guglielmi, G.; Hans, D. DXA parameters, Trabecular Bone Score (TBS) and Bone Mineral Density (BMD), in fracture risk prediction in endocrine-mediated secondary osteoporosis. Endocrine 2021, 74, 20–28. [Google Scholar] [CrossRef]
- Leitlinie Prophylaxe, Diagnostik und Therapie der Osteoporose bei Postmenopausalen Frauen und bei Männern, DVO Dachverband Osteologie. 2017. Available online: https://dv-osteologie.org/uploads/Leitlinie%202017/Finale%20Version%20Leitlinie%20Osteoporose%202017_end.pdf (accessed on 1 January 2021).
- Chin, K.Y.; Ng, B.N.; Rostam, M.K.I.; Muhammad Fadzil, N.F.D.; Raman, V.; Mohamed Yunus, F.; Syed Hashim, S.A.; Ekeuku, S.O. A Mini Review on Osteoporosis: From Biology to Pharmacological Management of Bone Loss. J. Clin. Med. 2022, 11, 6434. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ. Tech. Rep. Ser. 1994, 843, 1–129. [Google Scholar]
- Arboiro-Pinel, R.; Mahillo-Fernandez, I.; Diaz-Curiel, M. Bone Analysis Using Trabecular Bone Score and Dual-Energy X-Ray Absorptiometry-Based 3-Dimensional Modeling in Postmenopausal Women with Primary Hyperparathyroidism. Endocr. Pract. 2022, 28, 83–89. [Google Scholar] [CrossRef]
- Dudle, A.; Gugler, Y.; Pretterklieber, M.; Ferrari, S.; Lippuner, K.; Zysset, P. 2D-3D reconstruction of the proximal femur from DXA scans: Evaluation of the 3D-Shaper software. Front. Bioeng. Biotechnol. 2023, 11, 1111020. [Google Scholar] [CrossRef]
- Shuhart, C.R.; Yeap, S.S.; Anderson, P.A.; Jankowski, L.G.; Lewiecki, E.M.; Morse, L.R.; Rosen, H.N.; Weber, D.R.; Zemel, B.S.; Shepherd, J.A. Executive Summary of the 2019 ISCD Position Development Conference on Monitoring Treatment, DXA Cross-calibration and Least Significant Change, Spinal Cord Injury, Peri-prosthetic and Orthopedic Bone Health, Transgender Medicine, and Pediatrics. J. Clin. Densitom. 2019, 22, 453–471. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.C.; Broy, S.B.; Boutroy, S.; Schousboe, J.T.; Shepherd, J.A.; Leslie, W.D. Fracture Risk Prediction by Non-BMD DXA Measures: The 2015 ISCD Official Positions Part 2: Trabecular Bone Score. J. Clin. Densitom. 2015, 18, 309–330. [Google Scholar] [CrossRef] [PubMed]
- Mirza, F.; Canalis, E. Management of endocrine disease: Secondary osteoporosis: Pathophysiology and management. Eur. J. Endocrinol. 2015, 173, R131–R151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Favero, V.; Eller-Vainicher, C.; Chiodini, I. Secondary Osteoporosis: A Still Neglected Condition. Int. J. Mol. Sci. 2023, 24, 8558. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Kekatpure, A.L. Postmenopausal Osteoporosis: A Literature Review. Cureus 2022, 14, e29367. [Google Scholar] [CrossRef]
- Lee, J.W.; Kwon, B.C.; Choi, H.G. Analyses of the relationship between hyperuricemia and osteoporosis. Sci. Rep. 2021, 11, 12080. [Google Scholar] [CrossRef]
