Applicability of Fractal Analysis for Quantitative Evaluation of Midpalatal Suture Maturation
Abstract
1. Introduction
2. Materials and Methods
2.1. CBCT Scans
2.2. Fractal Analysis
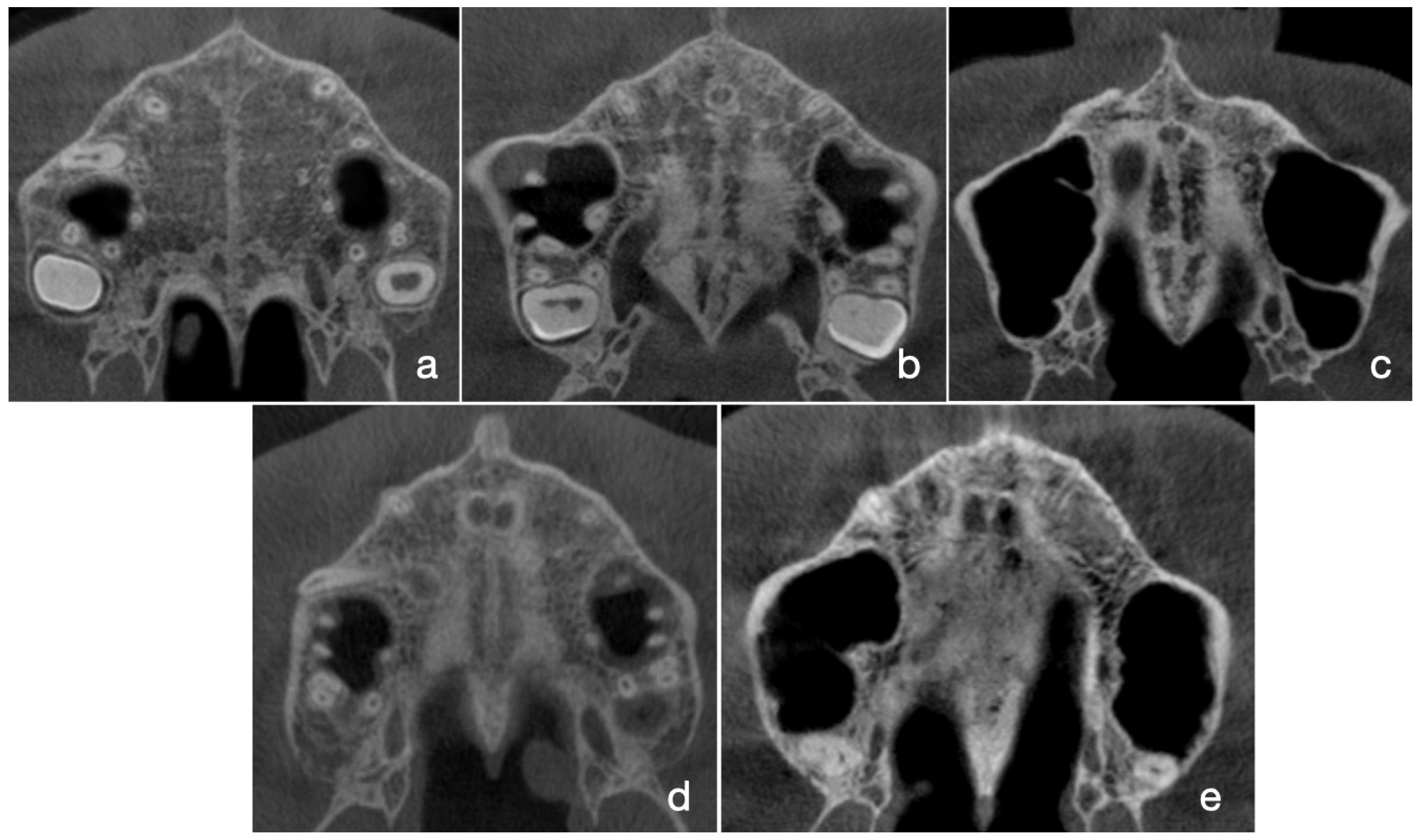
2.3. Assessment of MPS Maturation Stages According to Angelieri’s Classification Method
2.4. Statistical Analysis
3. Results
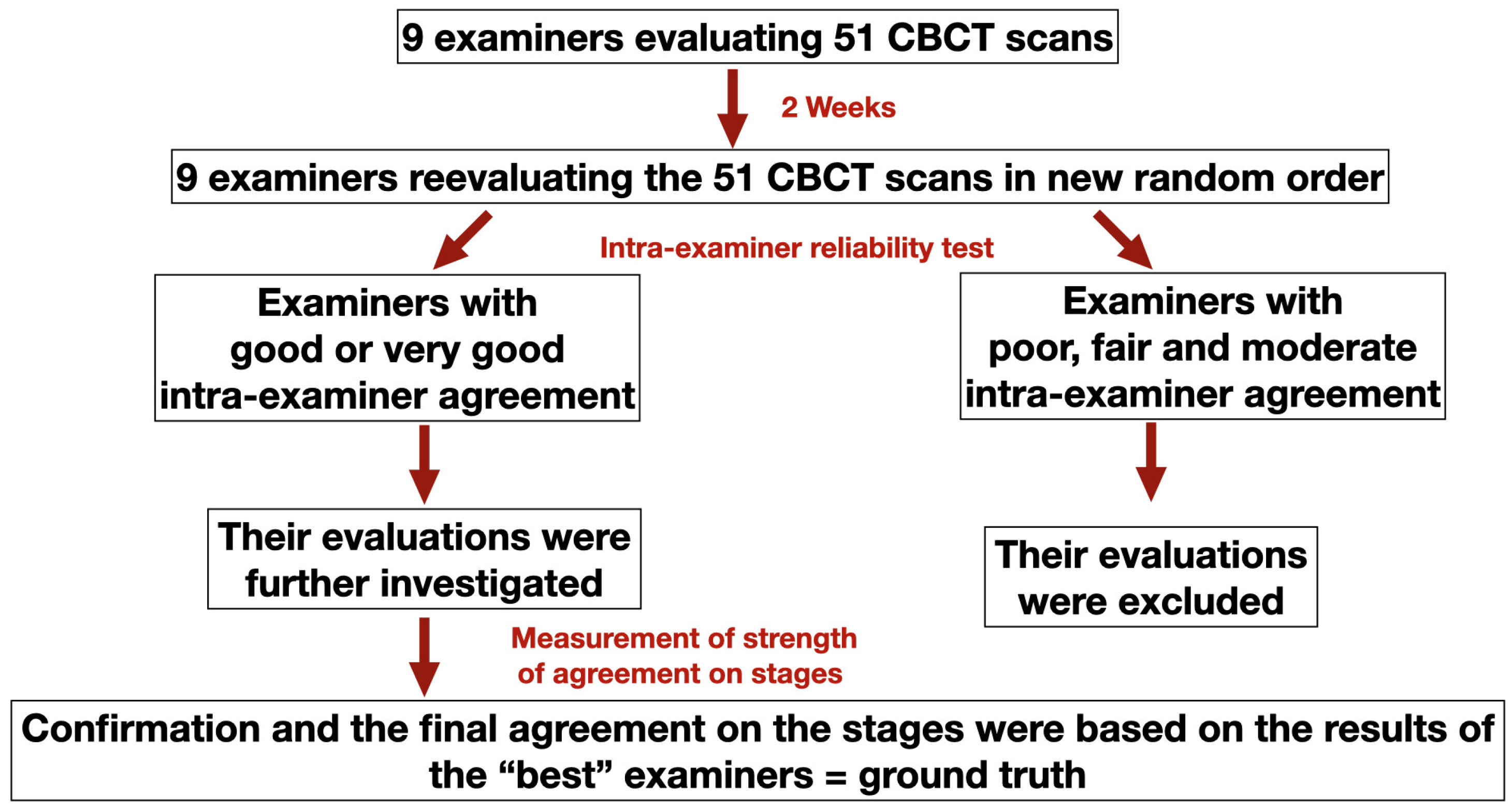
3.1. Intra- and Inter-Examiner Reliability of MPS Maturation
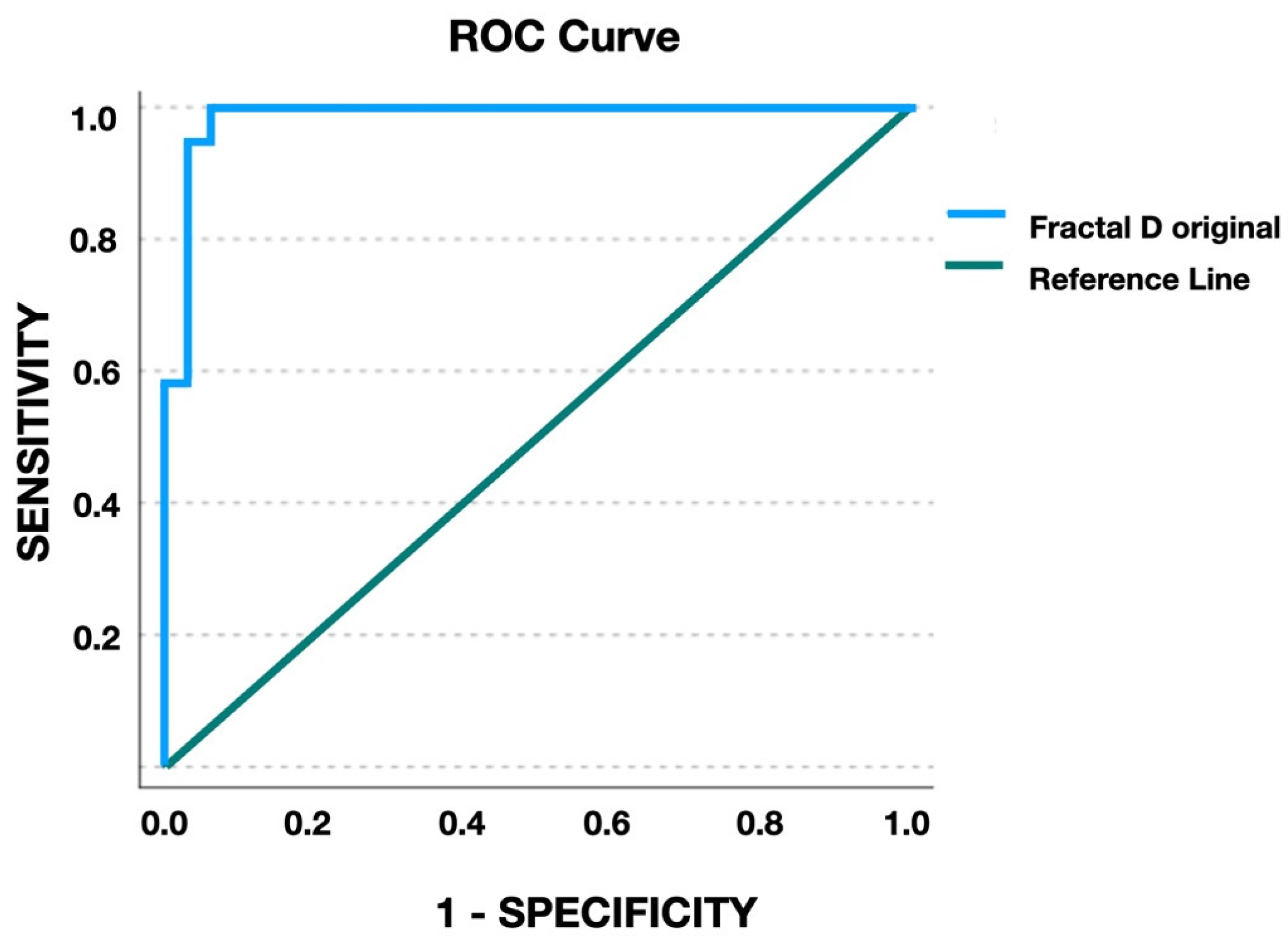
3.2. Agreement on MPS Stages and Fractal Dimension
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McNamara, J.A., Jr. Long-term adaptations to changes in the transverse dimension in children and adolescents: An overview. Am. J. Orthod. Dentofac. Orthop. 2006, 129 (Suppl. S4), S71–S74. [Google Scholar] [CrossRef] [PubMed]
- Lo Giudice, A.; Fastuca, R.; Portelli, M.; Militi, A.; Bellocchio, M.; Spinuzza, P.; Briguglio, F.; Caprioglio, A.; Nucera, R. Effects of rapid vs slow maxillary expansion on nasal cavity dimensions in growing subjects: A methodological and reproducibility study. Eur. J. Paediatr. Dent. 2017, 18, 299–304. [Google Scholar] [PubMed]
- Jimenez-Valdivia, L.M.; Malpartida-Carrillo, V.; Rodríguez-Cárdenas, Y.A.; Dias-Da Silveira, H.L.; Arriola-Guillén, L.E. Midpalatal suture maturation stage assessment in adolescents and young adults using cone-beam computed tomography. Prog. Orthod. 2019, 20, 38. [Google Scholar] [CrossRef] [PubMed]
- Bishara, S.E.; Staley, R.N. Maxillary expansion: Clinical implications. Am. J. Orthod. Dentofac. Orthop. 1987, 91, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Guest, S.S.; McNamara, J.A.; Baccetti, T., Jr.; Franchi, L. Improving Class II malocclusion as a side-effect of rapid maxillary expansion: A prospective clinical study. Am. J. Orthod. Dentofac. Orthop. 2010, 138, 582–591. [Google Scholar] [CrossRef]
