Cerebral Oxygenation Responses to Standing in Young Patients with Vasovagal Syncope
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting and Participants
2.2. Inclusion and Exclusion Criteria
2.3. Active Stand Testing
2.3.1. Protocol
2.3.2. Equipment
2.3.3. Cohort Clinical Characteristics
2.4. Data Analysis
2.4.1. Signal Processing
2.4.2. Statistical Analysis
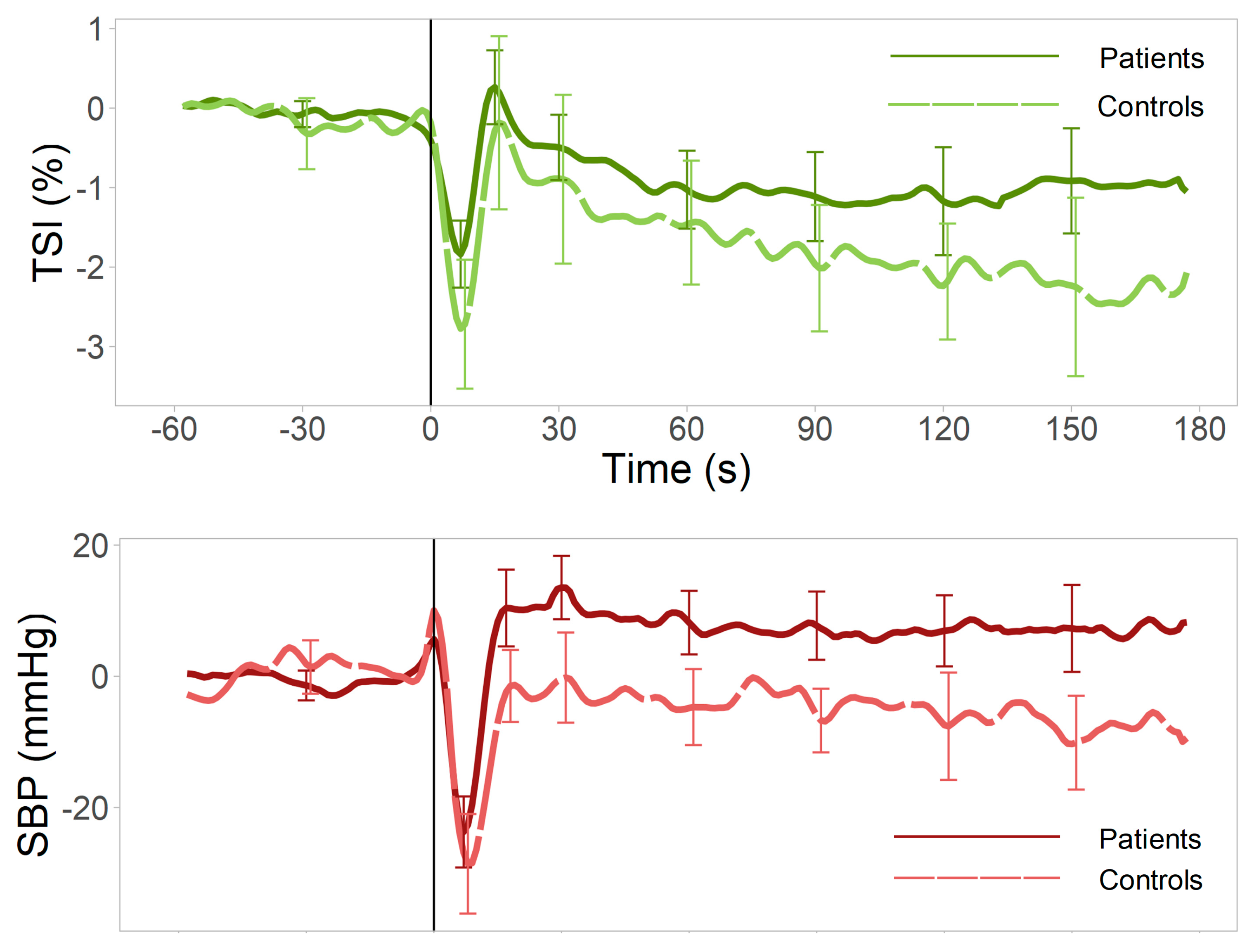
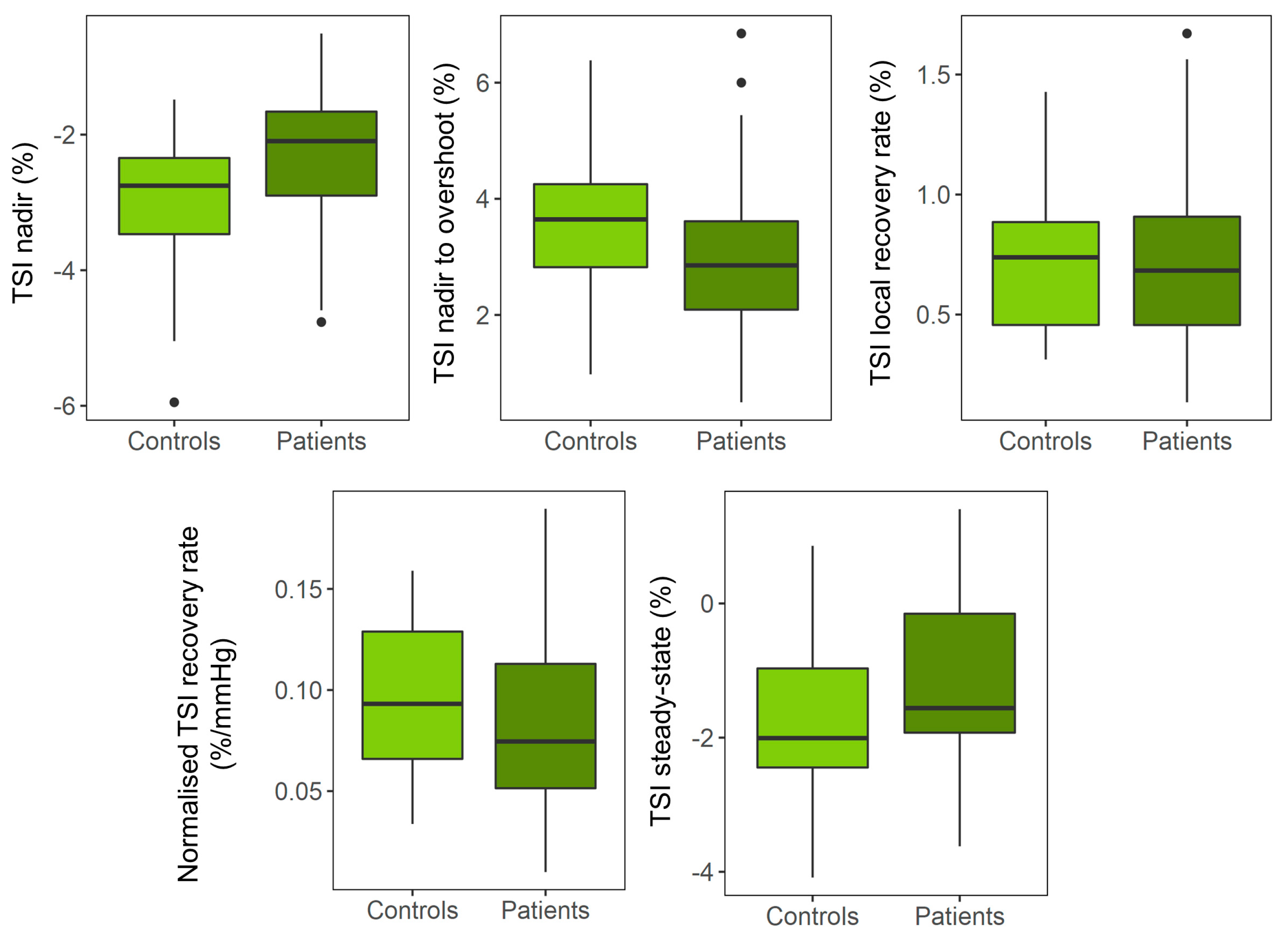
3. Results
3.1. Patients’ Characteristics
3.2. Univariate Analyses
3.3. Multivariate Analyses
3.4. Sensitivity Analyses
4. Discussion
4.1. Limitations
4.2. Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Variable | Units | Description | Calculation Method |
|---|---|---|---|
| SBP baseline | mmHg | Mean SBP 30–60 s before standing | |
| DBP baseline | mmHg | Mean DBP 30–60 s before standing | |
| HR baseline | bpm | Mean HR 30–60 s before standing | |
| TSI baseline | % | Mean TSI 30–60 s before standing | |
| Δ[O2Hb] baseline | µmol/L | Mean Δ[O2Hb] 30–60 s before standing | |
| Δ[HHb] baseline | µmol/L | Mean Δ[HHb] 30–60 s before standing | |
| SBP nadir | mmHg | Largest trough in SBP after standing (within 30 s) | |
| TSI nadir | % | Largest trough in TSI after standing (within 30 s) | |
| Δ[O2Hb] nadir | µmol/L | Largest trough in Δ[O2Hb] after standing (within 30 s) | |
| Δ[HHb] peak | µmol/L | Largest peak in Δ[HHb] after standing (within 30 s) | |
| SBP overshoot | mmHg | First peak found between 5 s after SBP nadir and 60 s after standing | |
| TSI overshoot | % | First peak found between 2 s after TSI nadir and 60 s after standing | |
| Δ[O2Hb] overshoot | µmol/L | First peak found between 2 s after Δ[O2Hb] nadir and 60 s after standing | |
| Δ[HHb] trough | µmol/L | First trough found between 2 s after Δ[HHb] peak and 60 s after standing | |
| SBP steady-state | mmHg | Mean SBP 60–120 s after standing | |
| TSI steady-state | mmHg | Mean TSI 60–120 s after standing | |
| Δ[O2Hb] steady-state | µmol/L | Mean Δ[O2Hb] 60–120 s after standing | |
| Δ[HHb] steady-state | µmol/L | Mean Δ[HHb] 60–120 s after standing | |
| Delta SBP | mmHg | SBP baseline—SBP nadir | SBPB − SBPN |
| Delta TSI | % | TSI baseline—TSI nadir | TSIB − TSIN |
| Delta Δ[O2Hb] | µmol/L | Δ[O2Hb] baseline—Δ[O2Hb] nadir | Δ[O2Hb]B − Δ[O2Hb]N |
| Delta Δ[HHb] | µmol/L | Δ[HHb] baseline—Δ[HHb]peak | Δ[HHb]B − Δ[HHb]P |
| SBP nadir to overshoot difference | mmHg | SBP overshoot—SBP nadir | SBPO − SBPN |
| TSI nadir to overshoot difference | % | TSI overshoot—TSI nadir | TSIO − TSIN |
| Δ[O2Hb] nadir to overshoot difference | µmol/L | Δ[O2Hb] overshoot—Δ[O2Hb] nadir | Δ[O2Hb]O − Δ[O2Hb]N |
| Δ[HHb] peak to through difference | µmol/L | Δ[HHb] trough—Δ[HHb] peak | Δ[HHb]T − Δ[HHb]P |
| SBP steady-state change | mmHg | SBP baseline—SBP steady-state | SBPB − SBPSS |
| TSI steady-state change | % | TSI baseline—TSI steady-state | TSIB − TSISS |
| Δ[O2Hb] steady-state change | µmol/L | Δ[O2Hb] baseline—Δ[O2Hb] steady-state | Δ[O2Hb]B − Δ[O2Hb]SS |
| Δ[HHb] steady-state change | µmol/L | Δ[HHb] baseline—Δ[HHb] steady-state | Δ[HHb]B − Δ[HHb]SS |
| Maximum SBP recovery rate | mmHg/s | In the range between SPB nadir and SBP overshoot: largest difference between consecutive points/time between points | Max((SBPi+1 − SBPi)/(1/fs)), i = nadir index,…, overshoot index |
| Maximum TSI recovery rate | %/s | In the range between TSI nadir and TSI overshoot: largest difference between consecutive points/time between points | Max((TSIi+1 − TSIi)/(1/fs)), i = nadir index,…, overshoot index |
