A Jordanian Multidisciplinary Consensus Statement on the Management of Dyslipidemia
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Step 1: Screening of Dyslipidemia
3.2. Step 2: Lipid Profile Measurement
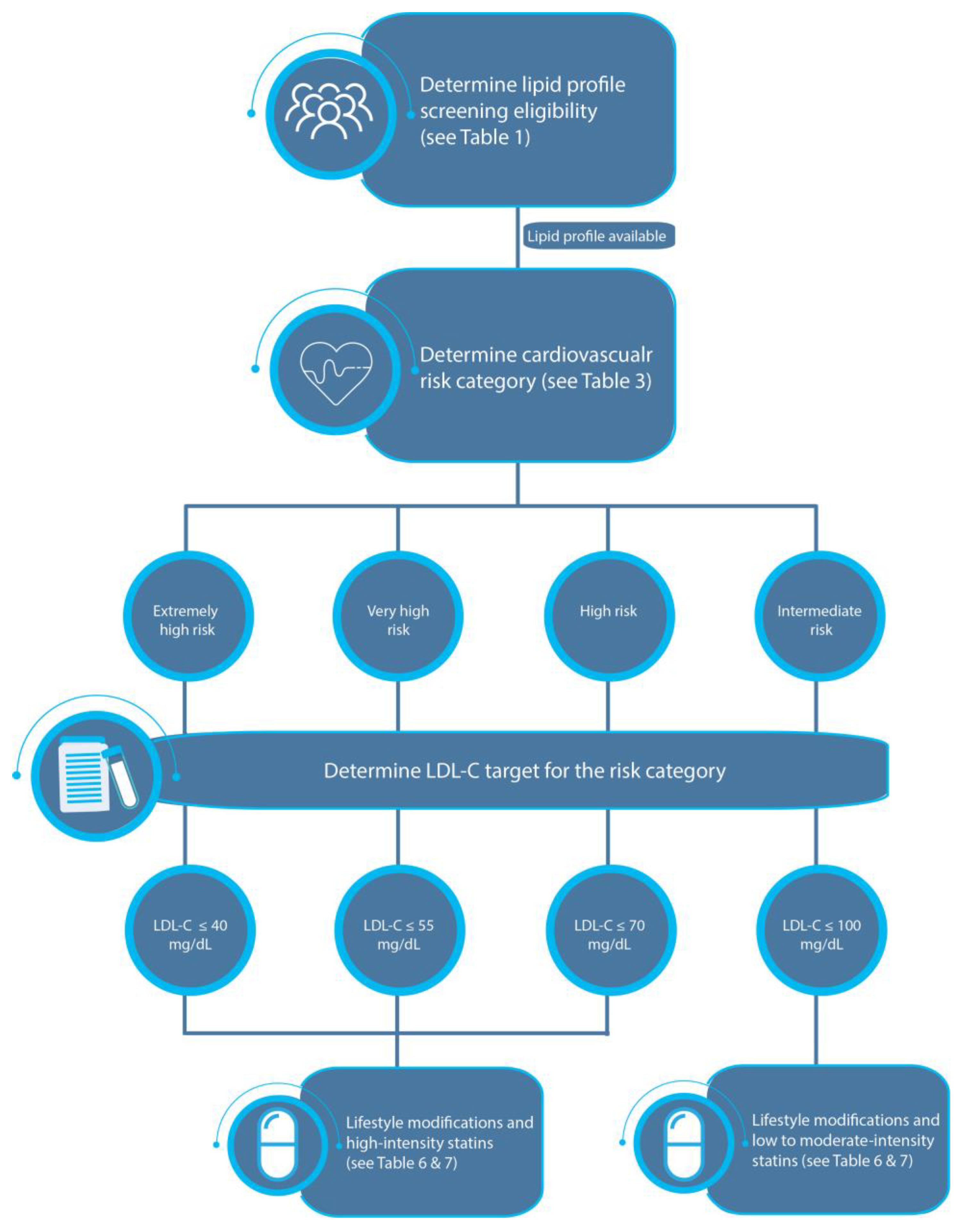
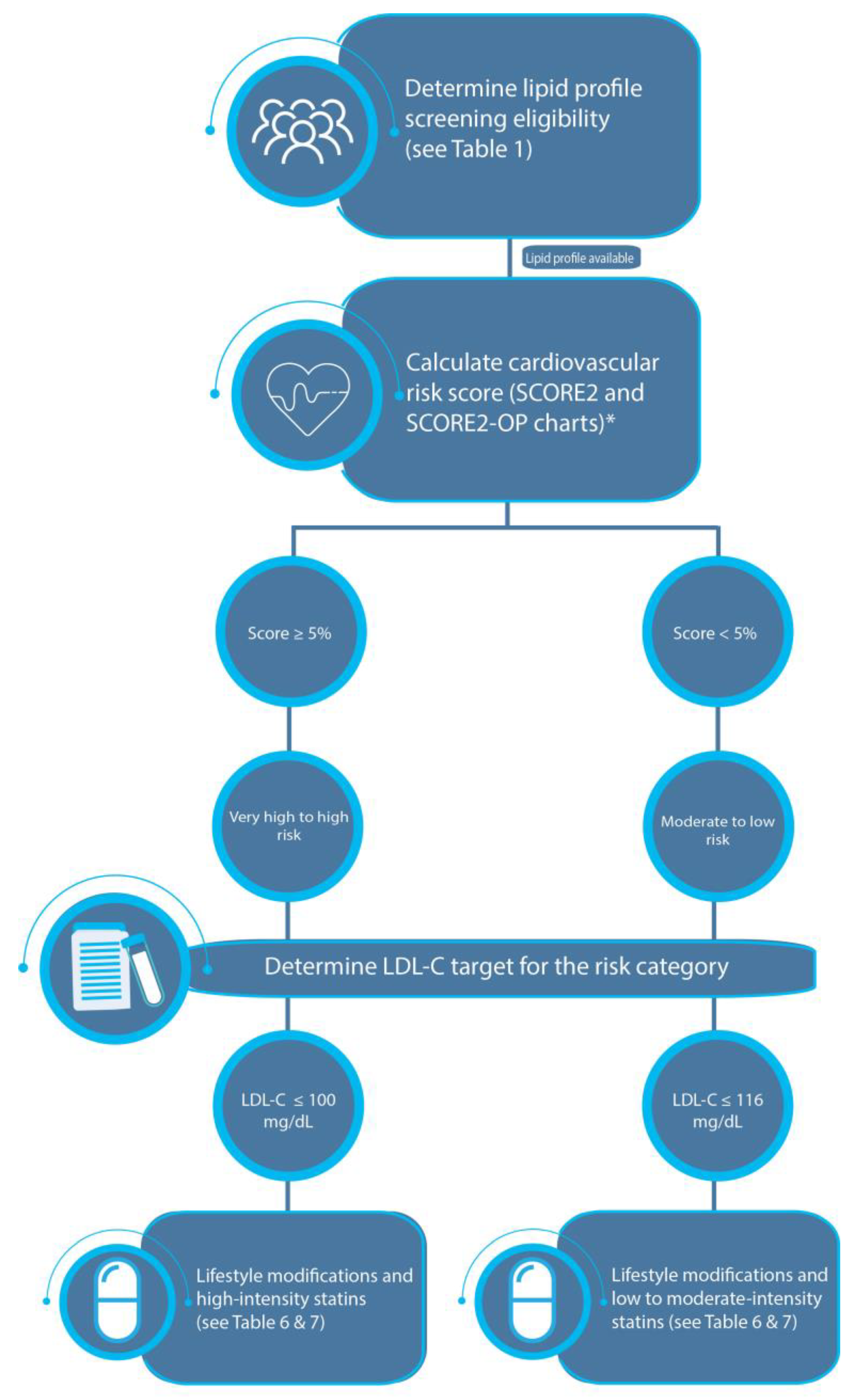
3.3. Step 3: Determine the Risk Category of the Individual
3.3.1. Risk Factors for ASCVD
| Major Risk Factors | Additional Risk Factors | Nontraditional Risk Factors |
|---|---|---|
| Advanced age | Obesity and abdominal obesity | ↑ Lipoprotein |
| ↑ TC | Family history of hyperlipidemia | ↑ Clotting factors |
| ↑ Non–HDL-C | ↑ Small, dense LDL-C | ↑ Inflammation markers (hsCRP and Lp-PLA2) |
| ↑ LDL-C | ↑ Apo B | ↑ Homocysteine levels |
| Low HDL-C | ↑ LDL particle concentration | Apo E4 isoform |
| DM | Fasting/postprandial hypertriglyceridemia | ↑ Uric acid |
| Hypertension | PCOS | ↑ TG-rich remnants |
| Chronic kidney disease | Dyslipidemia triad | |
| Smoker | ||
| Family history of ASCVD |
3.3.2. Estimation of the Total Cardiovascular Risk in Apparently Healthy Individuals
3.3.3. Cardiovascular Risk Categories
3.4. Step 4: Determine the Target LDL-C Serum Level to Be Reached
3.5. Step 5: Management
3.5.1. Lifestyle Modifications
3.5.2. Pharmacological Treatment
Statins
Cholesterol Absorption Inhibitors (Ezetimibe)
PCSK9 Inhibitors
Inclisiran
Other Lipid-Lowering Agents
Management of Hypertriglyceridemia
Treatment Algorithm
4. Conclusions
5. Top Ten Take-Home Messages
- ASCVD is the leading cause of death in Jordan. Dyslipidemia is one of the major modifiable risk factors for ASCVD. Lowering LDL-C levels has been shown to decrease cardiovascular mortality and morbidity.
