1. Introduction
According to WHO’s classification of soft tissue and bone tumors, a simple bone cyst (SBC) is an intramedullary, usually unilocular, cystic bone tumor lined by a fibrous membrane and filled with serous or serosanguineous fluid. The older term, unicameral bone cyst, is no longer recommended [
1]. The occurrence of SBC in the calcaneus is considered to be relatively rare, representing 2 to 14% of all SBCs [
1,
2,
3,
4,
5]. In our study on the distribution patterns of foot and ankle tumors observed at a university tumor institute between 1997 and 2015, SBC was the most common benign bone tumor, accounting for 50 (21.6%) out of 231 non-malignant bone tumors [
6]. In the largest review on foot tumors to date conducted by Ruggieri et al., there were 51 calcaneal cysts in 1170 cases of foot tumors. SBC accounted for 6.5% of cases in their subgroup of benign osseous lesions of the foot (consisting of 578 benign bone tumors and 201 pseudotumoral bone lesions) [
7].
SBC of the long bones most commonly occurs in children and adolescents, with a reported average age at diagnosis of 9–11 years [
5,
8]. On the other hand,
calcaneal simple bone cysts are observed later, usually during the second and third decades of life [
9,
10,
11]. In a review conducted by Levy et al. in 2015 including 10 studies comprising 171 patients with SBC, the mean age was reported as 25.7 ± 8.1 years, and males were affected in two-thirds of the cases [
12]. The average age of patients with
calcaneal SBC (
n = 42) in our series of foot and ankle tumors was 18.1 ± 6.8. There was a male predominance, with a m:f ratio of 2.2:1 [
6].
Localized in the lower extremity, SBCs may cause persistent pain and thus warrant surgical therapy. However, they are diagnosed as an incidental finding in approximately 30% of cases [
13,
14,
15]. In addition to pain, the risk of pathological fracture is another indication for surgical treatment [
14,
16]. According to the criteria established by Pogoda et al., the risk of a pathological fracture is increased when the cyst occupies 100% of the mediolateral cross-section in the coronary plane and more than 30% in the sagittal plane. Even in asymptomatic cases, surgery might be indicated if the critical size is reached [
17]. Rarely, surgery may also be considered due to tumor anxiety following incidental findings.
Lipoma of bone is a benign neoplasm composed of white adipocytes arising within or on the surface of bone. Intraosseous lipoma (IOL) commonly arises in the calcaneus and metaphysis of long tubular bones, especially the femur, tibia, and humerus. Approximately 70% of IOL cases involve the lower limb [
1]. Intraosseous lipoma is often considered to be one of the rarest primary bone tumors [
18] and may be asymptomatic (30%) or produce aching pain (70%). IOL is rare and accounts for <0.1% of primary bone tumors. The age of affected patients is reported to range from the second to eighth decades of life. Males are affected more frequently than females (m:f ratio of 1.3:1) [
1]. In our assessment of 413 foot and ankle tumors, there were 21 cases of IOL, all localized in the calcaneus, making it the fifth most common benign bone tumor in our series. The mean patient age was 39.4 ± 12.4, and there were 14 male and seven female patients affected by calcaneal IOL (m:f ratio 2:1). In a publication by Radl et al. including 29 cases of IOL, the mean patient age was 48 (20–75) [
19].
IOL can be misinterpreted as plantar fasciitis and other common causes of heel pain [
20,
21,
22]. In cases where a calcaneal SBC or IOL is detected on diagnostic imaging (often performed for unspecific heel pain), surgical intervention should only be considered after other causes for heel pain have been thoroughly assessed and excluded.
