The Relationship between Number of Supernumerary Blastocysts Cryopreserved and Probability of a Live Birth Outcome after Single Fresh Blastocyst Transfer: Analysis of over 10 Thousand Cycles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Ovarian Stimulation and Oocyte Retrieval
2.3. Embryo Assessment
2.4. Outcome Measure
2.5. Statistical Analysis
2.6. Ethics
3. Results
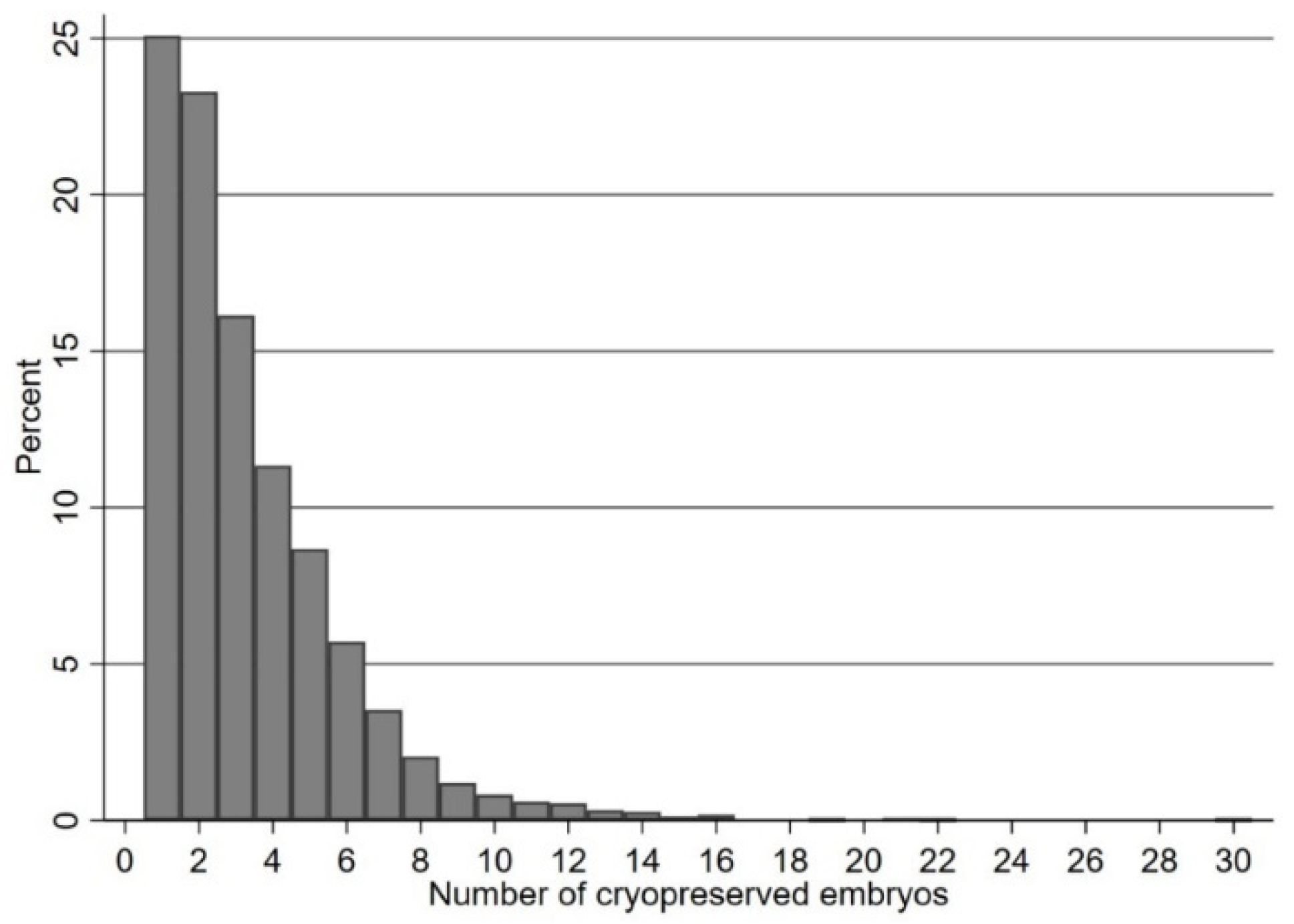
3.1. Association of Live Birth Rate with Number of Supernumerary Blastocysts Cryopreserved
3.2. Probability of Live Birth When Female Age Is below 35 Years
3.3. Probability of Live Birth When Female Age Is between 35 and 39 Years
3.4. Probability of Live Birth When Female Age Is Aged 40 Years and Above
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Veeck, L.L.; Amundson, C.H.; Brothman, L.J.; DeScisciolo, C.; Maloney, M.K.; Muasher, S.J.; Jones, H.W. Significantly enhanced pregnancy rates per cycle through cryopreservation and thaw of pronuclear stage oocytes. Fertil. Steril. 1993, 59, 1202–1207. [Google Scholar] [CrossRef] [PubMed]
- Tiitinen, A.; Halttunen, M.; Härkki, P.; Vuoristo, P.; Hyden-Granskog, C. Elective single embryo transfer: The value of cryopreservation. Hum. Reprod. 2001, 16, 1140–1144. [Google Scholar] [CrossRef] [PubMed]
- Marsh, C.A.; Farr, S.L.; Chang, J.; Kissin, D.M.; Grainger, D.A.; Posner, S.F.; Macaluso, M.; Jamieson, D.J. Trends and factors associated with the Day 5 embryo transfer, assisted reproductive technology surveillance, USA, 2001–2009. Hum. Reprod. 2012, 27, 2325–2331. [Google Scholar] [CrossRef]
- Sjögren, A.; Sjöblom, P.; Hamberger, L. Culture of human spare pre-embryos: Association between blastocyst formation and pregnancy. J. Assist. Reprod. Genet. 1992, 9, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Sunkara, S.; Rittenberg, V.; Raine-Fenning, N.; Bhattacharya, S.; Javier Zamora, J.; Coomarasamy, A. Association between the number of eggs and live birth in IVF treatment: An analysis of 400,135 treatment cycles. Hum. Reprod. 2011, 26, 1768–1774. [Google Scholar] [CrossRef] [Green Version]
- Steward, R.G.; Lan, L.; Shah, A.A.; Yeh, J.S.; Price, T.M.; Goldfarb, J.M.; Muasher, S.J. Oocyte number as a predictor for ovarian hyperstimulation syndrome and live birth: An analysis of 256,381 in vitro fertilization cycles. Fertil. Steril. 2014, 101, 967–973. [Google Scholar] [CrossRef]
- Baker, V.L.; Brown, M.B.; Luke, B.; Smith, G.W.; Ireland, J.J. Gonadotropin dose is negatively correlated with live birth rate: Analysis of more than 650,000 assisted reproductive technology cycles. Fertil. Steril. 2015, 104, 1145–1152. [Google Scholar] [CrossRef] [Green Version]
