Analysis of Electrocardiographic Criteria of Right Ventricular Hypertrophy in Patients with Chronic Thromboembolic Pulmonary Hypertension before and after Balloon Pulmonary Angioplasty
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design
Division into Subgroups
2.2. Electrocardiogram
Main Parameters
2.3. Right Heart Catheterization
2.4. Balloon Pulmonary Angioplasty
2.5. Statistics
3. Results
3.1. Patient Data
3.2. Haemodynamic Data
3.3. Electrocardiographic Data
3.3.1. General Parameters
3.3.2. QRS Axis
3.3.3. Atrial Parameters
3.3.4. Ventricular Parameters
3.3.5. Repolarisation Parameters
3.3.6. Main Parameters
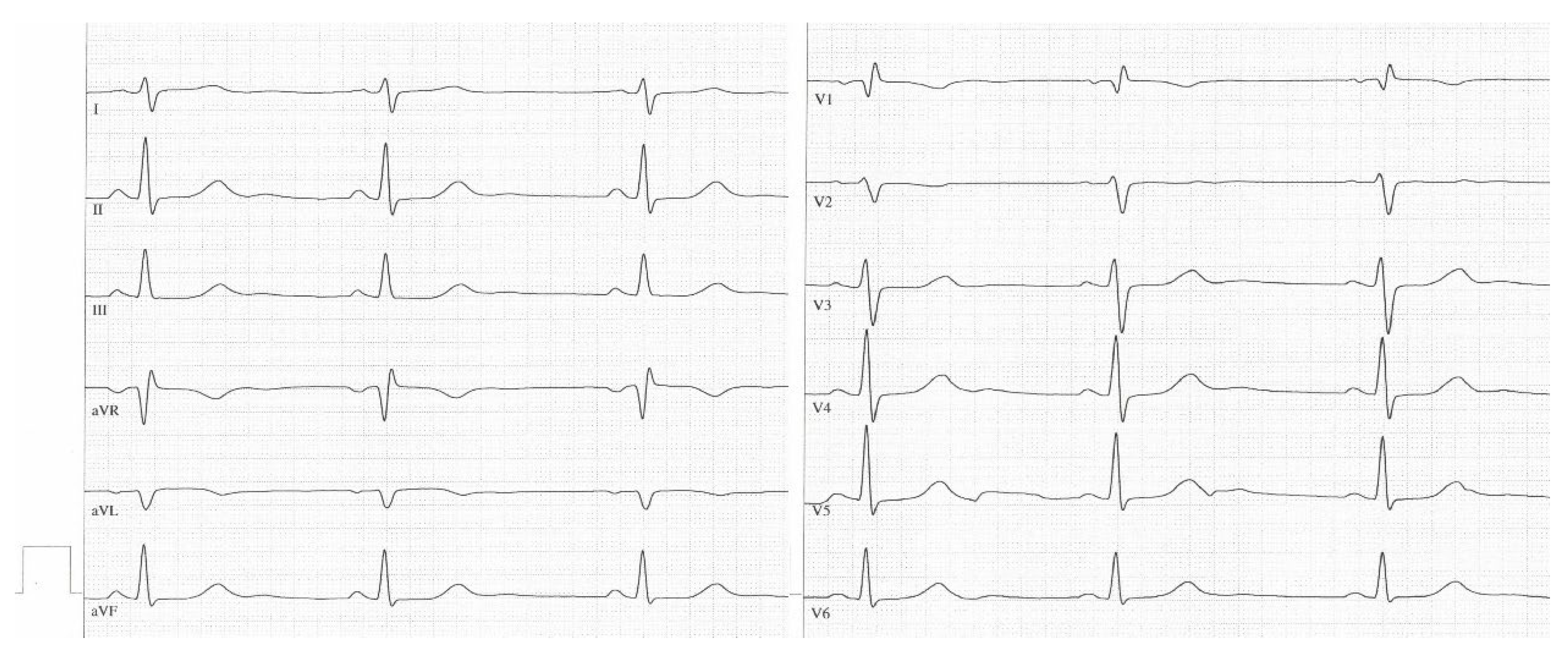
3.3.7. Sample ECGs
3.4. Correlation Analysis
3.5. Subgroup Analysis
3.6. Role of the Main Parameter “R V1, V2 + SI, aVL − S V1”
4. Discussion
4.1. Main Parameters in the Current Literature
4.2. Role of the Main Parameter “R V1, V2 + S I, aVL − S V1”
4.3. The Role of the Subgroups
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef] [PubMed]
- Simonneau, G.; Torbicki, A.; Dorfmüller, P.; Kim, N. The pathophysiology of chronic thromboembolic pulmonary hypertension. Eur. Respir. Rev. 2017, 26, 160112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pepke-Zaba, J.; Delcroix, M.; Lang, I.; Mayer, E.; Jansa, P.; Ambroz, D.; Treacy, C.; D’Armini, A.M.; Morsolini, M.; Snijder, R.; et al. Chronic thromboembolic pulmonary hypertension (CTEPH): Results from an international prospective registry. Circulation 2011, 124, 1973–1981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghofrani, H.-A.; D’Armini, A.M.; Kim, N.H.; Mayer, E.; Simonneau, G. Interventional and pharmacological management of chronic thromboembolic pulmonary hypertension. Respir. Med. 2021, 177, 106293. [Google Scholar] [CrossRef] [PubMed]
- Asano, R.; Ogo, T.; Ohta-Ogo, K.; Fukui, S.; Tsuji, A.; Ueda, J.; Konagai, N.; Fukuda, T.; Morita, Y.; Noguchi, T.; et al. Prolonged QRS duration as a predictor of right ventricular dysfunction after balloon pulmonary angioplasty. Int. J. Cardiol. 2019, 280, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Piłka, M.; Darocha, S.; Banaszkiewicz, M.; Florczyk, M.; Wieteska, M.; Dobosiewicz, A.; Mańczak, M.; Mańczak, R.; Pietrasik, A.; Pietura, R.; et al. The evolution of electrocardiographic signs of right ventricular overload after balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension. Pol. Arch. Intern. Med. 2019, 129, 451–459. [Google Scholar] [CrossRef] [Green Version]
- Nishiyama, T.; Takatsuki, S.; Kawakami, T.; Katsumata, Y.; Kimura, T.; Kataoka, M.; Tsuruta, H.; Itabashi, Y.; Murata, M.; Yuasa, S.; et al. Improvement in the electrocardiograms associated with right ventricular hypertrophy after balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension. Int. J. Cardiol. Heart Vasc. 2018, 19, 75–82. [Google Scholar] [CrossRef]