SBC and IOL are similar in many ways, including their location and radiological appearance, but their content differs. SBCs contain fluid, whereas lipomas contain fat, although some lesions may exhibit a mixed content [
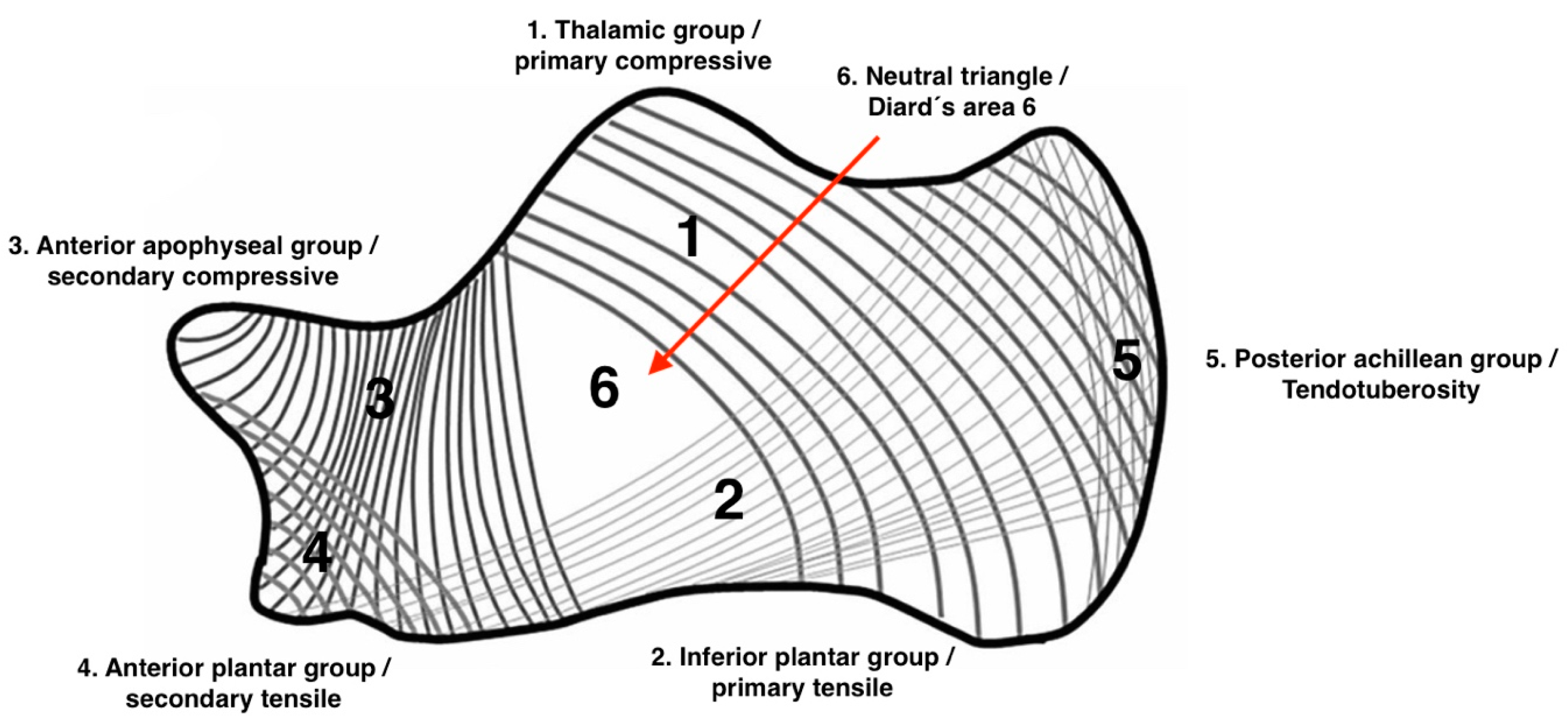
10]. Both calcaneal SBC and IOL are almost exclusively located in Diard’s Area 6, the ventral triangular area between the major trabecular groups under the calcaneal sulcus (
Figure 1), which is situated on the anteroinferior margin of the posterior articular surface [
3,
23,
24]. Their radiological appearance is also identical, forming a triangular area that lacks a trabecular structure. It is well delineated and sometimes surrounded by a sclerotic margin that is usually thin. The defect may display signs of moderate chronic expansion, most often predominating the infero-lateral trgion, with cortical thinning and bowing (visible on tangential axial radiographs) and no periosteal reaction. This appearance has been described for both simple bone cysts and intraosseous lipomas of the calcaneus [
10,
17,
18,
25,
26,
27,
28,
29,
30].
The anterior calcaneal location of SBC and IOL corresponds exactly to a triangular anatomic area in which the trabecular network is naturally much sparser (Diard’s Area 6,
Figure 1). This area is visible on 70% of any normal lateral radiograph and has a pseudocystic appearance in 7% of cases. The term “
pseudocyst” for this area of decreased radiopacity was first used by Irry et al. to describe these findings [
31]. Unlike cysts and lipomas, pseudocysts contain a sparse trabecular structure and do not exhibit peripheral sclerotic rims, signs of expansion, or central calcification [
32]. The term “
pseudocyst” may be confusing in the setting of simple bone cyst as pseudocyst is not lined by fibrous membrane and is not a true SBC. The pathogenesis of the two entities, SBC and IOL, is a subject of ongoing controversy. In their 2017 publication, Malghem et al. discussed in detail the most common hypotheses regarding the pathogenesis of calcaneal SBC and IOL [
10]. For SBC, various factors were discussed as possible causes, including development from a congenital remnant in the region of the primary ossification center [
33], degenerative processes in areas of bone rarefaction [
34], increased mechanical stress [
35], organized hemorrhages [
36], ischemic degeneration [
25,
28], and others. Additionally, the origin from ganglion cysts arising from the subtalar joint [
37] or the collapse of the sinus tarsi artery [
38] have been discussed. Cohen et al. indicated that the principal etiological factor of solitary bone cysts is the blockage of interstitial fluid drainage, hypothesizing that venous obstruction is the primary cause of simple bone cysts in patients with long bone cysts [
39,
40]. Similarly, Andermahr et al. proposed that the development of a calcaneal cyst is secondary to a disturbance in local intraosseous blood circulation [
41]. Our own findings support the hypothesis of Chigira and Andermahr, which suggests a disorder in physiologic intraosseous pressure and blood circulation [
41,
42,
43].