- Da Silva Filho, O.G.; Magro, A.C.; Capelozza Filho, L. Early treatment of the Class III malocclusion with rapid maxillary expansion and maxillary protraction. Am. J. Orthod. Dentofac. Orthop. 1998, 113, 196–203. [Google Scholar] [CrossRef]
- Barbosa, N.M.V.; Castro, A.C.; Conti, F.; Capelozza-Filho, L.; Almeida-Pedrin, R.R.; Cardoso, M.A. Reliability and reproducibility of the method of assessment of midpalatal suture maturation: A tomographic study. Angle Orthod. 2019, 89, 71–77. [Google Scholar] [CrossRef]
- Angelieri, F.; Cevidanes, L.H.; Franchi, L.; Gonçalves, J.R.; Benavides, E.; McNamara, J.A., Jr. Midpalatal suture maturation: Classification method for individual assessment before rapid maxillary expansion. Am. J. Orthod. Dentofac. Orthop. 2013, 144, 759–769. [Google Scholar] [CrossRef]
- Knaup, B.; Yildizhan, F.; Wehrbein, H. Age-related changes in the midpalatal suture. J. Orofac. Orthop. 2004, 65, 467–474. [Google Scholar] [CrossRef]
- Korbmacher, H.; Schilling, A.; Püschel, K.; Amling, M.; Kahl-Nieke, B. Age-dependent three-dimensional micro-computed tomography analysis of the human midpalatal suture. J. Orofac. Orthop. 2007, 68, 364–376. [Google Scholar] [CrossRef]
- Persson, M.; Thilander, B. Palatal suture closure in man from 15 to 35 years of age. Am. J. Orthod. 1977, 72, 42–52. [Google Scholar] [CrossRef]
- Grünheid, T.; Larson, C.E.; Larson, B.E. Midpalatal suture density ratio: A novel predictor of skeletal response to rapid maxillary expansion. Am. J. Orthod. Dentofac. Orthop. 2017, 151, 267–276. [Google Scholar] [CrossRef]
- Kawak, K.H.; Kim, S.S.; Kim, Y.I.; Kim, I.D. Quantitative evaluation of midpalatal suture maturation via fractal analysis. Korean J. Orthod. 2016, 46, 323–330. [Google Scholar] [CrossRef]