- Hill, M.J.; Richter, K.S.; Heitmann, R.J.; Lewis, T.D.; DeCherney, A.H.; Graham, J.R.; Widra, E.; Levy, M.J. Number of supernumerary vitrified blastocysts is positively correlated with implantation and live birth in single-blastocyst embryo transfers. Fertil. Steril. 2013, 99, 1631–1636. [Google Scholar] [CrossRef] [Green Version]
- Papanikolaou, E.; Chartomatsidou, T.; Timotheou, E.; Tatsi, P.; Katsoula, E.; Vlachou, C.; Asouchidou, I.; Zafeiratis, O.; Najdecki, R. In Freeze-All Strategy, Cumulative Live Birth Rate (CLBR) Is Increasing According to the Number of Blastocysts Formed in Women <40 Undergoing Intracytoplasmic Sperm Injection (ICSI). Front. Endocrinol. 2019, 10, 427. [Google Scholar] [CrossRef] [Green Version]
- Scheffer, J.B.; Scheffer, B.B.; de Carvalho, R.F.; Rodrigues, J.; Grynberg, M.; Lozano, D.H.M. Age as A Predictor of Embryo Quality Regardless of The Quantitative Ovarian Response. Int. J. Fertil. Steril. 2017, 11, 40–46. [Google Scholar] [CrossRef]
- Sunkara, S.K.; Coomarasamy, A.; Khalaf, Y.; Braude, P. A three-arm randomised controlled trial comparing Gonadotrophin Releasing Hormone (GnRH) agonist long regimen versus GnRH agonist short regimen versus GnRH antagonist regimen in women with a history of poor ovarian response undergoing in vitro fertilisation (IVF) treatment: Poor responders intervention trial (PRINT). Reprod. Health 2007, 4, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunkara, S.K.; Coomarasamy, A.; Faris, R.; Braude, P.; Khalaf, Y. Long gonadotropin-releasing hormone agonist versus short agonist versus antagonist regimens in poor responders undergoing in vitro fertilization: A randomized controlled trial. Fertil. Steril. 2014, 101, 147–153. [Google Scholar] [CrossRef] [PubMed]
- El-Toukhy, T.; Campo, R.; Khalaf, Y.; Tabanelli, C.; Gianaroli, L.; Gordts, S.S.; Gordts, S.; Mestdagh, G.; Mardesic, T.; Voboril, J.; et al. Hysteroscopy in recurrent in-vitro fertilisation failure (TROPHY): A multicentre, randomised controlled trial. Lancet 2016, 387, 2614–2621. [Google Scholar] [CrossRef] [Green Version]
- Naji, O.; Moska, N.; Dajani, Y.; El-Shirif, A.; El-Ashkar, H.; Hosni, M.M.; Khalil, M.; Khalaf, Y.; Bolton, V.; El-Toukhy, T. Early oocyte denudation does not compromise ICSI cycle outcome: A large retrospective cohort study. Reprod. Biomed. Online 2018, 37, 18–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardner, D.K.; Schoolcraft, W.B. In Vitro culture of human blastocyst. In Towards Reproductive Certainty: Fertility and Genetics Beyond 1999; Jansen, R., Mortimer, D., Eds.; Parthenon Publishing: Carnforth, UK, 1999; pp. 378–388. [Google Scholar]