- Wiebe, E.; Huscher, D.; Schaumburg, D.; Palmowski, A.; Hermann, S.; Buttgereit, T.; Biesen, R.; Burmester, G.R.; Palmowski, Y.; Boers, M.; et al. Optimising both disease control and glucocorticoid dosing is essential for bone protection in patients with rheumatic disease. Ann. Rheum. Dis. 2022, 81, 1313–1322. [Google Scholar] [CrossRef]
- Oelzner, P.; Eidner, T.; Pfeil, A. Glucocorticoid-induced osteoporosis-Focus treatment (part 1). Z Rheumatol. 2022, 81, 57–66. [Google Scholar] [CrossRef]
- Oelzner, P.; Eidner, T.; Pfeil, A. Glucocorticoid-induced osteoporosis-Focus treatment (part 2). Z Rheumatol. 2022, 81, 125–133. [Google Scholar] [CrossRef]
- Management of Osteoporosis in Postmenopausal Women: “The Position Statement of the North American Menopause Society” Editorial Panel. Management of osteoporosis in postmenopausal women: The 2021 position statement of the North American Menopause Society. Menopause 2021, 28, 973–997. [Google Scholar] [CrossRef]
- Maurel, D.B.; Boisseau, N.; Benhamou, C.L.; Jaffre, C. Alcohol and bone: Review of dose effects and mechanisms. Osteoporos. Int. 2012, 23, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, C.; Ix, J.H. Diagnosis and Management of Osteoporosis in Advanced Kidney Disease: A Review. Am. J. Kidney Dis. 2022, 79, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Hillier, T.A.; Cauley, J.A.; Rizzo, J.H.; Pedula, K.L.; Ensrud, K.E.; Bauer, D.C.; Lui, L.Y.; Vesco, K.K.; Black, D.M.; Donaldson, M.G.; et al. WHO absolute fracture risk models (FRAX): Do clinical risk factors improve fracture prediction in older women without osteoporosis? J. Bone Min. Res. 2011, 26, 1774–1782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hans, D.; Goertzen, A.L.; Krieg, M.A.; Leslie, W.D. Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: The Manitoba study. J. Bone Min. Res. 2011, 26, 2762–2769. [Google Scholar] [CrossRef]
- Cianferotti, L.; Cipriani, C.; Corbetta, S.; Corona, G.; Defeudis, G.; Lania, A.G.; Messina, C.; Napoli, N.; Mazziotti, G. Bone quality in endocrine diseases: Determinants and clinical relevance. J. Endocrinol. Investig. 2023, 46, 1283–1304. [Google Scholar] [CrossRef]
- Di Paola, M.; Gatti, D.; Viapiana, O.; Cianferotti, L.; Cavalli, L.; Caffarelli, C.; Conversano, F.; Quarta, E.; Pisani, P.; Girasole, G.; et al. Radiofrequency echographic multispectrometry compared with dual X-ray absorptiometry for osteoporosis diagnosis on lumbar spine and femoral neck. Osteoporos. Int. 2019, 30, 391–402. [Google Scholar] [CrossRef]
- Martineau, P.; Leslie, W.D. Trabecular bone score (TBS): Method and applications. Bone 2017, 104, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Martineau, P.; Leslie, W.D. The utility and limitations of using trabecular bone score with FRAX. Curr. Opin. Rheumatol. 2018, 30, 412–419. [Google Scholar] [CrossRef]