- Screening for dyslipidemia is recommended for those who are 20 years of age and older. Screening at a lower age is indicated for certain high-risk individuals.
- Screening for lipids can be performed in either a fasting or non-fasting state. However, if a non-fasting blood sample shows a TG level above 400 mg/dL, a fasting blood sample is indicated.
- Lowering LDL-C levels to set target levels is the main objective in reducing cardiovascular risk.
- Individual LDL-C serum level targets are determined by an individual’s level of cardiovascular risk (from extremely high risk to low risk).
- The level of cardiovascular risk (from extremely high to low risk) is readily determined by the presence of clinical disease (ASCVD, DM, or chronic kidney disease, among other clinical diseases). In individuals who do not have any of these features, the level of risk (high risk, moderate risk, or low risk) should be estimated by calculating a 10-year risk score that utilizes certain clinical and laboratory features.
- Lowering LDL-C levels should involve lifestyle modifications and lipid-lowering pharmacotherapy that includes statins and non-statins.
- Statin therapy, particularly, high-intensity statin therapy, is indicated to achieve the target LDL-C level. Ezetimibe is added to statin therapy if the target LDL-C level is not achieved after 3 months of the maximally tolerated statin dose.
- Two injectable lipid-lowering agents are available in Jordan: PCSK9i (Evolocumab), for SC administration using monthly or biweekly doses, and siRNA (inclisiran), administered subcutaneously twice yearly. Both medications are indicated for adult patients with primary hypercholesteremia or adults with mixed dyslipidemia.
- Treatment for high TG levels is indicated when an individual’s levels are above 500 mg/dL, primarily to prevent pancreatitis. In diabetic patients with TG levels ranging from 200–499 mg/dL, despite adequate glycemic control treatment with fibrates, treatment is recommended.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Mensah, G.A.; Roth, G.A.; Fuster, V. The Global Burden of Cardiovascular Diseases and Risk Factors: 2020 and Beyond. J. Am. Coll. Cardiol. 2019, 74, 2529–2532. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Fuster, V. The Global Burden of Cardiovascular Diseases and Risks: A Compass for Global Action. J. Am. Coll. Cardiol. 2020, 76, 2980–2981. [Google Scholar] [CrossRef]
- WHO. Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 28 March 2023).
- Bhagavathula, A.S.; Shehab, A.; Ullah, A.; Rahmani, J. The Burden of Cardiovascular Disease Risk Factors in the Middle East: A Systematic Review and Meta-Analysis Focusing on Primary Prevention. Curr. Vasc. Pharmacol. 2021, 19, 379–389. [Google Scholar] [CrossRef] [PubMed]
- WHO. Results of Jordan National STEPwise Survey (STEPs) of Noncommunicable Diseases and Their Risk Factors. 2019. Available online: https://www.emro.who.int/jor/jordan-infocus/jordan-implements-who-hearts-in-primary-health-care-to-strengthen-management-of-cardiovascular-diseases-and-related-risks.html (accessed on 28 March 2023).
- Yousif, M.A. In-home drug storage and utilization habits: A Sudanese study. East. Mediterr. Health J. 2002, 8, 422–431. [Google Scholar] [CrossRef]
- Yu, J.; Ma, Y.; Yang, S.; Pang, K.; Yu, Y.; Tao, Y.; Jin, L. Risk Factors for Cardiovascular Disease and Their Clustering among Adults in Jilin (China). Int. J. Environ. Res. Public Health 2016, 13, 70. [Google Scholar] [CrossRef]
- Yusuf, S.; Joseph, P.; Rangarajan, S.; Islam, S.; Mente, A.; Hystad, P.; Brauer, M.; Kutty, V.R.; Gupta, R.; Wielgosz, A.; et al. Modifiable risk factors, cardiovascular disease, and mortality in 155,722 individuals from 21 high-income, middle-income, and low-income countries (PURE): A prospective cohort study. Lancet 2020, 395, 795–808. [Google Scholar] [CrossRef]