Regarding IOL, most authors believe that they are true benign tumors resulting from adipocyte proliferation [
18,
44,
45]. Other authors have suggested IOL is the end result of a process of involution of other bone lesions [
28,
46,
47,
48,
49]. According to Milgram, IOL may present with varying features depending on its stage of evolution. Stage 1 lesions consist of solid fat cells and demonstrate a purely radiolucent lesion with resorption of the preexisting bone. Stage 2 lesions demonstrate similar features, but also contain localized regions of increased roentgenographic density due to calcified fat. Stage 3 lesions are characterized by reactive ossification around the calcified fat in the outer rim of the lesions. Moreover, many of Stage 3 lesions contain cystic regions [
18].
As stated by Malghem et al., the discussion of the pathogenesis of these two entities is confusing, especially because calcaneal cysts have been described as lipomas and vice versa. Lagier et al. believe that lipomas and cysts of the calcaneus may constitute two types of responses to a single mechanical or vascular stimulus [
47], while Mirra even uses the two terms to describe a single entity with a varying cyst- or lipoma-like histology [
2]. Thus, the possibility that the one may evolve into the other has been considered in both directions [
10,
30].
Previously, several authors have described a potential continuum between calcaneal cysts and lipomas, demonstrating through long-term MRI follow-ups that SBC can heal with fatty conversion of the cystic cavity, resulting in partly cystic remnants [
10,
30,
50]. Thus, it has been proposed that at least part of the so-called intraosseous lipomas are actually healed simple bone cysts [
50].
In our case series, the combination of preoperative MRI, intraoperative high-resolution endoscopic imaging of the lesion, and postoperative histopathological work-up of the resected specimen may contribute valuable information to this interesting discussion, suggesting that calcaneal SBC and IOL may share a common pathogenesis, and that (calcaneal) IOL may develop from SBC.
2. Materials and Methods
Between 2012 and 2019, a total of 25 benign osteolytic bone tumors consisting of 17 SBCs and 8 IOLs were treated by A.T. with endoscopic resection and grafting. All cases of SBC or IOL were confirmed histopathologically. We excluded all cases of calcaneal ganglion cysts and other (pseudo-) tumorous lesions which are commonly seen in Diard´s Area 6 and must be distinguished from calcaneal SBC and IOL [
37]. Overall, there were 17 male and 8 female patients with a mean age of 24.4y (range 12–62). There were 12 right and 13 left feet involved, and there were no bilateral cases. Patients with SBC (
n = 17) had a mean age of 17.0y (range 12–41), with 12 male and 6 female patients being affected. Patients with IOLs (
n = 8) had a mean age of 38.0 years old at the time of surgery (range 19–62), and there were 6 male and 2 female patients in this subgroup (
Table 1).
Thirteen out of the 25 cases were incidental findings (52%). In 7 of the 13 cases, an MRI was performed after an ankle sprain. The remaining 6 MRIs were performed for the following reasons: achillodynia (2×), after a medial malleolar fracture, insect bite, tarsal coalition, and after whole-body computed tomography in a polytrauma patient (
Table 1). For all cases, a preoperative MRI was performed in addition to plain radiographs. Due to their characteristic appearances on MRI, bioptical verification of the diagnosis was not required in any case. The analysis of the available imaging was performed by a musculoskeletal tumor radiologist with specialized training and the orthopedic tumor surgeon (AT). All cases were repeatedly discussed in a multi-disciplinary tumor board.
Surgery was indicated for the following reasons: pain (
n = 10), tumor size with increased risk of a pathological fracture (
n = 10), or at the explicit request of the patient in the case of tumor anxiety (
n = 5). In cases of heel pain, all relevant differential diagnoses had been excluded through repeated clinical assessment and imaging diagnostics. The critical size for an elevated risk of fracture was defined as 100% of the intracalcaneal cross-section in the coronary plane and at least 30% in the sagittal plane of the lesion, according to the definition of Pogoda [
17]. If the size of the lytic lesion did not warrant a recommendation for prophylactic stabilization, the affected patient was offered clinical and radiologic follow-up. As mentioned before, 5 patients with a relatively small tumor size opted for endoscopic tumor resection due to tumor anxiety after careful consideration of the available findings. Alternatively, bioptic verification of the diagnosis and follow-up controls were not requested.