- Gardner, D.K.; Balaban, B. Choosing Between Day 3 and Day 5 Embryo Transfers. Clin. Obstet. Gynecol. 2006, 49, 85–92. [Google Scholar] [CrossRef]
- Harbottle, S.; Hughes, C.; Cutting, R.; Roberts, S.; Brison, D.; On Behalf of The Association of Clinical Embryologists; The British Fertility Society. Elective Single Embryo Transfer: An update to UK Best Practice Guidelines. Hum. Fertil. 2015, 18, 165–183. [Google Scholar] [CrossRef] [Green Version]
- Lasalle, B.; Testart, J.; Renard, J. Human embryo features that influence the success of cryopreservation with the use of 1,2-propanediol. Fertil. Steril. 1985, 44, 645–651. [Google Scholar] [CrossRef]
- Edgar, D.; Bourne, H.; Speirs, A.; McBain, J. A quantitative analysis of the impact of cryopreservation on the implantation potential of human early cleavage stage embryos. Hum. Reprod. 2000, 15, 175–179. [Google Scholar] [CrossRef]
- StataCorp. Stata Statistical Software: Release 16; StataCorp LLC: College Station, TX, USA, 2019. [Google Scholar]
- Royston, P.; Altman, D.G. Regression Using Fractional Polynomials of Continuous Covariates: Parsimonious Parametric Modelling. J. R. Stat. Soc. Ser. C Appl. Stat. 1994, 43, 429. [Google Scholar] [CrossRef]
- Romanski, P.A.; Goldman, R.H.; Farland, L.V.; Srouji, S.S.; Racowsky, C. The association between quality of supernumerary embryos in a cohort and implantation potential of the transferred blastocyst. J. Assist. Reprod. Genet. 2018, 35, 1651–1656. [Google Scholar] [CrossRef]
- Drakopoulos, P.; Blockeel, C.; Stoop, D.; Camus, M.; de Vos, M.; Tournaye, H.; Polyzos, N. Conventional ovarian stimulation and single embryo transfer for IVF/ICSI. How many oocytes do we need to maximize cumulative live birth rates after utilization of all fresh and frozen embryos? Hum. Reprod. 2016, 31, 370–376. [Google Scholar] [CrossRef] [Green Version]
- Hariton, E.; Kim, K.; Mumford, S.L.; Palmor, M.; Bortoletto, P.; Cardozo, E.R.; Karmon, A.E.; Sabatini, M.E.; Styer, A.K. Total number of oocytes and zygotes are predictive of live birth pregnancy in fresh donor oocyte in vitro fertilization cycles. Fertil. Steril. 2017, 108, 262–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polyzos, N.P.; Drakopoulos, P.; Parra, J.; Pellicer, A.; Santos-Ribeiro, S.; Tournaye, H.; Bosch, E.; Garcia-Velasco, J. Cumulative live birth rates according to the number of oocytes retrieved after the first ovarian stimulation for in vitro fertilization/intracytoplasmic sperm injection: A multicenter multinational analysis including ~15,000 women. Fertil. Steril. 2018, 110, 661–670.e1. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.E.; Goldman, M.B.; Hatasaka, H.; MacKenzie, T.A.; Surrey, E.S.; Racowsky, C. Optimizing the number of cleavage stage embryos to transfer on day 3 in women 38 years of age and older: A Society for Assisted Reproductive Technology database study. Fertil. Steril. 2009, 91, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Mullin, C.; Berkeley, A.S.; Grifo, J.A. Supernumerary Blastocyst Cryopreservation: A key Prognostic Indicator for Patients Opting for an Elective Single Blastocyst Transfer (eSBT). J. Assist. Reprod. Genet. 2012, 29, 783–788. [Google Scholar] [CrossRef] [Green Version]
- Smeltzer, S.; Acharya, K.; Truong, T.; Pieper, C.; Muasher, S. Clinical pregnancy (CP) and live birth (LB) increase significantly with each additional fertilized oocyte up to nine, and decline after that: An analysis of 15,803 first fresh in vitro fertilization cycles from the Society for Assisted Reproductive Technology registry. Fertil. Steril. 2019, 112, 520–526. [Google Scholar]
- McLernon, D.J.; Steyerberg, E.W.; Velde, E.R.T.; Lee, A.J.; Bhattacharya, S. Predicting the chances of a live birth after one or more complete cycles of in vitro fertilisation: Population based study of linked cycle data from 113,873 women. BMJ 2016, 355, i5735. [Google Scholar] [CrossRef] [Green Version]
- Vaughan, D.A.; Leung, A.; Resetkova, N.; Ruthazer, R.; Penzias, A.S.; Sakkas, D.; Alper, M.M. How many oocytes are optimal to achieve multiple live births with one stimulation cycle? The one-and-done approach. Fertil. Steril. 2016, 107, 397–404.e3. [Google Scholar] [CrossRef] [Green Version]
- Ben-Nagi, J.; Jones, B.; Naja, R.; Amer, A.; Sunkara, S.; SenGupta, S.; Serhal, P. Live birth rate is associated with oocyte yield and number of biopsied and suitable blastocysts to transfer in preimplantation genetic testing (PGT) cycles for monogenic disorders and chromosomal structural rearrangements. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 10, 100055. [Google Scholar] [CrossRef]
- Zhang, M.; Bu, T.; Tian, H.; Li, X.; Wang, D.; Wan, X.; Wang, Q.; Mao, X.; La, X. Use of Cumulative Live Birth Rate per Total Number of Embryos to Calculate the Success of IVF in Consecutive IVF Cycles in Women Aged ≥35 Years. BioMed Res. Int. 2019, 2019, 6159793. [Google Scholar] [CrossRef] [Green Version]
- Munné, S.; Kaplan, B.; Frattarelli, J.L.; Child, T.; Nakhuda, G.; Shamma, F.N.; Silverberg, K.; Kalista, T.; Handyside, A.H.; Katz-Jaffe, M.; et al. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: A multicenter randomized clinical trial. Fertil. Steril. 2019, 112, 1071–1079.e7. [Google Scholar] [CrossRef] [PubMed]