| Δ[O2Hb] maximum recovery rate | µmol/L/s | In the range between Δ[O2Hb] nadir and Δ[O2Hb] overshoot: largest difference between consecutive points/time between points | Max((Δ[O2Hb]i+1 − Δ[O2Hb]i)/(1/fs)), i = nadir index,…, overshoot index |
| Δ[HHb] maximum recovery rate | µmol/L/s | In the range between Δ[HHb] nadir and Δ[HHb] overshoot: largest difference between consecutive points/time between points | Max((Δ[HHb] i+1 − Δ[HHb] i)/(1/fs)), i = nadir index,…, overshoot index |
| Normalized TSI recovery rate | %/mmHg | Maximum TSI recovery rate/maximum SBP recovery rate | TSIRR/SBPRR |
| Normalized Δ[O2Hb] recovery rate | µmol/L/mmHg | Δ[O2Hb] maximum recovery rate/maximum SBP recovery rate | Δ[O2Hb]RR/SBPRR |
| Normalized Δ[HHb] recovery rate | µmol/L/mmHg | Δ[HHb] maximum recovery rate/maximum SBP recovery rate | Δ[HHb]RR/SBPRR |
| β Coefficient (CI) | p-Value | FDR p-Value | |
| Model 1 | |||
| Δ[O2Hb] nadir (µmol/L) | 3.62 (0.05 7.19) | 0.047 * | 0.235 |
| Δ[O2Hb] nadir-overshoot difference (µmol/L) | 0.07 (−1.33 1.47) | 0.919 | 0.919 |
| Maximum Δ[O2Hb] recovery rate (µmol/L/s) | −0.10 (−0.47 0.26) | 0.572 | 0.715 |
| Normalized Δ[O2Hb] recovery rate (µmol/L·mmHg) | −0.02 (−0.06 0.02) | 0.292 | 0.487 |
| Δ[O2Hb] baseline to steady-state change (µmol/L) | 2.38 (−2.05 6.82) | 0.284 | 0.487 |
| Model 2 | |||
| Δ[O2Hb] nadir (µmol/L) | 2.87 (−0.60 6.35) | 0.102 | 0.432 |
| Δ[O2Hb] nadir-overshoot difference (µmol/L) | −0.17 (−1.56 1.23) | 0.810 | 0.810 |
| Maximum Δ[O2Hb] recovery rate (µmol/L/s) | −0.14 (−0.52 0.23) | 0.441 | 0.684 |
| Normalized Δ[O2Hb] recovery rate (µmol/L·mmHg) | −0.03 (−0.06 0.01) | 0.173 | 0.432 |
| Δ[O2Hb] baseline to steady-state change (µmol/L) | 1.33 (−3.09 5.74) | 0.547 | 0.684 |
| Model 3 | |||
| Δ[O2Hb] nadir (µmol/L) | 3.24 (−0.67 7.15) | 0.101 | 0.168 |
| Δ[O2Hb] nadir-overshoot difference (µmol/L) | −0.68 (−2.13 0.77) | 0.346 | 0.432 |
| Maximum Δ[O2Hb] recovery rate (µmol/L/s) | −0.37 (−0.72 −0.01) | 0.046 * | 0.168 |
| Normalized Δ[O2Hb] recovery rate (µmol/L·mmHg) | −0.04 (−0.08 0.00) | 0.070 | 0.168 |
| Δ[O2Hb] baseline to steady-state change (µmol/L) | 1.77 (−3.27 6.81) | 0.481 | 0.481 |
| Model 4 | |||
| Δ[O2Hb] nadir (µmol/L) | 2.53 (−1.38 6.45) | 0.197 | 0.263 |
| Δ[O2Hb] nadir-overshoot difference (µmol/L) | −0.88 (−2.22 0.46) | 0.191 | 0.263 |
| Maximum Δ[O2Hb] recovery rate (µmol/L/s) | −0.38 (−0.74 −0.02) | 0.042 * | 0.168 |
| Normalized Δ[O2Hb] recovery rate (µmol/L·mmHg) | - | - | |
| Δ[O2Hb] baseline to steady-state change (µmol/L) | 1.54 (−4.02 7.10) | 0.577 | 0.577 |
| β Coefficient (CI) | p-Value | FDR p-Value | |
|---|---|---|---|
| Model 1 | |||
| Δ[HHb] peak (µmol/L) | −1.09 (−2.07 −0.12) | 0.029 * | 0.036 * |
| Δ[HHb] peak-to-trough difference (µmol/L) | 0.93 (0.16 1.71) | 0.019 * | 0.032 * |
| Maximum Δ[HHb] recovery rate (µmol/L/s) | 0.29 (0.09 0.48) | 0.005 ** | 0.013 * |
| Normalized Δ[HHb] recovery rate (µmol/L·mmHg) | 0.05 (0.02 0.08) | 0.002 * | 0.010 * |
| Δ[HHb] baseline to steady-state change (µmol/L) | 0.34 (−1.85 2.52) | 0.756 | 0.756 |
| Model 2 | |||
| Δ[HHb] peak (µmol/L) | −1.20 (−2.19 −0.21) | 0.019 * | 0.024 * |
| Δ[HHb] peak-to-trough difference (µmol/L) | 1.02 (0.23 1.805) | 0.013 * | 0.022 * |
| Maximum Δ[HHb] recovery rate (µmol/L/s) | 0.30 (0.10 0.495) | 0.004 ** | 0.010 * |
| Normalized Δ[HHb] recovery rate (µmol/L·mmHg) | 0.05 (0.03 0.083) | 0.001 ** | 0.005 ** |