After a detailed explanation of the possible advantages and disadvantages of the respective filling materials, the affected patient (in the case of minors, their parents) could decide whether to use allogenic bone or injectable bone substitute. Our hypothesis was that the use of injectable bone substitute might decrease the surgery duration and allow for early full weight-bearing. Patients who requested early full weight-bearing were recommended to have IBS as the filling material. Moreover, some patients preferred to have artificial bone substitute over allogenic bone for personal reasons and therefore opted for IBS as a filling material. ACB was recommended for all other patients based on our previous experience regarding osseous integration and the current state of the literature on calcaneal SBC, which suggests that cancellous bone is beneficial in terms of recurrence [
12].
Autologous bone grafting was also discussed but not recommended due to the associated donor site morbidity. No randomization was performed.
A total of 12 patients with a mean age of 17.9 years (range 12–34) received allogenic cancellous bone (ACB, group A), and 13 patients with a mean age of 30.4 years (range 12–62) received injectable bone substitute (IBS, group B). In
Table 1, patients who received allogenic bone appear on white background, and cases with injectable bone substitute are grayed out.
For grafting, allogenic bone in the form of cancellous bone was used for plombage of the cavity in 10 cases (DIZG, Berlin, Germany and ReadiGraft® Cancellous Chips, LifeNet Health, Vienne, Austria), and injectable demineralized bone matrix was used in 2 cases (DIZG, Berlin, Germany). Four different types of injectable bone substitute were used: Cerament© (hydroxyapatite and calcium sulfate, Bonesupport, Lund, Sweden) in 10 cases, Pro-Dense© (calcium sulfate and calcium phosphate, Wright Medical, Memphis, TN, USA) in 1 case, QuickSet© calcium phosphate bone cement, Arthrex, Naples, FL, USA) in 1 case, and Innotere© (calcium phosphate bone cement, Arthrex, Naples, FL, USA) in 1 case.
The number of different bone graft substitutes can be explained by the availability and surgeons’ expectations regarding the osseous integration of the filling material. The first two cases using Pro-Dense and Quick-Set showed good applicability and handling but poor osteointegration over the initial course of time. The use of Cerament was expected to improve osseous integration. Due to its poor results with high complication rates, the use of this filling material was abandoned, and another IBS (Innotere) was used for the last case upon the patient’s request.
Depending on the product used, curing of the bone substitute differs slightly:
For Cerament©, the setting time is specified by the manufacturer as 15 min, and the initial compressive strength is 10–75 MPa (wet conditions–dry conditions). For Innotere©, a setting time of 24 h is given until the compression strength of cancellous bone is reached (10 MPa). For ProDense, the manufacturer specifies a compressive strength of 40 MPa after 2 h in wet conditions. Quickset is reported to reach a compression strength of 24 MPA at 24 h after implantation. After using injectable bone substitute, limited weight-bearing (15 kg) was recommended until wound healing was completed (2 weeks). When using allogenic bone for grafting, 6 weeks of limited weight bearing was indicated.
A radiologic follow-up was routinely performed 6 weeks, 12 weeks, and 12 months postop with plain radiography. To rule out recurrence, MRI imaging was indicated for all cases demonstrating a healing result with a defect on conventional radiographs (types Neer B–D).
The classification of the osseous healing outcome was based on the modified Neer classification (
Table 2). The modified Neer classification has previously been used for of any osseous localization [
51,
52,
53]. Type A describes a healed cyst filled with new bone, allowing a small radiolucent area of less than 1cm. With our modification of the classification, we only accepted complete osseus healing as type A (without any radiolucent area). Calcaneal SBC is sometimes only 3–4 cm in size. Therefore, we excluded any defect healing with a radiolucent area <1 cm for the type A classification, as this would correspond to a type B lesion in small SBCs.
Pre- and postoperative imaging with plain X-rays and MRI was retrospectively analyzed to assess tumor size, osseous consolidation, and tumor recurrence. A retrospective chart analysis focusing on adverse intra- and perioperative events and other complications associated to the surgical procedure was performed using the modified Clavien–Dindo classification [
54,
55,
56]. Complications were defined as any deviation from the normal postoperative course [
54]. Disturbed wound healing (DWH) was defined according to the definition of Dirschinger et al. for elective foot surgery [
57]. Approval for the study was obtained from our institutional review board.
The surgery duration was recorded in minutes. All but one ossoscopy was performed as a standalone procedure. In one case, endoscopic tumor resection was performed in combination with a joint preserving surgery for tarsal coalition. For this case, no isolated surgical time was available for the endoscopic procedure.