- Angelieri, F.; Franchi, L.; Cevidanes, L.H.S.; Gonçalves, J.R.; Nieri, M.; Wolford, L.M.; McNamara, J.A., Jr. Cone beam computed tomography evaluation of midpalatal suture maturation in adults. Int. J. Oral. Maxillofac. Surg. 2017, 46, 1557–1561. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, A.; Babay Ahari, S.; Moslemzadeh, H.; Rafighi, A.; Aghazadeh, Z. The reliability of clinical decisions based on the cervical vertebrae maturation staging method. Eur. J. Orthod. 2016, 38, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Dawson, B.; Trapp, R.G. Basic and clinical biostatistics. In Lange Medical Books/McGraw-Hill; Medical Publishing Division: New York, NY, USA, 2004. [Google Scholar]
- Hallgren, K.A. Computing inter-rater reliability for observational data: An overview and tutorial. Tutor. Quant. Methods Psychol. 2012, 8, 23–34. [Google Scholar] [CrossRef]
- Baccetti, T.; Franchi, L.; McNamara, J. The Cervical Vertebral Maturation (CVM) Method for the Assessment of Optimal Treatment Timing in Dentofacial Orthopedics. Semin. Orthod. 2005, 11, 119–129. [Google Scholar] [CrossRef]
- Menon, S.; Manerikar, R.; Sinha, R. Surgical management of transverse maxillary deficiency in adults. J. Maxillofac. Oral Surg. 2010, 9, 241–246. [Google Scholar] [CrossRef]
- Kapetanović, A.; Theodorou, C.I.; Bergé, S.J.; Schols, J.G.J.H.; Xi, T. Efficacy of Miniscrew-Assisted Rapid Palatal Expansion (MARPE) in late adolescents and adults: A systematic review and meta-analysis. Eur. J. Orthod. 2021, 43, 313–323. [Google Scholar] [CrossRef]
- Gonzálvez Moreno, A.M.; Garcovich, D.; Zhou Wu, A.; Alvarado Lorenzo, A.; Bernes Martinez, L.; Aiuto, R.; Dioguardi, M.; Re, D.; Paglia, L.; Adobes Martin, M. Cone Beam Computed Tomography evaluation of midpalatal suture maturationaccording to age and sex: A systematic review. Eur. J. Paediatr. Dent. 2022, 23, 44–50. [Google Scholar]