| Factor | No Blastocysts Cryopreserved | Supernumerary Blastocysts Cryopreserved | p-Value |
|---|---|---|---|
| Number | 5992 | 4023 | |
| Age, median (IQR) | 36.0 (33.0, 39.0) | 34.0 (31.5, 37.0) | <0.001 |
| Age Group | |||
| <35 | 2256 (38%) | 2119 (53%) | <0.001 |
| 35–39 | 2970 (49%) | 1728 (43%) | |
| ≥40 | 766 (13%) | 176 (4%) | |
| Baseline FSH (IU) Level, median (IQR) | 6.3 (5.3, 7.7) | 6.3 (5.3, 7.6) | 0.15 |
| Type of Gonadotrophins used | |||
| Recombinant | 5347 (89.2%) | 3688 (91.7%) | <0.001 |
| Urinary-derived | 645 (10.8%) | 335 (8.3%) | |
| Daily Dose of FSH, median (IQR) | 277 (150.0, 300.0) | 225.0 (150.0, 300.0) | <0.001 |
| Duration of Ovarian Stimulation, median (IQR) | 11.0 (10.0, 12.0) | 11.0 (10.0, 12.0) | 0.33 |
| Total Dose of FSH, median (IQR) | 2700 (1800, 3600) | 2250 (1650, 3150) | <0.001 |
| Number of Oocyctes Retrieved, median (IQR) | 9.0 (6.0, 14.0) | 14.0 (10.0, 18.0) | <0.001 |
| Method of Oocyte Fertilisation | |||
| IVF | 2105 (35%) | 1461 (36%) | |
| ICSI | 3887 (65%) | 2562 (64%) | |
| No. of Normally-Fertilised Oocytes, median (IQR) | 5.0 (3.0, 8.0) | 9.0 (6.0, 12.0) | <0.001 |
| Clinical Pregnancy | 1568 (28%) | 1612 (44%) | <0.001 |
| Live Birth | 1436 (24%) | 1518 (38%) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beebeejaun, Y.; Copeland, T.; Polanski, L.; El Toukhy, T. The Relationship between Number of Supernumerary Blastocysts Cryopreserved and Probability of a Live Birth Outcome after Single Fresh Blastocyst Transfer: Analysis of over 10 Thousand Cycles. J. Clin. Med. 2023, 12, 4172. https://doi.org/10.3390/jcm12134172
Beebeejaun Y, Copeland T, Polanski L, El Toukhy T. The Relationship between Number of Supernumerary Blastocysts Cryopreserved and Probability of a Live Birth Outcome after Single Fresh Blastocyst Transfer: Analysis of over 10 Thousand Cycles. Journal of Clinical Medicine. 2023; 12(13):4172. https://doi.org/10.3390/jcm12134172
Chicago/Turabian StyleBeebeejaun, Yusuf, Timothy Copeland, Lukasz Polanski, and Tarek El Toukhy. 2023. "The Relationship between Number of Supernumerary Blastocysts Cryopreserved and Probability of a Live Birth Outcome after Single Fresh Blastocyst Transfer: Analysis of over 10 Thousand Cycles" Journal of Clinical Medicine 12, no. 13: 4172. https://doi.org/10.3390/jcm12134172
APA StyleBeebeejaun, Y., Copeland, T., Polanski, L., & El Toukhy, T. (2023). The Relationship between Number of Supernumerary Blastocysts Cryopreserved and Probability of a Live Birth Outcome after Single Fresh Blastocyst Transfer: Analysis of over 10 Thousand Cycles. Journal of Clinical Medicine, 12(13), 4172. https://doi.org/10.3390/jcm12134172