2.1. Surgical Technique
The surgical technique has been previously described by the authors [
15,
24,
58]. The type of anesthetic procedure was determined by the patient and the anesthesiologist. The patient was placed in a stable lateral position on a radiolucent table. The dimensions of the bone lesion were marked on the skin of the lateral rear foot with a sterile pen under fluoroscopic control. The two portals for ossoscopy were marked according to the size of the cystic bone lesion. A tourniquet was used to reduce bleeding into the bone cavity and to facilitate visualization. After skin incision and blunt dissection of the underlying soft tissue, the thinned-out cortex was penetrated with a semi-sharp obturator before introducing the sheath for a 2.7 or 4 mm scope into the cavity. During blunt dissection to the lateral wall of the calcaneal bone, care was taken not to harm the sural nerve and the peroneal tendons. Contrary to ossoscopy of the calcaneal bone for a simple bone cyst, clear vision of the bone cavity in IOL can only be achieved after a second portal has been established and thorough endoscopic irrigation has been performed. Therefore, loose lipomatous tissue was washed out, and the typical calcified areas of intraosseous lipoma became visible. The calcifications were cleaned out using an arthroscopic shaver, and larger pieces were grasped using an arthroscopic punch or grasper (
Figure 2). Often, a tennisnet-like pseudo-membrane covered the walls of the cavity. This membrane is common for SBC but can also be present in calcaneal lipoma, suggesting their common etiology (
Figure 3). The membrane was completely resected to prevent tumor recurrence. Any visible tumor tissue was completely removed and sent for histopathological analysis. Injection of radiopaque contrast medium verified the integrity of the bone cavity (no accidental damage and leakage) and helped determine the amount of injectable bone substitute needed to completely fill the lesion.
Before grafting with allogenic bone or injectable bone substitute, the cavity was rinsed with 95% ethanol as a local adjuvant therapy, ensuring denaturation of the remaining microscopic cyst membranes and tumor tissue. The application time for the ethanol did not exceed 1–2 min, and after intermittent thorough irrigation with sterile saline solution, this procedure was repeated 2–3 times [
52,
59,
60,
61]. The ethanol did not come into contact with any soft tissue to avoid damage to sensible structures such as the sural nerve. For easy application of the allogenic cancellous bone chips, a small 4 mm
Hartmann ear speculum (Aesculap, Tuttlingen, Germany) was introduced through one of the ossoscopy portals (
Figure 4). In our experience, this device has proven to be easier to handle compared to previously used instruments such as a pedicle filler (borrowed from spine surgery) or a hollow bone punch originally used for biopsies. Under endoscopic vision through the second portal, impaction of the bone graft was performed intermittently. Alternatively, injectable bone substitute was applied under direct endoscopic and fluroroscopic control (
Figure 5). Finally, both portals were sealed with a collagen sponge to avoid accidental leakage of the graft material. After wound closure, the foot was immobilized in a semi-rigid lower leg orthosis. Partial weight bearing was advised for 6 weeks for all cases treated with allogenic bone and for two weeks for those treated with injectable bone substitute (until the removal of the skin sutures).
2.2. Statistics
The parameters were expressed as mean, standard deviation (SD) and range (min./max.). A Fisher´s exact test was used to determine the statistical significance of the differences between Group A and Group B, as well as between the different Neer types of osseous healing. A p-value < 0.05 was used to define statistical significance. The statistics were performed by an independent statistician using Microsoft Excel© and RStudio software.
4. Discussion
Are simple bone cyst and intraosseous lipoma two different entities?