- Yokokawa, T.; Sugimoto, K.; Nakazato, K.; Misaka, T.; Oikawa, M.; Kobayashi, A.; Yoshihisa, A.; Yamaki, T.; Kunii, H.; Ishida, T.; et al. Electrocardiographic Criteria of Right Ventricular Hypertrophy in Patients with Chronic Thromboembolic Pulmonary Hypertension after Balloon Pulmonary Angioplasty. Intern. Med. 2019, 58, 2139–2144. [Google Scholar] [CrossRef] [Green Version]
- Galiè, N.; Humbert, M.; Vachiery, J.-L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Noordegraaf, A.V.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Respir. J. 2015, 46, 903–975. [Google Scholar] [CrossRef]
- Kriechbaum, S.D.; Wiedenroth, C.B.; Wolter, J.S.; Hütz, R.; Haas, M.; Breithecker, A.; Roller, F.C.; Keller, T.; Guth, S.; Rolf, A.; et al. N-terminal pro-B-type natriuretic peptide for monitoring after balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2018, 37, 639–646. [Google Scholar] [CrossRef]
- Kriechbaum, S.D.; Wiedenroth, C.B.; Hesse, M.L.; Ajnwojner, R.; Keller, T.; Wolter, J.S.; Haas, M.; Roller, F.C.; Breithecker, A.; Rieth, A.J.; et al. Development of renal function during staged balloon pulmonary angioplasty for inoperable chronic thromboembolic pulmonary hypertension. Scand. J. Clin. Lab. Investig. 2019, 79, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Kriechbaum, S.D.; Wiedenroth, C.B.; Keller, T.; Wolter, J.S.; Ajnwojner, R.; Peters, K.; Haas, M.A.; Roller, F.C.; Breithecker, A.; Rieth, A.J.; et al. Dynamics of high-sensitivity cardiac troponin T during therapy with balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. PLoS ONE 2018, 13, e0204683. [Google Scholar] [CrossRef] [PubMed]
- Kriechbaum, S.D.; Wiedenroth, C.B.; Peters, K.; Barde, M.A.; Ajnwojner, R.; Wolter, J.-S.; Haas, M.; Roller, F.C.; Guth, S.; Rieth, A.J.; et al. Galectin-3, GDF-15, and sST2 for the assessment of disease severity and therapy response in patients suffering from inoperable chronic thromboembolic pulmonary hypertension. Biomark. Biochem. Indic. Expo. Response Susceptibility Chem. 2020, 25, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Olsson, K.M.; Wiedenroth, C.B.; Kamp, J.-C.; Breithecker, A.; Fuge, J.; Krombach, G.A.; Haas, M.; Hamm, C.; Kramm, T.; Guth, S.; et al. Balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension: The initial German experience. Eur. Respir. J. 2017, 49, 1602409. [Google Scholar] [CrossRef] [PubMed]
- Roller, F.C.; Kriechbaum, S.; Breithecker, A.; Liebetrau, C.; Haas, M.; Schneider, C.; Rolf, A.; Guth, S.; Mayer, E.; Hamm, C.; et al. Correlation of native T1 mapping with right ventricular function and pulmonary haemodynamics in patients with chronic thromboembolic pulmonary hypertension before and after balloon pulmonary angioplasty. Eur. Radiol. 2019, 29, 1565–1573. [Google Scholar] [CrossRef]