| Δ[HHb] baseline to steady-state change (µmol/L) | −0.26 (−2.40 1.876) | 0.807 | 0.807 |
| Model 3 | |||
| Δ[HHb] peak (µmol/L) | −0.85 (−1.94 0.24) | 0.123 | 0.154 |
| Δ[HHb] peak-to-trough difference (µmol/L) | 1.24 (0.36 2.12) | 0.007 ** | 0.012 * |
| Maximum Δ[HHb] recovery rate (µmol/L/s) | 0.38 (0.18 0.60) | 0.001 ** | 0.002 ** |
| Normalized Δ[HHb] recovery rate (µmol/L·mmHg) | 0.06 (0.03 0.09) | 0.001 ** | 0.002 ** |
| Δ[HHb] baseline to steady-state change (µmol/L) | −0.17 (−2.6 2.27) | 0.891 | 0.891 |
| Model 4 | |||
| Δ[HHb] peak (µmol/L) | −0.93 (−2.06 0.21) | 0.106 | 0.141 |
| Δ[HHb] peak-to-trough difference (µmol/L) | 1.21 (0.32 2.11) | 0.009 ** | 0.018 * |
| Maximum Δ[HHb] recovery rate (µmol/L/s) | 0.39 (0.17 0.60) | 0.001 ** | 0.004 ** |
| Normalized Δ[HHb] recovery rate (µmol/L·mmHg) | - | - | - |
| Δ[HHb] baseline to steady-state change (µmol/L) | 0.09 (−2.59 2.76) | 0.949 | 0.949 |
| β Coefficient (CI) | p-Value | FDR Corrected p-Value | |
|---|---|---|---|
| Model 1 | |||
| Delta SBP nadir (mmHg) | 5.67 (−4.13 15.47) | 0.249 | 0.249 |
| Delta SBP nadir to overshoot (mmHg) | 5.51 (−3.19 14.20) | 0.207 | 0.249 |
| Maximum SBP recovery rate (mmHg/s) | 1.22 (−0.70 3.14) | 0.205 | 0.249 |
| Delta SBP steady-state (mmHg) | 10.80 (3.42 18.17) | 0.005 * | 0.020 * |
| Model 2 | |||
| Delta SBP nadir (mmHg) | 3.36 (−6.40 13.12) | 0.490 | 0.490 |
| Delta SBP nadir to overshoot (mmHg) | 7.63 (−1.00 16.25) | 0.081 | 0.127 |
| Maximum SBP recovery rate (mmHg/s) | 1.63 (−0.30 3.56) | 0.095 | 0.127 |
| Delta SBP steady-state (mmHg) | 9.34 (1.88 16.80) | 0.016 | 0.064 |
| Model 3 | |||
| Delta SBP nadir (mmHg) | 4.99 (−5.52 15.49) | 0.342 | 0.438 |
| Delta SBP nadir to overshoot (mmHg) | 4.15 (−4.30 12.61) | 0.326 | 0.438 |
| Maximum SBP recovery rate (mmHg/s) | 0.60 (−0.95 2.15) | 0.438 | 0.438 |
| Delta SBP steady-state (mmHg) | 8.37 (0.44 16.30) | 0.039 * | 0.156 |
| β Coefficient (CI) | p-Value | FDR Corrected p-Value | |
|---|---|---|---|
| Delta TSI nadir (%) | 1.00 (0.05 1.94) | 0.039 * | 0.195 |
| Delta TSI nadir to overshoot (%) | −0.95 (−2.17 0.27) | 0.121 | 0.258 |
| Maximum TSI recovery rate (%/s) | −0.20 (−0.50 0.11) | 0.203 | 0.258 |
| Normalized TSI recovery rate (%/mmHg) | −0.02 (−0.06 0.02) | 0.283 | 0.283 |
| Delta TSI baseline to steady-state (%) | 0.76 (−0.44 1.95) | 0.206 | 0.258 |
| β Coefficient (CI) | p-Value | FDR Corrected p-Value | |
|---|---|---|---|
| Model 1 | |||
| TSI nadir (%) | 0.93 (0.12 1.74) | 0.026 * | 0.130 |
| TSI nadir to overshoot difference (%) | −0.53 (−1.55 0.49) | 0.300 | 0.375 |
| Average TSI recovery rate (%/s) | −0.04 (−0.19 0.11) | 0.587 | 0.587 |
| Normalized TSI recovery rate (%/mmHg) | −0.02 (−0.06 0.02) | 0.285 | 0.375 |
| TSI baseline to steady-state change (%) | 0.72 (−0.27 1.71) | 0.148 | 0.370 |
| Model 2 | |||
| TSI nadir (%) | 0.84 (0.00 1.68) | 0.050 | 0.250 |
| TSI nadir to overshoot difference (%) | −0.62 (−1.68 0.44) | 0.242 | 0.302 |
| Average TSI recovery rate (%/s) | −0.05 (−0.21 0.10) | 0.483 | 0.483 |
| Normalized TSI recovery rate (%/mmHg) | −0.03 (−0.07 0.01) | 0.116 | 0.290 |
| TSI baseline to steady-state change (%) | 0.66 (−0.37 1.69) | 0.202 | 0.302 |
| Model 3 | |||
| TSI nadir (%) | 0.84 (−0.13 1.80) | 0.088 | 0.240 |
| TSI nadir to overshoot difference (%) | −0.77 (−1.98 0.45) | 0.208 | 0.260 |
| Average TSI recovery rate (%/s) | −0.12 (−0.284 0.05) | 0.167 | 0.260 |