- McCloskey, E.V.; Oden, A.; Harvey, N.C.; Leslie, W.D.; Hans, D.; Johansson, H.; Barkmann, R.; Boutroy, S.; Brown, J.; Chapurlat, R.; et al. A Meta-Analysis of Trabecular Bone Score in Fracture Risk Prediction and Its Relationship to FRAX. J. Bone Min. Res. 2016, 31, 940–948. [Google Scholar] [CrossRef] [Green Version]
- Carey, J.J.; Buehring, B. Current imaging techniques in osteoporosis. Clin. Exp. Rheumatol. 2018, 36 (Suppl. S114), 115–126. [Google Scholar]
- Rajan, R.; Cherian, K.E.; Kapoor, N.; Paul, T.V. Trabecular Bone Score-An Emerging Tool in the Management of Osteoporosis. Indian J. Endocrinol. Metab 2020, 24, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Hans, D.; Stenova, E.; Lamy, O. The Trabecular Bone Score (TBS) Complements DXA and the FRAX as a Fracture Risk Assessment Tool in Routine Clinical Practice. Curr. Osteoporos. Rep. 2017, 15, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Harvey, N.C.; Gluer, C.C.; Binkley, N.; McCloskey, E.V.; Brandi, M.L.; Cooper, C.; Kendler, D.; Lamy, O.; Laslop, A.; Camargos, B.M.; et al. Trabecular bone score (TBS) as a new complementary approach for osteoporosis evaluation in clinical practice. Bone 2015, 78, 216–224. [Google Scholar] [CrossRef] [Green Version]
- Leib, E.S.; Winzenrieth, R. Bone status in glucocorticoid-treated men and women. Osteoporos. Int. 2016, 27, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Schacter, G.I.; Leslie, W.D. DXA-Based Measurements in Diabetes: Can They Predict Fracture Risk? Calcif. Tissue Int. 2017, 100, 150–164. [Google Scholar] [CrossRef]
- Ulivieri, F.M.; Silva, B.C.; Sardanelli, F.; Hans, D.; Bilezikian, J.P.; Caudarella, R. Utility of the trabecular bone score (TBS) in secondary osteoporosis. Endocrine 2014, 47, 435–448. [Google Scholar] [CrossRef]
- Therdyothin, A.; Amphansap, T.; Apiromyanont, K. Trabecular bone score as an additional therapeutic decision tool in osteoporosis and osteopenia. Osteoporos. Sarcopenia 2022, 8, 123–130. [Google Scholar] [CrossRef]
- Langsetmo, L.; Vo, T.N.; Ensrud, K.E.; Taylor, B.C.; Cawthon, P.M.; Schwartz, A.V.; Bauer, D.C.; Orwoll, E.S.; Lane, N.E.; Barrett-Connor, E.; et al. The Association Between Trabecular Bone Score and Lumbar Spine Volumetric BMD Is Attenuated Among Older Men with High Body Mass Index. J. Bone Min. Res. 2016, 31, 1820–1826. [Google Scholar] [CrossRef] [Green Version]
- Shin, Y.H.; Gong, H.S.; Lee, K.J.; Baek, G.H. Older Age and Higher Body Mass Index Are Associated with a More Degraded Trabecular Bone Score Compared to Bone Mineral Density. J. Clin. Densitom. 2019, 22, 266–271. [Google Scholar] [CrossRef]
- Kong, S.H.; Hong, N.; Kim, J.W.; Kim, D.Y.; Kim, J.H. Application of the Trabecular Bone Score in Clinical Practice. J. Bone Metab 2021, 28, 101–113. [Google Scholar] [CrossRef]