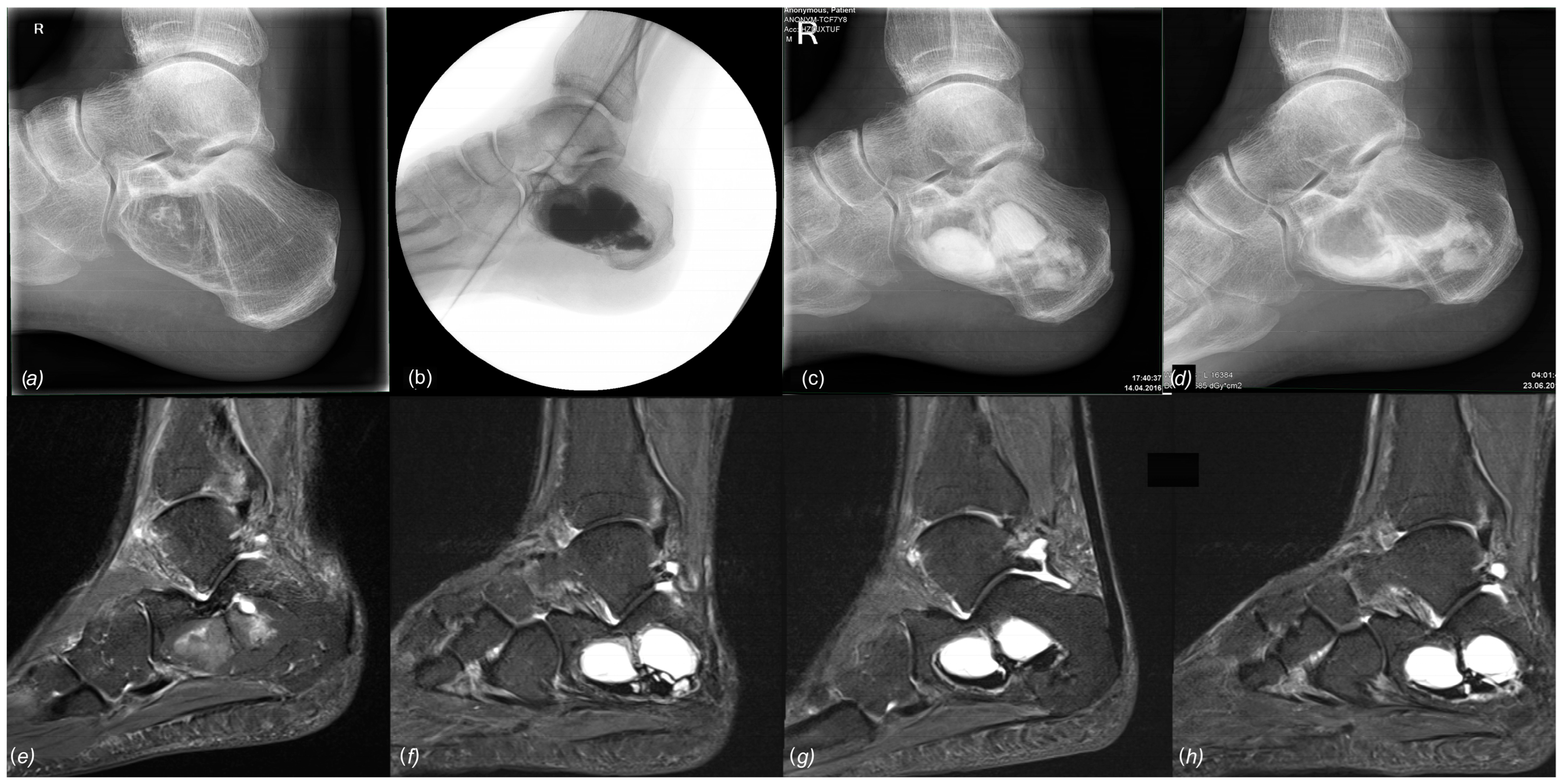
Preoperative imaging with plain radiographs and MRI led to the diagnosis of 17 SBCs and eight IOLs. Both entities exhibited a characteristic, almost pathognomonic appearance on MRI, making bioptic verification of the diagnosis unnecessary. Often times, MRI revealed a mixed form of SBC and IOL, showing lipomatous tissue enclosing central cystic areas (
Figure 8). This has been commonly described as the evolution of an IOL (Milgram Stage 3 [
18]) or involution from other preexisting bone lesions [
28,
36,
46,
47], suggesting that IOL develops from precursor lesions. It should be noted that in the current 2020 edition of the WHO classification of soft tissue and bone tumors, SBC and IOL are listed as separate entities and their etiology is considered unknown [
1]. However, several publications have documented the transition of SBC to IOL over time, suggesting that IOL may actually represent a developmental form of SBC [
10,
30,
50,
64].
While MRI allows differentiation between cystic from lipomatous areas of the lesion, histopathological analysis is required to verify the correct diagnosis. Direct endoscopic visualization of the lesion can help to correctly assess mixed and transitional forms of SBC and IOL macroscopically (
Figure 3). The high resolution of modern scopes (4K) and the magnification factor provide a much more detailed view of the findings during ossoscopy compared to open surgical resection. Thus, the tennis net-like membrane of SBC and other components of the lesion can clearly be identified.
Two cases initially diagnosed as IOLs based on MRI turned out to be SBC in the histopathological analysis (case 5 and 12). Another case of suspected IOL demonstrated membranous material with a histiocyte-resorptive reaction but no cholesterol clefts (which are typical for SBC) upon histopathological evaluation, resembling SBC (case 10).
Both our endoscopic and histopathological results support the theory proposed by other authors such as Malghem, Tins, and Kawaguchi that (calcaneal) IOL develops form SBC [
10,
30,
50,
64].
Which option should be used now: allogenic cancellous bone or injectable bone substitute?