- Melsen, B.; Melsen, F. The postnatal development of the palatomaxillary region studied on human autopsy material. Am. J. Orthod. 1982, 82, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Betts, N.J.; Vanarsdall, R.L.; Barber, H.D.; Higgins-Barber, K.; Fonseca, R.J. Diagnosis and treatment of transverse maxillary deficiency. Int. J. Adult Orthod. Orthognath. Surg. 1995, 10, 75–96. [Google Scholar]
- Rungcharassaeng, K.; Caruso, J.M.; Kan, J.Y.; Kim, J.; Taylor, G. Factors affecting buccal bone changes of maxillary posterior teeth after rapid maxillary expansion. Am. J. Orthod. Dentofac. Orthop. 2007, 132, 428.e1–428.e8. [Google Scholar] [CrossRef]
- Garib, D.G.; Henriques, J.F.; Janson, G.; de Freitas, M.R.; Fernandes, A.Y. Periodontal effects of rapid maxillary expansion with tooth-tissue-borne and tooth-borne expanders: A computed tomography evaluation. Am. J. Orthod. Dentofac. Orthop. 2006, 129, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Garrett, B.J.; Caruso, J.M.; Rungcharassaeng, K.; Farrage, J.R.; Kim, J.S.; Taylor, G.D. Skeletal effects to the maxilla after rapid maxillary expansion assessed with cone-beam computed tomography. Am. J. Orthod. Dentofac. Orthop. 2008, 134, 8–9. [Google Scholar] [CrossRef]
- Sayar, G.; Kılınç, D.D. Rapid maxillary expansion outcomes according to midpalatal suture maturationlevels. Prog. Orthod. 2019, 20, 27. [Google Scholar] [CrossRef] [PubMed]
- Shayani, A.; Vidal, P.S.; Carrasco, I.G.; Gerlach, M.M. Midpalatal Suture Maturation Method for the Assessment of Maturation before Maxillary Expansion: A Systematic Review. Diagnostics 2022, 12, 2774. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Chen, Z.; Zang, L.; Sun, Z.; Wang, Q.; Yu, G. Midpalatal Suture CBCT Image Quantitive Characteristics Analysis Based on Machine Learning Algorithm Construction and Optimization. Bioengineering 2022, 9, 316. [Google Scholar] [CrossRef]
- Sánchez, I.; Uzcátegui, G. Fractals in dentistry. J. Dent. 2011, 39, 273–292. [Google Scholar] [CrossRef]