- Roller, F.C.; Schüssler, A.; Hasse, A.; Kriechbaum, S.; Richter, M.; Guth, S.; Tello, K.; Breithecker, A.; Liebetrau, C.; Hamm, C.W.; et al. Effects of BPA on right ventricular mechanical dysfunction in patients with inoperable CTEPH—A cardiac magnetic resonance study. Eur. J. Radiol. 2022, 147, 110111. [Google Scholar] [CrossRef]
- Wiedenroth, C.B.; Rieth, A.J.; Kriechbaum, S.; Ghofrani, H.A.; Breithecker, A.; Haas, M.; Roller, F.; Richter, M.J.; Lankeit, M.; Mielzarek, L.; et al. Exercise right heart catheterization before and after balloon pulmonary angioplasty in inoperable patients with chronic thromboembolic pulmonary hypertension. Pulm. Circ. 2020, 10, 2045894020917884. [Google Scholar] [CrossRef] [Green Version]
- Wiedenroth, C.B.; Olsson, K.M.; Guth, S.; Breithecker, A.; Haas, M.; Kamp, J.; Fuge, J.; Hinrichs, J.B.; Roller, F.; Hamm, C.W.; et al. Balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic disease. Pulm. Circ. 2018, 8, 2045893217753122. [Google Scholar] [CrossRef] [Green Version]
- Hancock, E.W.; Deal, B.J.; Mirvis, D.M.; Okin, P.; Kligfield, P.; Gettes, L.S.; Bailey, J.J.; Childers, R.; Gorgels, A.; Josephson, M.; et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: Part V: Electrocardiogram changes associated with cardiac chamber hypertrophy: A scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J. Am. Coll. Cardiol. 2009, 53, 992–1002. [Google Scholar] [CrossRef] [Green Version]
- Piłka, M.; Darocha, S.; Banaszkiewicz, M.; Wieteska-Miłek, M.; Mańczak, M.; Mańczak, R.; Kędzierski, P.; Florczyk, M.; Dobosiewicz, A.; Torbicki, A.; et al. Assessment of electrocardiographic markers of acute and long-term hemodynamic improvement in patients with pulmonary hypertension. Ann. Noninvasive Electrocardiol. 2020, 25, e12758. [Google Scholar] [CrossRef] [Green Version]
- Igata, S.; Tahara, N.; Sugiyama, Y.; Bekki, M.; Kumanomido, J.; Tahara, A.; Honda, A.; Maeda, S.; Nashiki, K.; Nakamura, T.; et al. Utility of the amplitude of RV1+SV5/6 in assessment of pulmonary hypertension. PLoS ONE 2018, 13, e0206856. [Google Scholar] [CrossRef]
- Sato, S.; Ogawa, A.; Matsubara, H. Change in R wave in lead V1 predicts survival of patients with pulmonary arterial hypertension. Pulm. Circ. 2018, 8, 2045894018776496. [Google Scholar] [CrossRef] [Green Version]
- Waligóra, M.; Kopeć, G.; Jonas, K.; Tyrka, A.; Sarnecka, A.; Miszalski-Jamka, T.; Urbańczyk-Zawadzka, M.; Podolec, P. Mechanism and prognostic role of qR in V1 in patients with pulmonary arterial hypertension. J. Electrocardiol. 2017, 50, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Waligóra, M.; Tyrka, A.; Podolec, P.; Kopeć, G. ECG Markers of Hemodynamic Improvement in Patients with Pulmonary Hypertension. Biomed. Res. Int. 2018, 2018, 4606053. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.-L.; He, J.-G.; Liu, Z.-H.; Gu, Q.; Ni, X.-H.; Zhao, Z.-H.; Luo, Q.; Xiong, C.