| Normalized TSI recovery rate (%/mmHg) | −0.04 (−0.08 0.01) | 0.096 | 0.240 |
| TSI baseline to steady-state change (%) | −0.15 (−0.46 0.16) | 0.324 | 0.324 |
| Model 4 | |||
| TSI nadir (%) | 0.73 (−0.27 1.72) | 0.147 | 0.209 |
| TSI nadir to overshoot difference (%) | −0.86 (−2.06 0.35) | 0.157 | 0.209 |
| Average TSI recovery rate (%/s) | −0.13 (−0.30 0.04) | 0.132 | 0.209 |
| Normalized TSI recovery rate (%/mmHg) | - | - | - |
| TSI baseline to steady-state change (%) | 0.45 (−0.84 1.73) | 0.484 | 0.484 |
| β Coefficient (CI) | p-Value | FDR Corrected p-Value | |
|---|---|---|---|
| Model 1 | |||
| Delta TSI (%) | 0.81 (−0.22 1.846) | 0.117 | 0.454 |
| TSI nadir-overshoot difference (%) | −0.61 (−1.95 0.742) | 0.363 | 0.454 |
| TSI recovery rate (%/s) | −0.00 (−0.36 0.350) | 0.981 | 0.981 |
| Normalized TSI recovery rate (%/mmHg) | −0.02 (−0.06 0.020) | 0.343 | 0.454 |
| TSI baseline to steady-state change (%) | 0.54 (−0.58 1.655) | 0.333 | 0.454 |
| Model 3 | |||
| Delta TSI (%) | 0.73 (−0.52 1.98) | 0.238 | 0.697 |
| TSI nadir-overshoot difference (%) | −0.66 (−2.29 0.97) | 0.411 | 0.697 |
| TSI recovery rate (%/s) | −0.08 (−0.50 0.34) | 0.697 | 0.697 |
| Normalized TSI recovery rate (%/mmHg) | −0.01 (−0.05 0.03) | 0.647 | 0.697 |
| TSI baseline to steady-state change (%) | 0.51 (−0.86 1.88) | 0.445 | 0.697 |
| Model 4 | |||
| Delta TSI (%) | 0.68 (−0.62 1.98) | 0.287 | 0.614 |
| TSI nadir-overshoot difference (%) | −0.84 (−2.50 0.83) | 0.307 | 0.614 |
| TSI recovery rate (%/s) | −0.14 (−0.57 0.29) | 0.498 | 0.663 |
| Normalized TSI recovery rate (%/mmHg) | - | - | - |
| TSI baseline to steady-state change (%) | 0.32 (−1.19 1.83) | 0.663 | 0.663 |
| β Coefficient (CI) | p-Value | FDR Corrected p-Value | |
|---|---|---|---|
| Model 1 | |||
| Delta SBP (%) | 1.25 (−11.92 14.41) | 0.847 | 0.847 |
| SBP nadir-overshoot difference (%) | 10.31 (−0.73 21.35) | 0.066 | 0.088 |
| SBP recovery rate (%/s) | 2.53 (0.14 4.92) | 0.039 * | 0.088 |
| SBP baseline to steady-state change (%) | 9.05 (−0.48 18.58) | 0.062 | 0.088 |
| Model 3 | |||
| Delta SBP (%) | 2.87 (−11.56 17.30) | 0.684 | 0.684 |
| SBP nadir-overshoot difference (%) | 6.65 (−4.44 17.75) | 0.227 | 0.303 |
| SBP recovery rate (%/s) | 1.36 (−0.64 3.36) | 0.173 | 0.303 |
| SBP baseline to steady-state change (%) | 7.83 (−2.56 18.21) | 0.132 | 0.303 |
References
- Brignole, M.; Moya, A.; de Lange, F.J.; Deharo, J.C.; Elliott, P.M.; Fanciulli, A.; Fedorowski, A.; Furlan, R.; Kenny, R.A.; Martin, A.; et al. 2018 ESC Guidelines for the diagnosis and management of syncope. Eur. Heart J. 2018, 39, 1883–1948. [Google Scholar] [CrossRef] [PubMed]
- Colman, N.; Nahm, K.; Ganzeboom, K.S.; Shen, W.K.; Reitsma, J.; Linzer, M.; Wieling, W.; Kaufmann, H. Epidemiology of reflex syncope. Clin. Auton. Res. 2004, 14 (Suppl. S1), 9–17. [Google Scholar] [CrossRef]
- Carmody, M.; Finucane, C.; Nolan, H.; Kenny, R.A. Combining the Active Stand Test and Pattern Recognition Methods to Predict Vasovagal Syncope; Trinity College Dublin: Dublin, Ireland, 2013. [Google Scholar]
- Sybring, M.; Finucane, C.; Nolan, H.; Kenny, R.A. A Convenient Test for Vasovagal Syncope in Older Adults Combining Pattern Recognition and the Active Stand Test; Trinity College Dublin: Dublin, Ireland, 2014. [Google Scholar]
- Carmody, M.; Finucane, C.; Nolan, H.; O’Dwyer, C.; Kwok, M.; Kenny, R.A.; Fan, C.W. A Machine Learning Framework to Detect Syncope using the Active Stand. medRxiv 2020. [Google Scholar] [CrossRef]