- Senn, C.; Gunther, B.; Popp, A.W.; Perrelet, R.; Hans, D.; Lippuner, K. Comparative effects of teriparatide and ibandronate on spine bone mineral density (BMD) and microarchitecture (TBS) in postmenopausal women with osteoporosis: A 2-year open-label study. Osteoporos. Int. 2014, 25, 1945–1951. [Google Scholar] [CrossRef] [PubMed]
- Saag, K.G.; Agnusdei, D.; Hans, D.; Kohlmeier, L.A.; Krohn, K.D.; Leib, E.S.; MacLaughlin, E.J.; Alam, J.; Simonelli, C.; Taylor, K.A.; et al. Trabecular Bone Score in Patients with Chronic Glucocorticoid Therapy-Induced Osteoporosis Treated with Alendronate or Teriparatide. Arthritis Rheumatol. 2016, 68, 2122–2128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haeri, N.S.; Perera, S.; Ferreiro, I.; Hans, D.; Greenspan, S.L. Trabecular bone score in the hip: A new method to examine hip bone microarchitecture—A feasibility study. Arch. Osteoporos. 2022, 17, 126. [Google Scholar] [CrossRef] [PubMed]
- Brinjikji, W.; Luetmer, P.H.; Comstock, B.; Bresnahan, B.W.; Chen, L.E.; Deyo, R.A.; Halabi, S.; Turner, J.A.; Avins, A.L.; James, K.; et al. Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. AJNR Am. J. Neuroradiol. 2015, 36, 811–816. [Google Scholar] [CrossRef]
- Rand, T.; Seidl, G.; Kainberger, F.; Resch, A.; Hittmair, K.; Schneider, B.; Gluer, C.C.; Imhof, H. Impact of spinal degenerative changes on the evaluation of bone mineral density with dual energy X-ray absorptiometry (DXA). Calcif. Tissue Int. 1997, 60, 430–433. [Google Scholar] [CrossRef]
- Anderson, K.B.; Holloway-Kew, K.L.; Mohebbi, M.; Kotowicz, M.A.; Hans, D.; Pasco, J.A. Is trabecular bone score less affected by degenerative-changes at the spine than lumbar spine BMD? Arch. Osteoporos. 2018, 13, 127. [Google Scholar] [CrossRef] [PubMed]
- Padlina, I.; Gonzalez-Rodriguez, E.; Hans, D.; Metzger, M.; Stoll, D.; Aubry-Rozier, B.; Lamy, O. The lumbar spine age-related degenerative disease influences the BMD not the TBS: The Osteolaus cohort. Osteoporos. Int. 2017, 28, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Paz, R.D.; Henriquez, M.S.; Melian, K.A.; Martin, C.B. Prevalence of Poor Bone Quality in Patients Undergoing Spine Surgery: A Comprehensive Approach. Glob. Spine J. 2022, 12, 1412–1419. [Google Scholar] [CrossRef]
- Mosekilde, L. Age-related changes in vertebral trabecular bone architecture—Assessed by a new method. Bone 1988, 9, 247–250. [Google Scholar] [CrossRef]
- Leslie, W.D.; Aubry-Rozier, B.; Lamy, O.; Hans, D.; Manitoba Bone Density, P. TBS (trabecular bone score) and diabetes-related fracture risk. J. Clin. Endocrinol. Metab 2013, 98, 602–609. [Google Scholar] [CrossRef] [Green Version]