-M. The Value of the Electrocardiogram for Evaluating Prognosis in Patients with Idiopathic Pulmonary Arterial Hypertension. Lung 2017, 195, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Ghio, S.; Turco, A.; Klersy, C.; Scelsi, L.; Raineri, C.; Crescio, V.; Viscardi, A.; Grazioli, V.; Sciortino, A.; Visconti, L.O.; et al. Changes in surface electrocardiogram in patients with chronic thromboembolic pulmonary hypertension undergoing pulmonary endarterectomy. Correlations with hemodynamic and echocardiographic improvements after surgery. J. Electrocardiol. 2016, 49, 223–230. [Google Scholar] [CrossRef]
- Tonelli, A.R.; Baumgartner, M.; Alkukhun, L.; Minai, O.A.; Dweik, R.A. Electrocardiography at diagnosis and close to the time of death in pulmonary arterial hypertension. Ann. Noninvasive Electrocardiol. 2014, 19, 258–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopeć, G. Electrocardiography in pulmonary hypertension. Pol. Arch. Intern. Med. 2019, 129, 440–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagai, T.; Kohsaka, S.; Murata, M.; Okuda, S.; Anzai, T.; Fukuda, K.; Satoh, T. Significance of electrocardiographic right ventricular hypertrophy in patients with pulmonary hypertension with or without right ventricular systolic dysfunction. Intern. Med. 2012, 51, 2277–2283. [Google Scholar] [CrossRef] [Green Version]
- Henkens, I.R.; Gan, C.T.-J.; van Wolferen, S.A.; Hew, M.; Boonstra, A.; Twisk, J.W.R.; Kamp, O.; van der Wall, E.E.; Schalij, M.J.; Noordegraaf, A.V.; et al. ECG monitoring of treatment response in pulmonary arterial hypertension patients. Chest 2008, 134, 1250–1257. [Google Scholar] [CrossRef]
- Henkens, I.R.; Mouchaers, K.T.B.; Vonk-Noordegraaf, A.; Boonstra, A.; Swenne, C.A.; Maan, A.C.; Man, S.-C.; Twisk, J.W.R.; Van Der Wall, E.E.; Schalij, M.J.; et al. Improved ECG detection of presence and severity of right ventricular pressure load validated with cardiac magnetic resonance imaging. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H2150–H2157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Naamani, K.; Hijal, T.; Nguyen, V.; Andrew, S.; Nguyen, T.; Huynh, T. Predictive values of the electrocardiogram in diagnosing pulmonary hypertension. Int. J. Cardiol. 2008, 127, 214–218. [Google Scholar] [CrossRef]
- Ahearn, G.S.; Tapson, V.F.; Rebeiz, A.; Greenfield, J.C. Electrocardiography to define clinical status in primary pulmonary hypertension and pulmonary arterial hypertension secondary to collagen vascular disease. Chest 2002, 122, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Bossone, E.; Paciocco, G.; Iarussi, D.; Agretto, A.; Iacono, A.; Gillespie, B.W.; Rubenfire, M. The prognostic role of the ECG in primary pulmonary hypertension. Chest 2002, 121, 513–518. [Google Scholar] [CrossRef]
- Goldman, M.J. Principles of Clinical Electrocardiography, 9th ed.; Lange Medical Publications: Los Altos, CA, USA, 1976. [Google Scholar]
- Schamroth, L. An Introduction to Electrocardiography, 6th ed.; Blackwell: Oxford, UK, 1982. [Google Scholar]