- Kwok, M.; Nolan, H.; Fan, C.W.; O’Dwyer, C.; Kenny, R.A.; Finucane, C. Machine learning for analysis of active stand responses in older adults with vasovagal syncope. medRxiv 2020. [Google Scholar] [CrossRef]
- Novak, P. Orthostatic Cerebral Hypoperfusion Syndrome. Front. Aging Neurosci. 2016, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Claffey, P.; Pérez-Denia, L.; Rivasi, G.; Finucane, C.; Kenny, R.A. Near-infrared spectroscopy in evaluating psychogenic pseudosyncope—A novel diagnostic approach. QJM Int. J. Med. 2019, 113, 239–244. [Google Scholar] [CrossRef]
- Szufladowicz, E.; Maniewski, R.; Kozluk, E.; Zbiec, A.; Nosek, A.; Walczak, F. Near-infrared spectroscopy in evaluation of cerebral oxygenation during vasovagal syncope. Physiol. Meas. 2004, 25, 823–836. [Google Scholar] [CrossRef]
- Lieshout, J.J.V.; Wieling, W.; Karemaker, J.M.; Secher, N.H. Syncope, cerebral perfusion, and oxygenation. J. Appl. Physiol. 2003, 94, 833–848. [Google Scholar] [CrossRef]
- Carey, B.J.; Manktelow, B.N.; Panerai, R.B.; Potter, J.F. Cerebral autoregulatory responses to head-up tilt in normal subjects and patients with recurrent vasovagal syncope. Circulation 2001, 104, 898–902. [Google Scholar] [CrossRef]
- Mol, A.; Woltering, J.H.H.; Colier, W.; Maier, A.B.; Meskers, C.G.M.; van Wezel, R.J.A. Sensitivity and reliability of cerebral oxygenation responses to postural changes measured with near-infrared spectroscopy. Eur. J. Appl. Physiol. 2019, 119, 1117–1125. [Google Scholar] [CrossRef]
- Kim, Y.S.; Bogert, L.W.; Immink, R.V.; Harms, M.P.; Colier, W.N.; van Lieshout, J.J. Effects of aging on the cerebrovascular orthostatic response. Neurobiol. Aging 2011, 32, 344–353. [Google Scholar] [CrossRef]
- Immink, R.V.; Secher, N.H.; Roos, C.M.; Pott, F.; Madsen, P.L.; Lieshout, J.J. The postural reduction in middle cerebral artery blood velocity is not explained by PaCO2. Eur. J. Appl. Physiol. 2006, 96, 609–614. [Google Scholar] [CrossRef]
- Harms, M.; Colier, W.; Wieling, W.; Lenders, J.; Secher, N.; van Lieshout, J. Orthostatic Tolerance, Cerebral Oxygenation, and Blood Velocity in Humans with Sympathetic Failure. Stroke 2000, 31, 1608–1614. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-M.; Choi, J.-K.; Choi, M.; Ji, M.; Hwang, G.; Ko, S.-B.; Bae, H.-M. Assessment of cerebral autoregulation using continuous-wave near-infrared spectroscopy during squat-stand maneuvers in subjects with symptoms of orthostatic intolerance. Sci. Rep. 2018, 8, 13257. [Google Scholar] [CrossRef]
- Mc Nicholas, T.; Briggs, R.; Claffey, P.; Newman, L.; Tobin, K.; Kenny, R. Symptoms of orthostatic intolerance and cerebral perfusion-Data from The Irish Longitudinal Study on Ageing (TILDA). J. Hum. Hypertens. 2018, 32, 693–721. [Google Scholar]
- Endo, A.; Fujita, Y.; Fuchigami, T.; Takahashi, S.; Mugishima, H.; Skatani, K. Changes in cerebral blood oxygenation induced by active standing test in children with POTS and NMS. Adv. Exp. Med. Biol. 2014, 812, 253–261. [Google Scholar] [CrossRef]
- Kim, Y.T.; Tanaka, H.; Takaya, R.; Kajiura, M.; Tamai, H.; Arita, M. Quantitative study on cerebral blood volume determined by a near-infrared spectroscopy during postural change in children. Acta Paediatr. 2009, 98, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, T.; Fujita, Y.; Ishii, W.; Kimura, K.; Fukuda, A.; Fuchigami, T.; Morioka, I. Cerebral Blood Oxygenation Changes in Juvenile Patients with Delayed Orthostatic Hypotension During an Active Standing Test. Adv. Exp. Med. Biol. 2020, 1232, 85–90. [Google Scholar] [CrossRef]