- Joseph, J.J.; Deedwania, P.; Acharya, T.; Aguilar, D.; Bhatt, D.L.; Chyun, D.A.; Di Palo, K.E.; Golden, S.H.; Sperling, L.S. Comprehensive Management of Cardiovascular Risk Factors for Adults With Type 2 Diabetes: A Scientific Statement From the American Heart Association. Circulation 2022, 145, e722–e759. [Google Scholar] [CrossRef] [PubMed]
- Alsaud, W.; Tabbaa, M.J.; Kasabri, V.N.; Suyagh, M.F.; Abu Alsamen, M.A.; Haddad, H.M.; AO, A.L. Prevalence of Cardiovascular Diseases Risk Factors among Jordanians. J. Saudi Heart Assoc. 2020, 32, 324–333. [Google Scholar] [CrossRef]
- Qawasmeh, M.A.; Aldabbour, B.; Momani, A.; Obiedat, D.; Alhayek, K.; Kofahi, R.; Yassin, A.; El-Salem, K. Epidemiology, Risk Factors, and Predictors of Disability in a Cohort of Jordanian Patients with the First Ischemic Stroke. Stroke Res. Treat. 2020, 2020, 1920583. [Google Scholar] [CrossRef] [PubMed]
- Raffee, L.A.; Alawneh, K.Z.; Ibdah, R.K.; Rawashdeh, S.I.; Zoghoul, S.; Ewais, A.S.; Al-Mistarehi, A.H. Prevalence, Clinical Characteristics, and Risk Among Patients with Ischemic Heart Disease in the Young Jordanian Population. Open Access Emerg. Med. OAEM 2020, 12, 389–397. [Google Scholar] [CrossRef]
- Hammoudeh, A.J.; Al-Tarawneh, H.; Elharassis, A.; Haddad, J.; Mahadeen, Z.; Badran, N.; Izraiq, M.; Al-Mousa, E. Prevalence of conventional risk factors in Jordanians with coronary heart disease: The Jordan Hyperlipidemia and Related Targets Study (JoHARTS). Int. J. Cardiol. 2006, 110, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R. Approach to the Patient with Dyslipidemia. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Cooney, M.T.; Dudina, A.L.; Graham, I.M. Value and limitations of existing scores for the assessment of cardiovascular risk: A review for clinicians. J. Am. Coll. Cardiol. 2009, 54, 1209–1227. [Google Scholar] [CrossRef] [PubMed]
- Hajifathalian, K.; Ueda, P.; Lu, Y.; Woodward, M.; Ahmadvand, A.; Aguilar-Salinas, C.A.; Azizi, F.; Cifkova, R.; Di Cesare, M.; Eriksen, L.; et al. A novel risk score to predict cardiovascular disease risk in national populations (Globorisk): A pooled analysis of prospective cohorts and health examination surveys. Lancet Diabetes Endocrinol. 2015, 3, 339–355. [Google Scholar] [CrossRef] [PubMed]
- Cooney, M.T.; Dudina, A.; D’Agostino, R.; Graham, I.M. Cardiovascular risk-estimation systems in primary prevention: Do they differ? Do they make a difference? Can we see the future? Circulation 2010, 122, 300–310. [Google Scholar] [CrossRef]
- Alshamiri, M.; Ghanaim, M.M.A.; Barter, P.; Chang, K.C.; Li, J.J.; Matawaran, B.J.; Santoso, A.; Shaheen, S.; Suastika, K.; Thongtang, N.; et al. Expert opinion on the applicability of dyslipidemia guidelines in Asia and the Middle East. Int. J. Gen. Med. 2018, 11, 313–322. [Google Scholar] [CrossRef]
- AlRahimi, J.; AlSaif, S.; Alasnag, M.; Awan, Z.; Almutairi, F.; Al Mudaiheem, H.; Gencer, B.; Catapano, A.L.; Mach, F.; Tash, A.J.H.V. 2022 Saudi guidelines for the management of dyslipidemia. Heart Views 2023, 24, 67. [Google Scholar]
- Pappan, N.; Rehman, A. Dyslipidemia. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Nicholls, S.; Lundman, P. The emerging role of lipoproteins in atherogenesis: Beyond LDL cholesterol. Semin. Vasc. Med. 2004, 4, 187–195. [Google Scholar] [CrossRef]
- Pengpid, S.; Peltzer, K. Prevalence, awareness, treatment, and control of dyslipidemia and associated factors among adults in Jordan: Results of a national cross-sectional survey in 2019. Prev. Med. Rep. 2022, 28, 101874. [Google Scholar] [CrossRef]
- Abujbara, M.; Batieha, A.; Khader, Y.; Jaddou, H.; El-Khateeb, M.; Ajlouni, K. The Prevalence of Dyslipidemia among Jordanians. J. Lipids 2018, 2018, 6298739. [Google Scholar] [CrossRef]