- Kopeć, G.; Tyrka, A.; Miszalski-Jamka, T.; Sobien, M.; Waligóra, M.; Brózda, M.; Podolec, P. Electrocardiogram for the diagnosis of right ventricular hypertrophy and dilation in idiopathic pulmonary arterial hypertension. Circ. J. Off. J. Jpn. Circ. Soc. 2012, 76, 1744–1749. [Google Scholar] [CrossRef] [Green Version]
- Sławek-Szmyt, S.; Araszkiewicz, A.; Jankiewicz, S.; Smukowska-Gorynia, A.; Grygier, M.; Janus, M.; Lesiak, M.; Mularek-Kubzdela, T. Association of Electrocardiographic Signs of Right Ventricular Hypertrophy and Clot Localization in Chronic Thromboembolic Pulmonary Hypertension. J. Clin. Med. 2022, 11, 625. [Google Scholar] [CrossRef]
- Bossone, E.; Butera, G.; Bodini, B.D.; Rubenfire, M. The interpretation of the electrocardiogram in patients with pulmonary hypertension: The need for clinical correlation. Ital. Heart J. 2003, 4, 850–854. [Google Scholar] [PubMed]
- Macfarlane, P.W.; van Oosterom, A.; Pahlm, O.; Kligfield, P.; Janse, M.; Camm, J. (Eds.) Comprehensive Electrocardiology, 2nd ed.; Springer: London, UK, 2010. [Google Scholar]
- Pollack, M.L. ECG manifestations of selected extracardiac diseases. Emerg. Med. Clin. N. Am. 2006, 24, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Surawicz, B.; Knilans, T.K. Chou’s Electrocardiography in Clinical Practice; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Lewczuk, J.; Ajlan, A.W.; Piszko, P.; Jagas, J.; Mikulewicz, M.; Wrabec, K. Electrocardiographic signs of right ventricular overload in patients who underwent pulmonary embolism event(s). Are they useful in diagnosis of chronic thromboembolic pulmonary hypertension? J. Electrocardiol. 2004, 37, 219–225. [Google Scholar] [CrossRef]
- Lewis, C.; Lambiase, P. The electrocardiogram in pulmonary hypertension. Br. J. Hosp. Med. 2005, 66, M62–M63. [Google Scholar] [CrossRef]
- Medvegy, M.; Antalóczy, Z.; Préda, I. Connection between right ventricular pressure and the ECG. J. Electrocardiol. 1994, 27, 23–27. [Google Scholar] [CrossRef]
- Frost, A.; Badesch, D.; Gibbs, J.S.R.; Gopalan, D.; Khanna, D.; Manes, A.; Oudiz, R.; Satoh, T.; Torres, F.; Torbicki, A. Diagnosis of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blyth, K.G.; Kinsella, J.; Hakacova, N.; McLure, L.E.; Siddiqui, A.M.; Wagner, G.S.; Peacock, A.J. Quantitative estimation of right ventricular hypertrophy using ECG criteria in patients with pulmonary hypertension: A comparison with cardiac MRI. Pulm. Circ. 2011, 1, 470–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miura, M.; Ikeda, S.; Yoshida, T.; Yamagata, Y.; Nakata, T.; Koga, S.; Koidet, Y.; Kalwano, H.; Maemura, K. Deeper S Wave in Lead V5 and Broader Extent of T Wave Inversions in the Precordial Leads are Clinically Useful Electrocardiographic Parameters for Predicting Pulmonary Hypertension. Int. Heart J. 2018, 59, 136–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Baseline | Follow-Up | |
|---|---|---|