- Finucane, C.; van Wijnen, V.K.; Fan, C.W.; Soraghan, C.; Byrne, L.; Westerhof, B.E.; Freeman, R.; Fedorowski, A.; Harms, M.P.M.; Wieling, W.; et al. A practical guide to active stand testing and analysis using continuous beat-to-beat non-invasive blood pressure monitoring. Clin. Auton. Res. 2019, 29, 427–441. [Google Scholar] [CrossRef] [PubMed]
- Klem, G.H.; Lüders, H.O.; Jasper, H.H.; Elger, C. The ten-twenty electrode system of the International Federation. The International Federation of Clinical Neurophysiology. Electroencephalogr. Clin. Neurophysiol. Suppl. 1999, 52, 3–6. [Google Scholar]
- O’Connor, J.D.; O’Connell, M.D.L.; Nolan, H.; Newman, L.; Knight, S.P.; Kenny, R.A. Impact of Standing Speed on the Peripheral and Central Hemodynamic Response to Orthostasis. Hypertension 2020, 75, 524–531. [Google Scholar] [CrossRef]
- Soraghan, C.J.; Fan, C.W.; Hayakawa, T.; Cronin, H.; Foran, T.; Boyle, G.; Kenny, R.-A.; Finucane, C. TILDA Signal Processing Framework (SPF) for the analysis of BP responses to standing in epidemiological and clinical studies. In Proceedings of the IEEE-EMBS International Conference on Biomedical and Health Informatics (BHI), Valencia, Spain, 1–4 June 2014; pp. 793–796. [Google Scholar]
- Pérez-Denia, L.; Claffey, P.; Byrne, L.; Rice, C.; Kenny, R.A.; Finucane, C. Increased multimorbidity is associated with impaired cerebral and peripheral hemodynamic stabilization during active standing. J. Am. Geriatr. Soc. 2022, 70, 1973–1986. [Google Scholar] [CrossRef] [PubMed]
- Field, A.; Miles, J.; Field, Z. Discovering Statistics Using R; Sage: Los Angeles, CA, USA, 2013. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Society. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Medow, M.S.; Kothari, M.L.; Goetz, A.M.; O’Donnell-Smith, M.B.; Terilli, C.; Stewart, J.M. Decreasing cerebral oxygen consumption during upright tilt in vasovagal syncope. Physiol. Rep. 2017, 5, e13286. [Google Scholar] [CrossRef]
- Aaslid, R.; Markwalder, T.M.; Nornes, H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J. Neurosurg. 1982, 57, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Sung, R.Y.; Du, Z.D.; Yu, C.W.; Yam, M.C.; Fok, T.F. Cerebral blood flow during vasovagal syncope induced by active standing or head up tilt. Arch. Dis. Child. 2000, 82, 154–158. [Google Scholar] [CrossRef]
- Mankovsky, B.N.; Piolot, R.; Mankovsky, O.L.; Ziegler, D. Impairment of cerebral autoregulation in diabetic patients with cardiovascular autonomic neuropathy and orthostatic hypotension. Diabet. Med. 2003, 20, 119–126. [Google Scholar] [CrossRef]
- Harms, M.P.; van Lieshout, J.J. Cerebrovascular and cardiovascular responses associated with orthostatic intolerance and tachycardia. Clin. Auton. Res. 2001, 11, 35–38. [Google Scholar] [CrossRef]
- Nakamura, K.; Shiroto, Y.; Tamura, Y.; Koyama, K.; Takeuchi, K.; Amanuma, M.; Nagasawa, T.; Ozawa, S. An increase in the deoxygenated hemoglobin concentration induced by a working memory task during the refractory period in the hemodynamic response in the human cerebral cortex. Neurosci. Lett. 2020, 714, 134531. [Google Scholar] [CrossRef]