- Dajani, R.; Khader, Y.S.; Hakooz, N.; Fatahalla, R.; Quadan, F. Metabolic syndrome between two ethnic minority groups (Circassians and Chechens) and the original inhabitants of Jordan. Endocrine 2013, 43, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Khader, Y.S.; Batieha, A.; El-Khateeb, M.; Al Omari, M.; Ajlouni, K. Prevalence of dyslipidemia and its associated factors among Jordanian adults. J. Clin. Lipidol. 2010, 4, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Halcox, J.P.; Banegas, J.R.; Roy, C.; Dallongeville, J.; De Backer, G.; Guallar, E.; Perk, J.; Hajage, D.; Henriksson, K.M.; Borghi, C. Prevalence and treatment of atherogenic dyslipidemia in the primary prevention of cardiovascular disease in Europe: EURIKA, a cross-sectional observational study. BMC Cardiovasc. Disord. 2017, 17, 160. [Google Scholar] [CrossRef]
- Bilitou, A.; Were, J.; Farrer, A.; Rabe, A.; Ming, S.W.Y.; Haq, I.; Dunton, K. Prevalence and Patient Outcomes of Adult Primary Hypercholesterolemia and Dyslipidemia in the UK: Longitudinal Retrospective Study Using a Primary Care Dataset from 2009 to 2019. Clin. Outcomes Res. CEOR 2022, 14, 189–203. [Google Scholar] [CrossRef]
- Hammoudeh, A.J.; Izraiq, M.; Al-Mousa, E.; Al-Tarawneh, H.; Elharassis, A.; Mahadeen, Z.; Badran, N.; Haddad, J. Serum lipid profiles with and without CAD: Jordan Hyperlipidaemia and Related Targets Study (JoHARTS-1). East. Mediterr. Health J. 2008, 14, 24–32. [Google Scholar] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Pearson, G.J.; Thanassoulis, G.; Anderson, T.J.; Barry, A.R.; Couture, P.; Dayan, N.; Francis, G.A.; Genest, J.; Grégoire, J.; Grover, S.A.; et al. 2021 Canadian Cardiovascular Society Guidelines for the Management of Dyslipidemia for the Prevention of Cardiovascular Disease in Adults. Can. J. Cardiol. 2021, 37, 1129–1150. [Google Scholar] [CrossRef]
- Banach, M.; Burchardt, P.; Chlebus, K.; Dobrowolski, P.; Dudek, D.; Dyrbuś, K.; Gąsior, M.; Jankowski, P.; Jóźwiak, J.; Kłosiewicz-Latoszek, L.; et al. PoLA/CFPiP/PCS/PSLD/PSD/PSH guidelines on diagnosis and therapy of lipid disorders in Poland 2021. Arch. Med. Sci. AMS 2021, 17, 1447–1547. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; Ferranti, S.d.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. J. Am. Coll. Cardiol. 2019, 73, e285–e350. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef]
- Jarrah, M.I.; Ababneh, M.J.; Tawalbeh, L.I.; Hammoudeh, A.J.; Barukba, H.M.; Othman, A. Statin eligibility based on the ACC/AHA guidelines among Middle Eastern patients with diabetes mellitus presenting with acute myocardial infarction. Ann. Med. Surg. 2021, 61, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Collins Bradley, L.; Gupta, A.; Fatima, A.; Qamar, A.; Biery, D.; Baez, J.; Cawley, M.; Klein, J.; Hainer, J.; et al. Cardiovascular Risk and Statin Eligibility of Young Adults After an MI. J. Am. Coll. Cardiol. 2018, 71, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.J.; Grégoire, J.; Pearson, G.J.; Barry, A.R.; Couture, P.; Dawes, M.; Francis, G.A.; Genest, J., Jr.; Grover, S.; Gupta, M.; et al. 2016 Canadian Cardiovascular Society Guidelines for the Management of Dyslipidemia for the Prevention of Cardiovascular Disease in the Adult. Can. J. Cardiol. 2016, 32, 1263–1282. [Google Scholar] [CrossRef]
- Sathiyakumar, V.; Park, J.; Golozar, A.; Lazo, M.; Quispe, R.; Guallar, E.; Blumenthal, R.S.; Jones, S.R.; Martin, S.S. Fasting Versus Nonfasting and Low-Density Lipoprotein Cholesterol Accuracy. Circulation 2018, 137, 10–19. [Google Scholar] [CrossRef]
- Mora, S.; Rifai, N.; Buring, J.E.; Ridker, P.M. Fasting compared with nonfasting lipids and apolipoproteins for predicting incident cardiovascular events. Circulation 2008, 118, 993–1001. [Google Scholar] [CrossRef]
- Chapman, M.J.; Ginsberg, H.N.; Amarenco, P.; Andreotti, F.; Borén, J.; Catapano, A.L.; Descamps, O.S.; Fisher, E.; Kovanen, P.T.; Kuivenhoven, J.A.; et al. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: Evidence and guidance for management. Eur. Heart J. 2011, 32, 1345–1361. [Google Scholar] [CrossRef]