A systematic review on the treatment of unicameral bone cysts of the calcaneus conducted by Levy et al. from 2015 concluded that open curettage with autograft bone augmentation is the most effective procedure. Unsurprisingly, autograft procedures resulted in significantly greater radiographic healing compared with allografting. The review included only one publication on endoscopic curettage and calcium–phosphate injection, and two more reports with two cases each treated with open curettage and calcium phosphate or calcium sulfate, respectively [
12].
In a recent study by Ma et al.,
45S5 Bioglass was reported to better facilitate the formation of new bone with a faster drying time of the skin incision than allogenic bone in open curettage of 31 patients, including 18 cases of SBC and six cases of IOL with a f/u of 12 months [
65]. Only plain radiographs were used to assess osseous integration of the filling material.
Karr et al. reported the use of Cerament© calcium sulfate/calcium phosphate bone void filler in a patient with bilateral calcaneal SBC, mixing the injectable bone substitute with ACB on one side, demonstrating complete trabecular regeneration at 2.3 years (right foot) and three years (left foot) postoperatively [
66].
Aycan et al. reported on intralesional curettage and defect filling with cortico-spongeous allograft in 14 patients with calcaneal intraosseous lipoma. After a mean f/u of 84 months (range 18–108), no recurrence was observed [
67]. Ulucay et al. reported on 21 cases of calcaneal IOL treated with cancellous iliac crest autografts after open curettage, and no recurrence was observed after a mean f/u of 94 months (range 45–143) [
27].
Previously, we reported our findings of endoscopic resection and allografting for four cases of calcaneal IOL and six cases of SBC, all demonstrating complete osseous healing after a mean f/u of 19.8 months (range 9.5–39.3) [
15].
In 2011, Yildirim performed a comparative study including 26 cases of calcaneal SBC. Thirteen feet underwent open surgical curettage, and 13 cases underwent endoscopic curettage. Both groups received allografting. The operating time and mean length of stay were significantly shorter in the endoscopic group. The time to healing was similar in both groups. The overall success rate was higher for the endoscopic group (100%, 13 of 13 vs. 92.3%, 12 of 13 cases), and there were no statistically significant differences regarding radiological healing [
68].
Similarly, Nishimaura et al. published their findings on 16 calcaneal SBCs in 2016. Eight patients underwent an open procedure, and eight cases were treated with an endoscopic procedure. No significant difference between the two groups was observed in the operative time. The surgery duration for the endoscopic group was 56.1 ± 13.8 min, and no adverse effects were observed. However, the open group experienced one temporary irritation in the sural nerve area and one calcium phosphate cement leakage along the peroneal tendon sheath. The interval for a return to sports was significantly shorter in the endoscopic group. The authors concluded that endoscopic surgery is a useful approach for the treatment of calcaneal bone cysts, allowing early rehabilitation and an early return to sports without any adverse effects.
For better comparison,
Table 6 lists all available studies on endoscopic resection of calcaneal SBC and/or IOL. To the best of our knowledge, our case series is the only study that routinely included MRI in follow-ups. As conventional radiography is insufficient for sufficiently assessing osseous integration of the graft or tumor recurrence, we strongly recommend including MRI in future studies.
A publication by Choi et al. on endoscopic tumor treatment of the skeleton was not included because the author failed to indicate the anatomic location of nine cases of SBC [
80]. Surprisingly, no complications were reported in 14 publications that included a total of 61 cases of calcaneal SBC or IOL, with a mean f/u of 30.86 months (no recurrence, no DWH, no suralis neuropraxia). This discrepancy may be attributed to the type of imaging used in follow-ups (X-rays vs. MRI), the different definitions of osseous integration and recurrence, and the duration of the f/u.
Apart from the publication of Nishimura et al., only Farouk et al. provided information on their surgery duration for three cases of endoscopic resection of calcaneal IOL (45, 40, and 15 min, respectively). Grafting was performed in 2/3 cases with PMMA, while the third case did not receive any filling of the bone cavity. The mean surgery time in our series was 75.2 min. (range 35–133) and 71.7 min (range 35–103) for group A (ACB), and 84.2 min (range 45–133) for group B (IBS). Our hypothesis that the use of injectable bone substitute would shorten the duration of the procedure compared with allogeneic bone grafting was not confirmed.
Complications were predominantly related to the use of Cerament©, leading to a 90% complication rate (9/10) in patients treated with this particular injectable bone substitute. These complications included DWH with “white-out” in 7/10 cases, one case of DWH without “white-out”, one revision surgery, and one suralis neuropraxia. As the “white-out” phenomenon has been repeatedly observed in various non-tumorous indications in our clinical practice, and excessive white drainage after the use of Cerament© does not seem to be limited to its use in aneurysmal bone cysts, we have stopped using this product entirely.