- Eller-Vainicher, C.; Filopanti, M.; Palmieri, S.; Ulivieri, F.M.; Morelli, V.; Zhukouskaya, V.V.; Cairoli, E.; Pino, R.; Naccarato, A.; Verga, U.; et al. Bone quality, as measured by trabecular bone score, in patients with primary hyperparathyroidism. Eur. J. Endocrinol. 2013, 169, 155–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cormier, C.; Koumakis, E. Bone and primary hyperparathyroidism. Jt. Bone Spine 2022, 89, 105129. [Google Scholar] [CrossRef] [PubMed]
- Esposti, L.D.; Perrone, V.; Sella, S.; Arcidiacono, G.; Bertoldo, F.; Giustina, A.; Minisola, S.; Napoli, N.; Passeri, G.; Rossini, M.; et al. The Potential Impact of Inducing a Restriction in Reimbursement Criteria on Vitamin D Supplementation in Osteoporotic Patients with or without Fractures. Nutrients 2022, 14, 1877. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Kar, R.; Gluhak-Heinrich, J.; Yao, W.; Lane, N.E.; Bonewald, L.F.; Biswas, S.K.; Lo, W.K.; Jiang, J.X. Glucocorticoid-induced autophagy in osteocytes. J. Bone Min. Res. 2010, 25, 2479–2488. [Google Scholar] [CrossRef] [PubMed]
- Breban, S.; Briot, K.; Kolta, S.; Paternotte, S.; Ghazi, M.; Fechtenbaum, J.; Roux, C. Identification of rheumatoid arthritis patients with vertebral fractures using bone mineral density and trabecular bone score. J. Clin. Densitom. 2012, 15, 260–266. [Google Scholar] [CrossRef] [PubMed]
- van Staa, T.P. The pathogenesis, epidemiology and management of glucocorticoid-induced osteoporosis. Calcif. Tissue Int. 2006, 79, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Florez, H.; Hernandez-Rodriguez, J.; Muxi, A.; Carrasco, J.L.; Prieto-Gonzalez, S.; Cid, M.C.; Espinosa, G.; Gomez-Puerta, J.A.; Monegal, A.; Guanabens, N.; et al. Trabecular bone score improves fracture risk assessment in glucocorticoid-induced osteoporosis. Rheumatology 2020, 59, 1574–1580. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Wang, B.; Wang, R.; Gong, S.; Chen, G.; Xu, W. The shift in the balance between osteoblastogenesis and adipogenesis of mesenchymal stem cells mediated by glucocorticoid receptor. Stem Cell Res. 2019, 10, 377. [Google Scholar] [CrossRef] [Green Version]
| Type of Parameter | Parameter |
|---|---|
| Demographics and general information | Age, sex, height, weight, BMI |
| Underlying disease | Inflammatory rheumatic diseases (rheumatoid arthritis, psoriatic arthritis, spondyloarthritis, connective tissue diseases, vasculitis, polymyalgia rheumatica); endocrine diseases (primary hyperthyroidism, hypogonadism, crushing’s disease, hypophysitis, hyperthyreosis, Diabetes mellitus); Other diseases (monoclonal gammopathy of undetermined significance (MGUS), therapy with aromatas inhibitors after breast cancer). |
| Antiosteoporotic medication | Vitamin D supplementation, treatment with raloxifene, bisphosphonates (oral, i.v.), denosumab, teriparatide |
| Bone-relevant laboratory parameters | Vitamin D levels, bone alkaline phosphatase, deoxypyridinoline, C-telopeptide crosslaps of type I collagen (CTX-1), osteocalcin |