- Board, J.B.S. Joint British Societies’ consensus recommendations for the prevention of cardiovascular disease (JBS3). Heart (Br. Card. Soc.) 2014, 100 (Suppl. S2), ii1–ii67. [Google Scholar] [CrossRef]
- Nauck, M.; Warnick, G.R.; Rifai, N. Methods for measurement of LDL-cholesterol: A critical assessment of direct measurement by homogeneous assays versus calculation. Clin. Chem. 2002, 48, 236–254. [Google Scholar] [CrossRef] [PubMed]
- Sampson, M.; Ling, C.; Sun, Q.; Harb, R.; Ashmaig, M.; Warnick, R.; Sethi, A.; Fleming, J.K.; Otvos, J.D.; Meeusen, J.W.; et al. A New Equation for Calculation of Low-Density Lipoprotein Cholesterol in Patients with Normolipidemia and/or Hypertriglyceridemia. JAMA Cardiol. 2020, 5, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Al-Makhamreh, H.K.; Shaban, A.E.; AlHaddadin, S.S.; AlSharif, A.A.; Ghalayni, R.A.; Daoud, L.F.; Alshraideh, B.M. Is Lipoprotein (a) a Risk Factor for Coronary Artery Ectasia? Cardiol. Res. 2020, 11, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Handelsman, Y.; Jellinger, P.S.; Guerin, C.K.; Bloomgarden, Z.T.; Brinton, E.A.; Budoff, M.J.; Davidson, M.H.; Einhorn, D.; Fazio, S.; Fonseca, V.A.; et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Management of Dyslipidemia and Prevention of Cardiovascular Disease Algorithm—2020 Executive Summary. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2020, 26, 1196–1224. [Google Scholar] [CrossRef] [PubMed]
- Cartier, L.J.; Collins, C.; Lagacé, M.; Douville, P. Comparison of fasting and non-fasting lipid profiles in a large cohort of patients presenting at a community hospital. Clin. Biochem. 2018, 52, 61–66. [Google Scholar] [CrossRef]
- D’Agostino, R.B., Sr.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef]
- Multiple Risk Factor Intervention Trial. Risk factor changes and mortality results. Multiple Risk Factor Intervention Trial Research Group. JAMA 1982, 248, 1465–1477. [Google Scholar] [CrossRef]
- Yusuf, S.; Hawken, S.; Ounpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef] [PubMed]
- Hyassat, D.; Al-Saeksaek, S.; Naji, D.; Mahasneh, A.; Khader, Y.; Abujbara, M.; El-Khateeb, M.; Ajlouni, K. Dyslipidemia among patients with type 2 diabetes in Jordan: Prevalence, pattern, and associated factors. Front. Public Health 2022, 10, 1002466. [Google Scholar] [CrossRef] [PubMed]
- Haddad, F.H.; Mahafza, S.M. Impact of metabolic syndrome’s components on the development of cardiovascular disease in a Jordanian cohort with metabolic syndrome. Saudi Med. J. 2008, 29, 1299–1305. [Google Scholar]
- Spagnoli, L.G.; Bonanno, E.; Sangiorgi, G.; Mauriello, A. Role of Inflammation in Atherosclerosis. J. Nucl. Med. 2007, 48, 1800–1815. [Google Scholar] [CrossRef]
- Conroy, R.M.; Pyörälä, K.; Fitzgerald, A.P.; Sans, S.; Menotti, A.; De Backer, G.; De Bacquer, D.; Ducimetière, P.; Jousilahti, P.; Keil, U.; et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: The SCORE project. Eur. Heart J. 2003, 24, 987–1003. [Google Scholar] [CrossRef]
- Morris, N.S.; MacLean, C.D.; Chew, L.D.; Littenberg, B. The Single Item Literacy Screener: Evaluation of a brief instrument to identify limited reading ability. BMC Fam. Pract. 2006, 7, 21. [Google Scholar] [CrossRef]
- Karagiannis, A.D.; Mehta, A.; Dhindsa, D.S.; Virani, S.S.; Orringer, C.E.; Blumenthal, R.S.; Stone, N.J.; Sperling, L.S. How low is safe? The frontier of very low (<30 mg/dL) LDL cholesterol. Eur. Heart J. 2021, 42, 2154–2169. [Google Scholar] [CrossRef]