- Huang, C.C.; Chen, J.C.; Chang, Y.C.; Jeng, J.H.; Chen, C.M. A fractal dimensional approach to successful evaluation of apical healing. Int. Endod. J. 2013, 46, 523–529. [Google Scholar] [CrossRef]
- Güngör, E.; Yildirim, D.; Çevik, R. Evaluation of osteoporosis in jaw bones using cone beam CT and dual-energy X-ray absorptiometry. J. Oral Sci. 2016, 58, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Umemori, S.; Tonami, K.; Nitta, H.; Mataki, S.; Araki, K. The possibility of digital imaging in the diagnosis of occlusal caries. Int. J. Dent. 2010, 2010, 860515. [Google Scholar] [CrossRef] [PubMed]
- Kato, C.N.; Barra, S.G.; Tavares, N.P.; Amaral, T.M.; Brasileiro, C.B.; Mesquita, R.A.; Abreu, L.G. Use of fractal analysis in dental images: A systematic review. Dentomaxillofac. Radiol. 2020, 49, 20180457. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Lee, J.; Chung, C.Y.; Ahn, S.; Sung, K.H.; Kim, T.W.; Lee, H.J.; Park, M.S. Pitfalls and important issues in testing reliability using intraclass correlation coefficients in orthopaedic research. Clin. Orthop. Surg. 2012, 4, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Isfeld, D.; Lagravere, M.; Leon-Salazar, V.; Flores-Mir, C. Novel methodologies and technologies to assess mid-palatal suturematuration: A systematic review. Head. Face Med. 2017, 13, 13. [Google Scholar] [CrossRef]

| Men | Women | |
|---|---|---|
| Number of subjects | 19 | 32 |
| Average age (years) | 21.79 ± 6.6 | 23.1 ± 6.26 |
| Age 9–15 (years) | 3 | 3 |
| Age 16–20 (years) | 6 | 8 |
| Age 21–25 (years) | 6 | 10 |
| Age 26–30 (years) | 2 | 8 |
| Age 31–40 (years) | 2 | 3 |
| Value of Kappa | Interpretation (Strength of Agreement) |
|---|---|
| 0 | No agreement |
| <0.20 | Poor |
| 0.21–0.40 | Fair |
| 0.41–0.60 | Moderate |
| 0.61–0.80 | Good |
| 0.81–1.00 | Very good |
| Strength of Agreement (Kappa Value) | |
|---|---|
| Test # 1 | 0.268 (Fair) |