- Brignole, M.; Rivasi, G.; Sutton, R.; Kenny, R.A.; Morillo, C.A.; Sheldon, R.; Raj, S.R.; Ungar, A.; Furlan, R.; van Dijk, G.; et al. Low-blood pressure phenotype underpins the tendency to reflex syncope. J. Hypertens. 2021, 39, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-L.; Wagner, J.; Heugel, N.; Sugar, J.; Lee, Y.-W.; Conant, L.; Malloy, M.; Heffernan, J.; Quirk, B.; Zinos, A.; et al. Functional Near-Infrared Spectroscopy and Its Clinical Application in the Field of Neuroscience: Advances and Future Directions. Front. Neurosci. 2020, 14, 724. [Google Scholar] [CrossRef] [PubMed]
- Yoshitani, K.; Kawaguchi, M.; Miura, N.; Okuno, T.; Kanoda, T.; Ohnishi, Y.; Kuro, M. Effects of Hemoglobin Concentration, Skull Thickness, and the Area of the Cerebrospinal Fluid Layer on Near-infrared Spectroscopy Measurements. Anesthesiol. J. Am. Soc. Anesthesiol. 2007, 106, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Davie, S.N.; Grocott, H.P. Impact of extracranial contamination on regional cerebral oxygen saturation: A comparison of three cerebral oximetry technologies. Anesthesiology 2012, 116, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.N.; Cotter, J.D.; Galvin, S.D.; Williams, M.J.A.; Willie, C.K.; Ainslie, P.N. Initial orthostatic hypotension is unrelated to orthostatic tolerance in healthy young subjects. J. Appl. Physiol. 2009, 107, 506–517. [Google Scholar] [CrossRef]
- Lieshout, J.J.v.; Pott, F.; Madsen, P.L.; Goudoever, J.v.; Secher, N.H. Muscle Tensing During Standing. Stroke 2001, 32, 1546–1551. [Google Scholar] [CrossRef]
- Immink, R.V.; Pott, F.C.; Secher, N.H.; van Lieshout, J.J. Hyperventilation, cerebral perfusion, and syncope. J. Appl. Physiol. 2014, 116, 844–851. [Google Scholar] [CrossRef]
- Sorond, F.A.; Serrador, J.M.; Jones, R.N.; Shaffer, M.L.; Lipsitz, L.A. The sit-to-stand technique for the measurement of dynamic cerebral autoregulation. Ultrasound Med. Biol. 2009, 35, 21–29. [Google Scholar] [CrossRef]
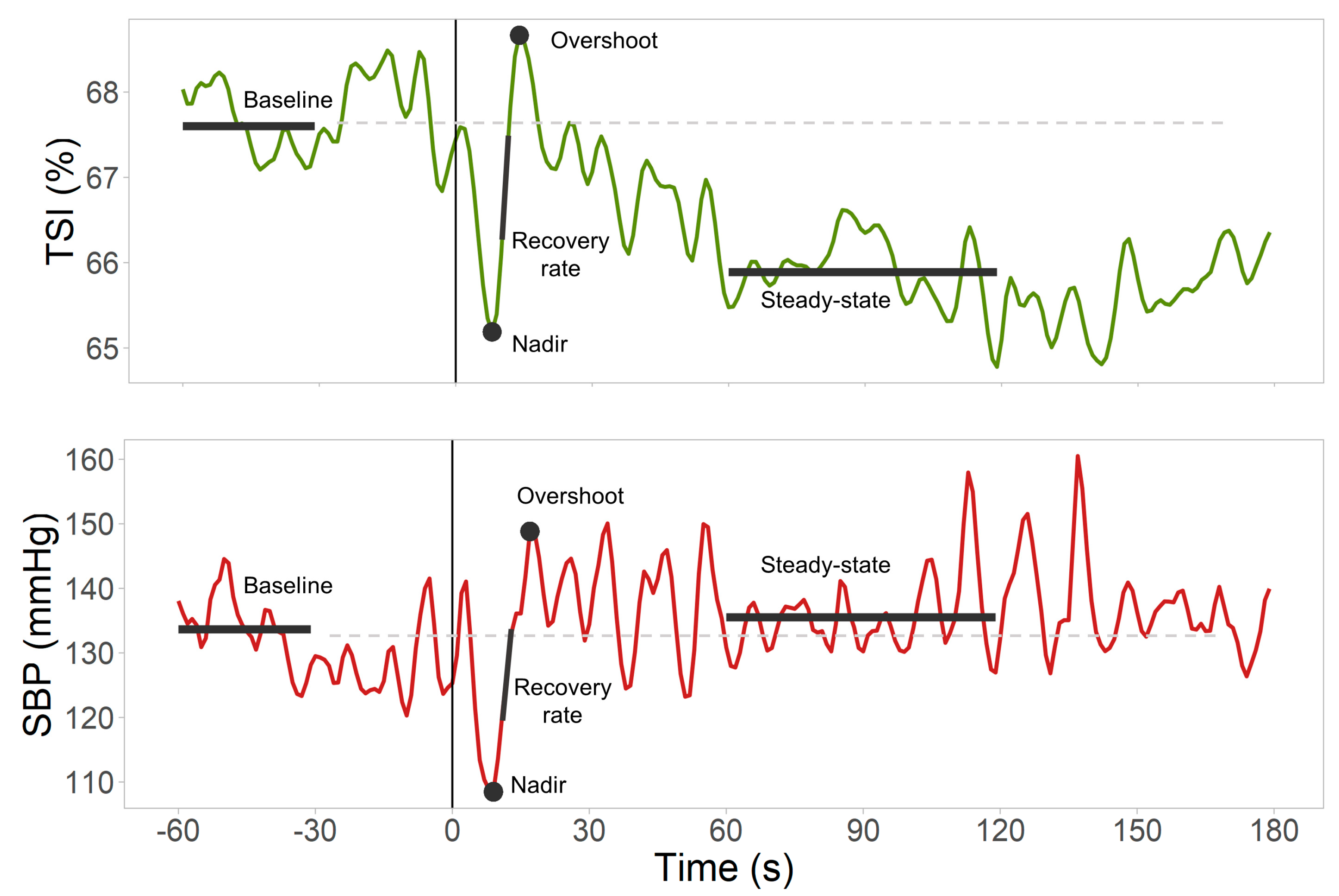
| Biosignal (Units) | Variable | Description |
|---|---|---|
| SBP (mmHg), HR (bpm), TSI (%) | Baseline | Mean value 30–60 s before standing |
| SBP (mmHg), TSI (%) | Nadir | Largest trough after standing (in first 30 s) |
| SBP (mmHg) | Overshoot | First peak found between SBP nadir time +5 s and 60 s after standing |
| TSI (%) | Overshoot | First peak found between TSI nadir time +2 s and 60 s after standing |
| SBP (mmHg), TSI (%) | Steady-state | Mean value 60–120 s after standing |
| SBP (mmHg), TSI (%) | Delta | Nadir—Baseline |
| SBP (mmHg), TSI (%) | Nadir to overshoot | Overshoot—Nadir |
| SBP (mmHg), TSI (%) | Steady-state change | Steady-state—Baseline |
| SBP (mmHg/s), TSI (%/s) | Maximum recovery rate | Largest difference between consecutive points divided by the time between points (within the time interval between the nadir and overshoot) |
| TSI/SBP (%/mmHg) | Normalized TSI recovery rate | Maximum TSI recovery rate/maximum SBP recovery rate |
| Covariates | |
|---|---|
| Model 1 | None |
| Model 2 | Age + Gender |
| Model 3 | Age + Gender + BMI + HR baseline |
| Model 4 | Age + Gender + BMI + HR baseline + SBP concurrent feature * |
| Controls (n = 13) | Patients (n = 27) | p-Value | |
|---|---|---|---|
| Age (years) | 22 (1) | 21 (6) | 0.220 |
| Female, % (n) | 54 (7) | 78 (21) | 0.122 |
| Weight (kg) | 75 (15) | 69 (17) | 0.496 |
| Height (cm) | 174 (11) | 168 (13) | 0.310 |
| BMI (kg/m2) | 23 (3) | 23 (5) | 0.806 |
| SBP baseline (mmHg) | 132 (22) | 130 (27) | 0.479 |
| DBP baseline (mmHg) | 81 (5) | 77 (18) | 0.875 |
| MAP baseline (mmHg) | 101 (11) | 102 (23) | 0.977 |
| HR baseline (bpm) | 66 (16) | 76 (14) | 0.039 * |