- Boekholdt, S.M.; Hovingh, G.K.; Mora, S.; Arsenault, B.J.; Amarenco, P.; Pedersen, T.R.; LaRosa, J.C.; Waters, D.D.; DeMicco, D.A.; Simes, R.J.; et al. Very low levels of atherogenic lipoproteins and the risk for cardiovascular events: A meta-analysis of statin trials. J. Am. Coll. Cardiol. 2014, 64, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D. Natural trans fat, dairy fat, partially hydrogenated oils, and cardiometabolic health: The Ludwigshafen Risk and Cardiovascular Health Study. Eur. Heart J. 2016, 37, 1079–1081. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, Y.; Sun, Q.; Pan, A.; Manson, J.E.; Rexrode, K.M.; Willett, W.C.; Rimm, E.B.; Hu, F.B. Dairy fat and risk of cardiovascular disease in 3 cohorts of US adults. Am. J. Clin. Nutr. 2016, 104, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Penson, P.E.; Banach, M. Natural compounds as anti-atherogenic agents: Clinical evidence for improved cardiovascular outcomes. Atherosclerosis 2021, 316, 58–65. [Google Scholar] [CrossRef]
- Alkhalidy, H.; Orabi, A.; Alnaser, K.; Al-Shami, I.; Alzboun, T.; Obeidat, M.D.; Liu, D. Obesity Measures as Predictors of Type 2 Diabetes and Cardiovascular Diseases among the Jordanian Population: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2021, 18, 12187. [Google Scholar] [CrossRef] [PubMed]
- Alboqai, O.K.; Suleiman, A.A.; Al-Natour, M.Q.; Al-Hourani, H.M.; Abuirmeileh, N.M. Estimated risk of coronary heart disease in obese adult males in Northern Jordan. Saudi Med. J. 2006, 27, 681–686. [Google Scholar]
- Mosleh, S.M.; Darawad, M. Patients’ Adherence to Healthy Behavior in Coronary Heart Disease: Risk Factor Management Among Jordanian Patients. J. Cardiovasc. Nurs. 2015, 30, 471–478. [Google Scholar] [CrossRef]
- Aljabery, M.A.; Rajeh Saifan, A.; Alrimawi, I.; Alzoubi, A.M.; Atout, M. The Associations Between Patients’ Characteristics and the Quality of Life Among Acute Coronary Syndrome Patients in Jordan: A Cross-Sectional Study. SAGE Open Nurs. 2022, 8, 23779608221129129. [Google Scholar] [CrossRef] [PubMed]
- Shajrawi, A.; Granat, M.; Jones, I.; Astin, F. Physical Activity and Cardiac Self-Efficacy Levels during Early Recovery after Acute Myocardial Infarction: A Jordanian Study. J. Nurs. Res. JNR 2020, 29, e131. [Google Scholar] [CrossRef]
- Tayyem, R.F.; Al-Shudifat, A.E.; Hammad, S.; Agraib, L.M.; Azab, M.; Bawadi, H. Fat intake and the risk of coronary heart disease among Jordanians. Nutr. Hosp. 2020, 37, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Azar, S.T.; Hantash, H.A.; Jambart, S.; El-Zaheri, M.M.; Rachoin, R.; Chalfoun, A.; Lahoud, L.; Okkeh, O.; Bramlage, P.; Brudi, P.; et al. Factors influencing dyslipidemia in statin-treated patients in Lebanon and Jordan: Results of the Dyslipidemia International Study. Vasc. Health Risk Manag. 2014, 10, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Stancu, C.; Sima, A. Statins: Mechanism of action and effects. J. Cell. Mol. Med. 2001, 5, 378–387. [Google Scholar] [CrossRef]
- Genser, B.; März, W. Low density lipoprotein cholesterol, statins and cardiovascular events: A meta-analysis. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2006, 95, 393–404. [Google Scholar] [CrossRef]
- Mills, E.J.; Wu, P.; Chong, G.; Ghement, I.; Singh, S.; Akl, E.A.; Eyawo, O.; Guyatt, G.; Berwanger, O.; Briel, M. Efficacy and safety of statin treatment for cardiovascular disease: A network meta-analysis of 170,255 patients from 76 randomized trials. QJM Mon. J. Assoc. Physicians 2011, 104, 109–124. [Google Scholar] [CrossRef]
- Ray, K.K.; Seshasai, S.R.; Erqou, S.; Sever, P.; Jukema, J.W.; Ford, I.; Sattar, N. Statins and all-cause mortality in high-risk primary prevention: A meta-analysis of 11 randomized controlled trials involving 65,229 participants. Arch. Intern. Med. 2010, 170, 1024–1031. [Google Scholar] [CrossRef]
- Ford, I.; Murray, H.; McCowan, C.; Packard, C.J. Long-Term Safety and Efficacy of Lowering Low-Density Lipoprotein Cholesterol with Statin Therapy: 20-Year Follow-Up of West of Scotland Coronary Prevention Study. Circulation 2016, 133, 1073–1080. [Google Scholar] [CrossRef]