| sex, m/f, n (%) | 71 (47.3%), 79 (52.7%) | 71 (47.3%), 79 (52.7%) |
| age, years, median (IQR) | 63.5 (18.8) | 65.3 (18.6) |
| age, m/f, years, median (IQR) | 60.6 (19.9)/65.8 (15.8) | 62.6 (20.7)/67.1 (15.8) |
| Baseline | Follow-Up | p | |
|---|---|---|---|
| RAP, mmHg, median (IQR) | 6 (4) | 5 (3) | <0.001 |
| mPAP, mmHg, median (IQR) | 40 (13.8) | 29 (12) | <0.001 |
| CO, L/min, median (IQR) | 4.7 (1.6) | 5 (1.4) | 0.004 |
| PVR, dyn∗sec∗cm−5, median (IQR) | 536 (312) | 304 (206) | <0.001 |
| ECG Parameter | Baseline | Follow-Up | p-Value |
|---|---|---|---|
| Sinus rhythm, n (%) | 148 (99%) | 143 (95%) | 0.025 |
| Heart rate, bpm, median (IQR) | 84 (21.8) | 78 (19) | <0.001 |
| QRS axis > 90°, n (%) | 63 (42%) | 34 (23%) | <0.001 |
| QRS axis > 120°, n (%) | 23 (12%) | 10 (7%) | 0.002 |
| SISIISIII type, n (%) | 11 (7%) | 12 (8%) | 0.705 |
| SIQIII type, n (%) | 10 (7%) | 2 (1%) | 0.005 |
| QRS axis associated with right heart strain, n (%) | 84 (56%) | 48 (32%) | <0.001 |
| Left axis deviation, n (%) | 12 (8%) | 22 (15%) | 0.004 |
| Normal QRS axis, n (%) | 53 (35%) | 80 (53%) | <0.001 |
| P dextroatriale, n (%) | 60 (40%) | 24 (16%) | <0.001 |
| P biatriale, n (%) | 10 (7%) | 3 (2%) | 0.008 |
| P-wave amplitude in II, mV, median (IQR) | 0.2 (0.1) | 0.2 (0.05) | <0.001 |
| Right ventricular hypertrophy (Sokolow–Lyon index), n (%) | 67 (45%) | 40 (27%) | <0.001 |
| Biventricular hypertrophy (Sokolow–Lyon index), n (%) | 0 (0%) | 1 (1%) | |
| qR pattern in V1, n (%) | 24 (16%) | 19 (13%) | 0.297 |
| Right bundle branch block, n (%) | 50 (33%) | 42 (28%) | 0.074 |
| Incomplete right bundle branch block, n (%) | 28 (19%) | 23 (15%) | 0.275 |
| Complete right bundle branch block, n (%) | 22 (15%) | 19 (13%) | 0.405 |
| R-wave amplitude in V1, mV, median (IQR) | 0.3 (0.35) | 0.2 (0.25) | <0.001 |
| R-wave amplitude in V2, mV, median (IQR) | 0.3 (0.3) | 0.25 (0.25) | 0.003 |
| S-wave amplitude in V5, mV, median (IQR) | 0.5 (0.45) | 0.4 (0.4) | <0.001 |
| S-wave amplitude in V6, mV, median (IQR) | 0.3 (0.4) | 0.2 (0.29) | <0.001 |
| R/S in V1, median (IQR) | 1 (2) | 0.4 (0.8) | <0.001 |
| R/S in V5, median (IQR) | 1.7 (2) | 2.4 (2.7) | <0.001 |
| R/S in V6, median (IQR) | 2.4 (2.6) | 3.3 (4.4) | <0.001 |
| R V1, V2 + S I, V6 − S V1, mV, median (IQR) | 0.6 (0.89) | 0.28 (0.7) | <0.001 |
| R V1 + S V5, V6, mV, median (IQR) | 0.85 (0.73) | 0.65 (0.56) | <0.001 |
| R peak time V1 (QRS duration < 120 ms), ms, median (IQR) | 50 (30) | 43 (40) | <0.001 |
| QT interval, ms, median (IQR) | 390 (70) | 380 (40) | <0.001 |
| QTc interval (Bazett), ms, median (IQR) | 454 (85) | 432 (44) | <0.001 |
| T-wave inversion in II, n (%) | 46 (31%) | 19 (13%) | <0.001 |
| T-wave inversion in III, n (%) | 73 (49%) | 49 (33%) | <0.001 |
| T-wave inversion in aVF, n (%) | 61 (41%) | 26 (17%) | <0.001 |
| T-wave inversion in V1, n (%) | 130 (87%) | 133 (89%) | 0.414 |