| Total n = 265 | Glucocorticoid Therapy n = 48 | Rheumatic Diseases n = 56 | Endocrine Diseases n = 62 | Elderly Patients/Primary Osteoporosis n = 141 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | ||
| Sex | |||||||||||
| Male | 49 | 18.5 | 12 | 25.0 | 10 | 17.9 | 13 | 21.0 | 23 | 16.3 | |
| Female | 216 | 81.5 | 36 | 75.0 | 46 | 82.1 | 49 | 79.0 | 118 | 83.7 | |
| Age, mean (Sd) | 62.6 (14.9) | 63.8 (13.2) | 65.0 (12.6) | 55.8 (18.8) | 64.3 (13.0) | ||||||
| ≤55 years | 75 | 28.3 | 11 | 22.9 | 10 | 17.9 | 31 | 50.0 | 33 | 23.4 | |
| 56–75 years | 122 | 46.0 | 24 | 50.0 | 31 | 55.4 | 15 | 24.2 | 74 | 52.5 | |
| 76+ years | 68 | 25.7 | 13 | 27.1 | 15 | 26.8 | 16 | 25.8 | 34 | 24.1 | |
| Height in m, mean (SD) | 1.66 (0.09) | 1.65 (0.10) | 1.65 (0.10) | 1.68 (0.10) | 1.65 (0.08) | ||||||
| Weight in kg, mean (SD) | 69.3 (14.7) | 72.5 (15.6) | 71.1 (14.4) | 69.2 (16.2) | 68.5 (14.2) | ||||||
| BMI in kg/m2, mean (SD) | 25.2 (4.8) | 26.5 (5.2) | 26.3 (4.9) | 24.3 (4.7) | 25.0 (4.6) | ||||||
| Bone alkaline phosphatase U/L mean (SD), normal range 4.7–27.1 | 18.7 (8.3) | 19.0 (10.0) | 18.8 (8.8) | 19.2 (8.7) | 18.4 (7.5) | ||||||
| 25-OH-Vitamin D nmol/L, mean (SD), normal range 19.0–139.0 | 78.9 (67.5) | 76.9 (33.3) | 79.3 (30.0) | 64.2 (25.8) | 85.5 (89.8) | ||||||
| Deoxypyridnoline nmol/mmol, mean (SD), normal range 3.0–7.4 | 7.4 (5.9) | 6.9 (2.6) | 7.3 (2.3) | 9.7 (12.8) | 6.9 (3.4) | ||||||
| Total N = 265 | Male N = 49 | Female N = 216 | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Median | Mean (SD) | Median | Mean (SD) | Median | p Value | ||
| Total | ||||||||
| T-score L1–L4 | −1.11 (1.60) | −1.25 | −1.44 (1.53) | −1.30 | −1.04 (1.61) | −1.20 | 0.107 | |
| T-score femoral neck | −1.30 (1.11) | −1.40 | −1.83 (1.09) | −2.00 | −1.17 (1.09) | −1.30 | <0.001 | |
| T-score femur | −1.23 (1.16) | −1.30 | −1.57 (1.12) | −1.65 | −1.15 (1.15) | −1.30 | 0.021 | |
| TBS T-score | −2.23 (1.43) | −2.10 | −1.82 (1.49) | −1.70 | −2.32 (1.40) | −2.20 | 0.034 | |
| TBS Z-score | −0.27 (1.25) | −0.20 | −0.63 (1.31) | −0.50 | −0.18 (1.22) | −0.10 | 0.045 | |
| TBS value | 1.25 (0.13) | 1.27 | 1.25 (0.15) | 1.26 | 1.25 (0.12) | 1.27 | 0.774 | |
| Glucocorticoid therapy | ||||||||
| T-score L1–L4 | −1.02 (1.59) | −1.20 | −1.28 (1.50) | −1.20 | −0.92 (1.64) | −1.20 | 0.496 | |
| T-score femoral neck | −1.25 (1.06) | −1.40 | −1.46 (1.02) | −1.90 | −1.18 (1.07) | −1.30 | 0.434 | |
| T-score femur | −1.08 (0.96) | −1.20 | −1.20 (1.01) | −1.30 | −1.05 (0.96) | −1.00 | 0.666 | |
| TBS T-score | −2.36 (1.53) | −2.15 | −1.58 (1.73) | −1.30 | −2.62 (1.39) | −2.30 | 0.079 | |
| TBS Z-score | −0.51 (1.43) | −0.20 | −0.75 (1.34) | −0.50 | −0.44 (1.47) | −0.15 | 0.537 | |
| TBS value | 1.24 (0.14) | 1.27 | 1.28 (0.19) | 1.31 | 1.23 (0.12) | 1.26 | 0.440 | |
| Rheumatic diseases | ||||||||
| T-score L1–L4 | −0.62 (1.64) | −0.80 | −1.11 (1.88) | −0.90 | −0.50 (1.58) | −0.80 | 0.364 | |