| Cardiovascular diseases, % (n) | 0 | 4 (1) | - |
| Cholesterol, % (n) | 0 | 4 (1) | - |
| Chronic obstructive airway disease, % (n) | 0 | 15 (4) | - |
| Migraine, % (n) | 0 | 15 (4) | - |
| Depression/anxiety, % (n) | 0 | 30 (8) | - |
| Current smokers, % (n) | 23 (3) | 15 (4) | 0.519 |
| Previous smokers, % (n) | 0 | 0 | - |
| History of syncope, % (n) | 0 | 85 (23) | - |
| History of recurrent syncope, % (n) * | 0 | 59 (16) | - |
| History of presyncope, % (n) | 0 | 52 (14) | - |
| History of recurrent presyncope, % (n) * | 0 | 41 (11) | - |
| Presence of OH on standing | 8 (1) | 0 | - |
| Beta-blockers, % (n) | 8 (1) | 4 (1) | - |
| Antidepressants, % (n) | 8 (1) | 11 (3) | - |
| Antihypertensives, % (n) | 0 | 0 | - |
| β Coefficient (CI) | p-Value | FDR Corrected p-Value | |
|---|---|---|---|
| Model 1 | |||
| TSI nadir (%) | 0.93 (0.12 1.74) | 0.026 * | 0.130 |
| TSI nadir to overshoot difference (%) | −0.53 (−1.55 0.49) | 0.300 | 0.500 |
| Maximum TSI recovery rate (%/s) | −0.04 (−0.30 0.23) | 0.790 | 0.790 |
| Normalized TSI recovery rate (%/mmHg) | −0.01 (−0.04 0.02) | 0.426 | 0.532 |
| TSI baseline to steady-state change (%) | 0.72 (−0.27 1.71) | 0.148 | 0.370 |
| Model 2 | |||
| TSI nadir (%) | 0.84 (0.00 1.68) | 0.050 | 0.250 |
| TSI nadir to overshoot difference (%) | −0.62 (−1.68 0.44) | 0.242 | 0.318 |
| Maximum TSI recovery rate (%/s) | −0.06 (−0.33 0.22) | 0.688 | 0.688 |
| Normalized TSI recovery rate (%/mmHg) | −0.02 (−0.05 0.01) | 0.254 | 0.318 |
| TSI baseline to steady-state change (%) | 0.66 (−0.37 1.69) | 0.202 | 0.318 |
| Model 3 | |||
| TSI nadir (%) | 0.84 (−0.13 1.80) | 0.088 | 0.401 |
| TSI nadir to overshoot difference (%) | −0.77 (−1.98 0.45) | 0.208 | 0.401 |
| Maximum TSI recovery rate (%/s) | −0.15 (−0.46 0.16) | 0.324 | 0.401 |
| Normalized TSI recovery rate (%/mmHg) | −0.02 (−0.05 0.02) | 0.401 | 0.401 |
| TSI baseline to steady-state change (%) | −0.15 (−0.46 0.16) | 0.324 | 0.401 |
| Model 4 | |||
| TSI nadir (%) | 0.73 (−0.27 1.72) | 0.147 | 0.314 |
| TSI nadir to overshoot difference (%) | −0.86 (−2.06 0.35) | 0.157 | 0.314 |
| Maximum TSI recovery rate (%/s) | −0.17 (−0.47 0.14) | 0.282 | 0.376 |
| Normalized TSI recovery rate (%/mmHg) | - | - | - |
| TSI baseline to steady-state change (%) | 0.45 (−0.84 1.73) | 0.484 | 0.484 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Denia, L.; Claffey, P.; O’Reilly, A.; Delgado-Ortet, M.; Rice, C.; Kenny, R.A.; Finucane, C. Cerebral Oxygenation Responses to Standing in Young Patients with Vasovagal Syncope. J. Clin. Med. 2023, 12, 4202. https://doi.org/10.3390/jcm12134202
Pérez-Denia L, Claffey P, O’Reilly A, Delgado-Ortet M, Rice C, Kenny RA, Finucane C. Cerebral Oxygenation Responses to Standing in Young Patients with Vasovagal Syncope. Journal of Clinical Medicine. 2023; 12(13):4202. https://doi.org/10.3390/jcm12134202
Chicago/Turabian StylePérez-Denia, Laura, Paul Claffey, Ailbhe O’Reilly, Maria Delgado-Ortet, Ciara Rice, Rose Anne Kenny, and Ciarán Finucane. 2023. "Cerebral Oxygenation Responses to Standing in Young Patients with Vasovagal Syncope" Journal of Clinical Medicine 12, no. 13: 4202. https://doi.org/10.3390/jcm12134202
APA StylePérez-Denia, L., Claffey, P., O’Reilly, A., Delgado-Ortet, M., Rice, C., Kenny, R. A., & Finucane, C. (2023). Cerebral Oxygenation Responses to Standing in Young Patients with Vasovagal Syncope. Journal of Clinical Medicine, 12(13), 4202. https://doi.org/10.3390/jcm12134202