| T-wave inversion in V2, n (%) | 76 (51%) | 68 (45%) | 0.144 |
| T-wave inversion in V3, n (%) | 90 (60%) | 66 (44%) | <0.001 |
| ECG Parameter | Cut-Off Value | Baseline | Follow-Up | p-Value |
|---|---|---|---|---|
| P-wave amplitude in II, n (%) | ≥0.25 mV | 48 (32%) | 17 (11%) | <0.001 |
| R-wave amplitude in V1, n (%) | >0.6 mV | 22 (15%) | 13 (9%) | 0.029 |
| S-wave amplitude in V5, n (%) | >1.0 mV | 13 (9%) | 5 (3%) | 0.021 |
| S-wave amplitude in V6, n (%) | >0.3 mV | 68 (45%) | 44 (29%) | <0.001 |
| R/S in V1, n (%) | >1 | 55 (37%) | 25 (17%) | <0.001 |
| R/S in V5, n (%) | <0.75 | 18 (12%) | 13 (9%) | 0.251 |
| R/S in V6, n (%) | <0.4 | 3 (2%) | 2 (1%) | 0.655 |
| R V1, V2 + S I, V6 − S V1, n (%) | >0.6 mV | 69 (46%) | 40 (27%) | <0.001 |
| R V1 + S V5, V6, n (%) | >1.05 mV | 48 (32%) | 27 (18%) | <0.001 |
| R peak time V1 (QRS duration < 120 ms), n (%) | >35 ms | 86 (57%) | 67 (45%) | 0.002 |
| ECG Parameter | Cut-Off Value | Baseline | Follow-Up | p-Value |
|---|---|---|---|---|
| S > R or S > 40 ms in I, II, III, n (%) | positive | 104 (69%) | 81 (54%) | <0.001 * |
| S > R or S > 40 ms in V6, n (%) | positive | 35 (23%) | 29 (19%) | 0.157 |
| R/S V1 > R/S V3, V4, n (%) | positive | 36 (24%) | 15 (10%) | <0.001 * |
| R/S V5: R/S V1, n (%) | <0.04 | 0 (0%) | 0 (0%) | |
| (RI + SIII) − (SI + RIII), n (%) | <1.5 mV | 148 (99%) | 147 (98%) | 0.655 |
| R V1, V2 + S I, aVL − S V1, n (%) | >0.6 mV | 71 (47%) | 44 (29%) | <0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ley, L.; Wiedenroth, C.B.; Ghofrani, H.A.; Hoeltgen, R.; Bandorski, D. Analysis of Electrocardiographic Criteria of Right Ventricular Hypertrophy in Patients with Chronic Thromboembolic Pulmonary Hypertension before and after Balloon Pulmonary Angioplasty. J. Clin. Med. 2023, 12, 4196. https://doi.org/10.3390/jcm12134196
Ley L, Wiedenroth CB, Ghofrani HA, Hoeltgen R, Bandorski D. Analysis of Electrocardiographic Criteria of Right Ventricular Hypertrophy in Patients with Chronic Thromboembolic Pulmonary Hypertension before and after Balloon Pulmonary Angioplasty. Journal of Clinical Medicine. 2023; 12(13):4196. https://doi.org/10.3390/jcm12134196
Chicago/Turabian StyleLey, Lukas, Christoph B. Wiedenroth, Hossein Ardeschir Ghofrani, Reinhard Hoeltgen, and Dirk Bandorski. 2023. "Analysis of Electrocardiographic Criteria of Right Ventricular Hypertrophy in Patients with Chronic Thromboembolic Pulmonary Hypertension before and after Balloon Pulmonary Angioplasty" Journal of Clinical Medicine 12, no. 13: 4196. https://doi.org/10.3390/jcm12134196
APA StyleLey, L., Wiedenroth, C. B., Ghofrani, H. A., Hoeltgen, R., & Bandorski, D. (2023). Analysis of Electrocardiographic Criteria of Right Ventricular Hypertrophy in Patients with Chronic Thromboembolic Pulmonary Hypertension before and after Balloon Pulmonary Angioplasty. Journal of Clinical Medicine, 12(13), 4196. https://doi.org/10.3390/jcm12134196