| T-score femoral neck | −1.10 (1.09) | −1.20 | −1.61 (1.28) | −2.00 | −1.00 (1.04) | −1.05 | 0.205 | |
| T-Score femur | −0.92 (1.08) | −1.00 | −1.22 (0.96) | −1.30 | −0.86 (1.10) | −1.00 | 0.335 | |
| TBS T-score | −2.11 (1.49) | −2.15 | −1.88 (1.94) | −2.00 | −2.16 (1.39) | −2.15 | 0.669 | |
| TBS Z score | −0.13 (1.32) | 0.00 | −0.59 (1.41) | −0.20 | −0.03 (1.30) | 0.05 | 0.298 | |
| TBS value | 1.27 (0.14) | 1.27 | 1.26 (0.20) | 1.25 | 1.27 (0.13) | 1.27 | 0.935 | |
| Endocrine diseases | ||||||||
| T-score L1–L4 | −1.04 (1.78) | −1.00 | −1.28 (1.51) | −1.40 | −0.98 (1.86) | −0.95 | 0.556 | |
| T-score femoral neck | −1.12 (1.32) | −1.30 | −1.47 (1.19) | −1.50 | −1.02 (1.34) | −1.30 | 0.253 | |
| T-score femur | −1.19 (1.37) | −1.20 | −1.31 (1.29) | −1.10 | −1.16 (1.40) | −1.30 | 0.721 | |
| TBS T-score | −2.23 (1.58) | −2.00 | −1.24 (0.99) | −1.00 | −2.50 (1.60) | −2.20 | 0.001 | |
| TBS Z-score | −0.62 (1.23) | −0.35 | −0.26 (0.91) | −0.10 | −0.72 (1.30) | −0.40 | 0.233 | |
| TBS value | 1.26 (0.14) | 1.28 | 1.32 (0.11) | 1.34 | 1.24 (0.14) | 1.27 | 0.053 | |
| Elderly patients/primary osteoporosis | ||||||||
| T-score L1–L4 | −1.31 (1.48) | −1.40 | −1.75 (1.47) | −1.60 | −1.22 (1.47) | −1.40 | 0.136 | |
| T-score femoral neck | −1.42 (1.02) | −1.60 | −2.12 (0.98) | −2.10 | −1.28 (0.98) | −1.40 | 0.001 | |
| T-score femur | −1.34 (1.08) | −1.45 | −1.83 (1.10) | −1.80 | −1.24 (1.06) | −1.30 | 0.024 | |
| TBS T-score | −2.26 (1.35) | −2.10 | −2.07 (1.54) | −1.80 | −2.30 (1.31) | −2.20 | 0.519 | |
| TBS Z-score | −0.19 (1.21) | −0.20 | −0.75 (1.48) | −0.60 | −0.06 (1.11) | −0.10 | 0.045 | |
| TBS value | 1.25 (0.12) | 1.26 | 1.21 (0.15) | 1.23 | 1.26 (0.11) | 1.27 | 0.149 | |
| T-Score L1–L4 | T-Score Femoral Neck | T-Score Total Femur | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Beta (95% CI) | p-Value | R2 | Beta (95% CI) | p-Value | R2 | Beta (95% CI) | p-Value | R2 | ||
| Univariate | Bone alkaline phosphatase | −0.12 (−0.25, 0.02) | 0.093 | 0.012 | −0.02 (−0.14, 0.10) | 0.758 | 0.000 | −0.09 (−0.22, 0.04) | 0.186 | 0.008 |
| 25-OH-vitamin D | 0.01 (−0.09, 0.11) | 0.837 | 0.000 | −0.04 (−0.13, 0.05) | 0.376 | 0.002 | 0.03 (−0.08, 0.13) | 0.587 | 0.001 | |
| Deoxypyridinoline | −0.01 (−0.18, 0.17) | 0.943 | 0.000 | −0.08 (−0.19, 0.04) | 0.210 | 0.006 | −0.08 (−0.25, 0.10) | 0.374 | 0.006 | |
| Multivariable | Bone alkaline phosphatase | −0.05 (−0.20, 0.10) | 0.480 | −0.03 (−0.18, 0.12) | 0.682 | −0.12 (−0.28 0.04) | 0.142 | |||
| 25-OH-vitamin D | 0.03 (−0.06, 0.12) | 0.532 | 0.005 | −0.01 (−0.07, 0.04) | 0.595 | 0.003 | 0.08 (0.04, 0.13) | <0.001 | 0.031 | |
| Deoxypyridinoline | 0.04 (−0.07, 0.15) | 0.504 | −0.04 (−0.12, 0.04) | 0.332 | −0.03 (−0.14, 0.08) | 0.600 | ||||
| Univariate | TBS T-score | 0.31 (0.19, 0.44) | <0.001 | 0.095 | 0.37 (0.25, 0.48) | <0.001 | 0.130 | 0.38 (0.26, 0.50) | <0.001 | 0.139 |