Figure 9 and
Figure 10 show examples of the gradual loss of Cerament© over time, leading to “white-out”, insufficient stabilization of the bone cavity, and cyst recurrence. These specific complications did not occur with other injectable bone substitutes. However, repeated MRI performed after treatment with two different types of calcium phosphate bone cement (case 6 and 25, Quickset© and Innotere©) did not demonstrate any postoperative signs of osseous ingrowth or bony transformation at 36 and 91 months (
Figure 6b and
Figure 11), respectively.
Aiba et al. proposed the use of curettage without additional grafting for simple bone cysts in various locations. They reported a recurrence rate of 18.9% (7/37 patients) when using endoscopic curettage as a stand-alone procedure. It is important to note that all recurrences occurred in tubular (
n = 6) or flat bones (
n = 1), and not in the calcaneus [
53] (
Table 6). Farouk et al. reported on one case of calcaneal SBC treated with endoscopic curettage without grafting [
77]. Other reports of curettage without grafting exist for different locations, but are mostly limited to case reports [
81,
82]. Apart from the six cases of calcaneal SBC described in Aiba’s publication and one case published by Farouk et al., to our knowledge, there is no other literature supporting curettage without grafting at this site [
53,
77].
For large SBCs in the lower weight-bearing extremities, grafting is considered advantageous in regard to actual osseous healing. This hypothesis is supported by the findings of the only systematic review available on calcaneal SBC published by Levy et al. in 2015 [
12]. Open curettage with bone augmentation demonstrated the best outcomes in their review. Nearly 80% of patients in the bone augmentation group experienced heel pain that had completely resolved after a mean duration of 3.3 ± 1.3 years. No clear distinction was found between autografting and allografting. No patients in either group experienced recurrence, complications, or reactions suggestive of graft rejection [
12].
Intralesional tumor resection with thorough curettage of the simple bone cyst with complete removal of the inner cyst wall and cyst membrane is generally regarded as the key to preventing recurrence. The current literature on calcaneal SBC favors additional cancellous bone grafting over IBS or techniques without grafting (e.g., cannulated screw decompression or corticosteroid injections) [
12,
58].
Strengths and Limitations
Although this is the largest case series on endoscopic resection of calcaneal SBC and IOL, identifying an exact difference between the two groups (statistical power) would require much larger sample sizes. This is true for most rare diseases and is unlikely to change in the foreseeable future. Calcaneal SBC proved to be the most common benign bone tumor in our publication on the distribution patterns of foot and ankle tumors (50 SBC out of 413 tumors overall) [
6], but it was observed less frequently in Ruggieri’s analysis (51 SBC out of 1170 tumors) [
7]. Nonetheless, it remains exceedingly rare outside of a university tumor center. A significant portion of SBC and IOL cases are diagnosed as incidental findings and do not require any form of therapy. This is why even a university musculoskeletal tumor center can only report a limited number of cases over a period of almost ten years. All surgeries were performed by the same surgeon (A.T.), ensuring a certain standard and reproducibility of the procedure and eliminating heterogeneous treatment algorithms that could possibly influence outcome.
One limitation is the use of different types of injectable bone graft substitutes, preventing any general recommendation for or against their use in calcaneal ossoscopy. Nevertheless, IBS regularly showed prolonged wound drainage (Cerament©) or showed no signs of osseous integration after several years of f/u (ProDense©, Innotere©). The follow-up was inconsistent, both in terms of the type of imaging and the duration of follow-up. MRI was only routinely indicated if plain radiographs at 12 months post-op suggested incomplete healing. Still, 10/25 patients (40%) had (repeated) MRI with a mean f/u of 40.3 months (range 12–91) after surgery, providing a medium-term radiological outcome including MRI for a rare condition treated with an even rarer surgical procedure.
Most publications on calcaneal IOL consist of case reports with one or two cases. To the best of our knowledge, there are only three publications with a larger case load of calcaneal IOL, all of them reporting open surgical tumor resection [
27,
67,
83]. Regarding endoscopic treatment of calcaneal IOL, this is the largest study to date.
Another limitation of this study is its retrospective, non-randomized design. Conducting a prospective, single-center study with sufficient statistical power is not feasible within a reasonable time frame for rare diseases such as calcaneal IOL or SBC. Patients were able to choose the respective filling material after careful consideration of possible advantages and disadvantages. Although both groups (ACB vs. IBS) had almost identical sample size, the inclusion of two different tumor entities could be criticized. Several publications and our own findings suggest that SBC undergoes a continuous lipomatous evolution over time, eventually transforming into IOL [
10,
30,
50,
64,
84,
85]. In fact, both tumors might be considered as different developmental stages of the same entity.