| Test # 2 | 0.349 (Fair) |
| Examiner | Strength of Agreement (Kappa Value) |
|---|---|
| 1 | 0.625 (Good) |
| 2 | 0.631 (Good) |
| 3 | 0.412 (Moderate) |
| 4 | 0.711 (Good) |
| 5 | 0.306 (Fair) |
| 6 | 0.138 (Poor) |
| 7 | 0.381 (Moderate) |
| 8 | 0.126 (Poor) |
| 9 | 0.715 (Good) |
| MPS Stage | Strength of Agreement on Individual Stages |
|---|---|
| Stage A | 0.345 |
| Stage B | 0.460 |
| Stage C | 0.474 |
| Stage E | 0.438 |
| Stage D | 0.690 |
| MPS Stage | Fractal Dimension (mean ± SD) | 95% CI Lower Bound | 95% CI Upper Bound |
|---|---|---|---|
| Stage A | 1.267 ± 0.015 | 1.133 | 1.399 |
| Stage B | 1.197 ± 0.039 | 1.161 | 1.233 |
| Stage C | 1.095 ± 0.29 | 1.082 | 1.108 |
| Stage E | 1.017 ± 0.019 | 1.001 | 1.029 |
| Stage D | 0.973 ± 0.034 | 0.938 | 1.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darawsheh, A.F.; Kolarovszki, B.; Hong, D.H.; Farkas, N.; Taheri, S.; Frank, D. Applicability of Fractal Analysis for Quantitative Evaluation of Midpalatal Suture Maturation. J. Clin. Med. 2023, 12, 4189. https://doi.org/10.3390/jcm12134189
Darawsheh AF, Kolarovszki B, Hong DH, Farkas N, Taheri S, Frank D. Applicability of Fractal Analysis for Quantitative Evaluation of Midpalatal Suture Maturation. Journal of Clinical Medicine. 2023; 12(13):4189. https://doi.org/10.3390/jcm12134189
Chicago/Turabian StyleDarawsheh, Ali Farid, Béla Kolarovszki, Da Hye Hong, Nelli Farkas, Soroush Taheri, and Dorottya Frank. 2023. "Applicability of Fractal Analysis for Quantitative Evaluation of Midpalatal Suture Maturation" Journal of Clinical Medicine 12, no. 13: 4189. https://doi.org/10.3390/jcm12134189
APA StyleDarawsheh, A. F., Kolarovszki, B., Hong, D. H., Farkas, N., Taheri, S., & Frank, D. (2023). Applicability of Fractal Analysis for Quantitative Evaluation of Midpalatal Suture Maturation. Journal of Clinical Medicine, 12(13), 4189. https://doi.org/10.3390/jcm12134189