| TBS Z-score | 0.29 (0.14, 0.43) | <0.001 | 0.079 | 0.24 (0.12, 0.37) | <0.001 | 0.070 | 0.26 (0.13, 0.39) | <0.001 | 0.075 | |
| TBS value | 0.32 (0.20, 0.45) | <0.001 | 0.101 | 0.37 (0.26, 0.48) | <0.001 | 0.135 | 0.37 (0.25, 0.49) | <0.001 | 0.133 | |
| Multivariable | TBS T-score | 0.26 (0.05, 0.47) | 0.018 | 0.068 | 0.36 (0.16, 0.55) | 0.001 | 0.119 | 0.37 (0.19, 0.55) | <0.001 | 0.158 |
| Bone alkaline phosphatase | −0.03 (−0.17, 0.11) | 0.693 | 0.04 (−0.10, 0.19) | 0.545 | −0.03 (−0.17, 0.10) | 0.645 | ||||
| 25-OH-vitamin D | 0.06 (−0.03, 0.16) | 0.204 | 0.03 (−0.02, 0.08) | 0.192 | 0.13 (0.09, 0.18) | <0.001 | ||||
| Deoxypyridinoline | 0.06 (−0.04, 0.17) | 0.215 | −0.01 (−0.09, 0.08) | 0.850 | 0.01 (−0.10, 0.11) | 0.876 | ||||
| Multivariable | TBS Z-score | 0.23 (−0.03, 0.48) | 0.079 | 0.052 | 0.18 (−0.04, 0.41) | 0.115 | 0.035 | 0.22 (−0.01, 0.44) | 0.058 | 0.083 |
| Bone alkaline phosphatase | −0.03 (−0.18, 0.12) | 0.709 | 0.00 (−0.14, 0.14) | 0.978 | −0.07 (−0.22, 0.07) | 0.327 | ||||
| 25-OH-vitamin D | 0.05 (−0.06, 0.15) | 0.370 | 0.02 (−0.03, 0.06) | 0.521 | 0.11 (0.06, 0.16) | <0.001 | ||||
| Deoxypyridinoline | 0.07 (−0.02, 0.17) | 0.141 | 0.01 (−0.07, 0.10) | 0.761 | 0.02 (−0.06, 0.11) | 0.594 | ||||
| Multivariable | TBS value | 0.31 (0.11, 0.52) | 0.003 | 0.094 | 0.40 (0.20, 0.60) | <0.001 | 0.138 | 0.40 (0.21, 0.58) | <0.001 | 0.164 |
| Bone alkaline phosphatase | −0.03 (−0.17, 0.11) | 0.642 | 0.04 (−0.11, 0.18) | 0.623 | −0.04 (−0.18, 0.09) | 0.530 | ||||
| 25-OH-vitamin D | 0.07 (−0.03, 0.16) | 0.161 | 0.04 (−0.01, 0.09) | 0.150 | 0.13 (0.09, 0.18) | <0.001 | ||||
| Deoxypyridinoline | 0.07 (−0.03, 0.17) | 0.195 | −0.01 (−0.09, 0.07) | 0.813 | 0.00 (−0.10, 0.11) | 0.926 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Hashimi, L.; Klotsche, J.; Ohrndorf, S.; Gaber, T.; Hoff, P. Trabecular Bone Score Significantly Influences Treatment Decisions in Secondary Osteoporosis. J. Clin. Med. 2023, 12, 4147. https://doi.org/10.3390/jcm12124147
Al-Hashimi L, Klotsche J, Ohrndorf S, Gaber T, Hoff P. Trabecular Bone Score Significantly Influences Treatment Decisions in Secondary Osteoporosis. Journal of Clinical Medicine. 2023; 12(12):4147. https://doi.org/10.3390/jcm12124147
Chicago/Turabian StyleAl-Hashimi, Leith, Jens Klotsche, Sarah Ohrndorf, Timo Gaber, and Paula Hoff. 2023. "Trabecular Bone Score Significantly Influences Treatment Decisions in Secondary Osteoporosis" Journal of Clinical Medicine 12, no. 12: 4147. https://doi.org/10.3390/jcm12124147
APA StyleAl-Hashimi, L., Klotsche, J., Ohrndorf, S., Gaber, T., & Hoff, P. (2023). Trabecular Bone Score Significantly Influences Treatment Decisions in Secondary Osteoporosis. Journal of Clinical Medicine, 12(12), 4147. https://doi.org/10.3390/jcm12124147