- Herrington, W.G.; Emberson, J.; Mihaylova, B.; Blackwell, L.; Reith, C.; Solbu, M.D.; Mark, P.B.; Fellström, B.; Jardine, A.G.; Wanner, C.; et al. Impact of renal function on the effects of LDL cholesterol lowering with statin-based regimens: A meta-analysis of individual participant data from 28 randomised trials. Lancet Diabetes Endocrinol. 2016, 4, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Baigent, C.; Blackwell, L.; Emberson, J.; Holland, L.E.; Reith, C.; Bhala, N.; Peto, R.; Barnes, E.H.; Keech, A.; Simes, J.; et al. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar] [CrossRef]
- Fulcher, J.; O’Connell, R.; Voysey, M.; Emberson, J.; Blackwell, L.; Mihaylova, B.; Simes, J.; Collins, R.; Kirby, A.; Colhoun, H.; et al. Efficacy and safety of LDL-lowering therapy among men and women: Meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet 2015, 385, 1397–1405. [Google Scholar] [CrossRef]
- Mihaylova, B.; Emberson, J.; Blackwell, L.; Keech, A.; Simes, J.; Barnes, E.H.; Voysey, M.; Gray, A.; Collins, R.; Baigent, C. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: Meta-analysis of individual data from 27 randomised trials. Lancet 2012, 380, 581–590. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rifai, N.; Clearfield, M.; Downs, J.R.; Weis, S.E.; Miles, J.S.; Gotto, A.M., Jr. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N. Engl. J. Med. 2001, 344, 1959–1965. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Rifai, N.; Pfeffer, M.A.; Sacks, F.; Braunwald, E. Long-term effects of pravastatin on plasma concentration of C-reactive protein. The Cholesterol and Recurrent Events (CARE) Investigators. Circulation 1999, 100, 230–235. [Google Scholar] [CrossRef]
- Albert, M.A.; Danielson, E.; Rifai, N.; Ridker, P.M. Effect of statin therapy on C-reactive protein levels: The pravastatin inflammation/CRP evaluation (PRINCE): A randomized trial and cohort study. JAMA 2001, 286, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Sever, P.S.; Dahlöf, B.; Poulter, N.R.; Wedel, H.; Beevers, G.; Caulfield, M.; Collins, R.; Kjeldsen, S.E.; Kristinsson, A.; McInnes, G.T.; et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid Lowering Arm (ASCOT-LLA): A multicentre randomised controlled trial. Lancet 2003, 361, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Kearney, P.M.; Blackwell, L.; Collins, R.; Keech, A.; Simes, J.; Peto, R.; Armitage, J.; Baigent, C. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: A meta-analysis. Lancet 2008, 371, 117–125. [Google Scholar] [CrossRef]
- de Lemos, J.A.; Blazing, M.A.; Wiviott, S.D.; Lewis, E.F.; Fox, K.A.; White, H.D.; Rouleau, J.L.; Pedersen, T.R.; Gardner, L.H.; Mukherjee, R.; et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: Phase Z of the A to Z trial. JAMA 2004, 292, 1307–1316. [Google Scholar] [CrossRef]
- Bruckert, E.; Hayem, G.; Dejager, S.; Yau, C.; Bégaud, B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—The PRIMO study. Cardiovasc. Drugs Ther. 2005, 19, 403–414. [Google Scholar] [CrossRef]
- Al-Makhamreh, H.K.; Toubasi, A.A.; Obaid, Y.Y.; Albustanji, F.H. Significance of Statin-Associated Muscle Symptoms and Its Impact on Patients Adherence and Outcomes. J. Cardiovasc. Pharmacol. 2023, 81, 185–191. [Google Scholar] [CrossRef]
- Cheeley, M.K.; Saseen, J.J.; Agarwala, A.; Ravilla, S.; Ciffone, N.; Jacobson, T.A.; Dixon, D.L.; Maki, K.C. NLA scientific statement on statin intolerance: A new definition and key considerations for ASCVD risk reduction in the statin intolerant patient. J. Clin. Lipidol. 2022, 16, 361–375. [Google Scholar] [CrossRef]
- Knopp, R.H.; Gitter, H.; Truitt, T.; Bays, H.; Manion, C.V.; Lipka, L.J.; LeBeaut, A.P.; Suresh, R.; Yang, B.; Veltri, E.P. Effects of ezetimibe, a new cholesterol absorption inhibitor, on plasma lipids in patients with primary hypercholesterolemia. Eur. Heart J. 2003, 24, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.S.; Karnoub, M.C.; Devlin, D.J.; Arreaza, M.G.; Qiu, P.; Monks, S.A.; Severino, M.E.; Deutsch, P.; Palmisano, J.; Sachs, A.B.; et al. Sequence variation in NPC1L1 and association with improved LDL-cholesterol lowering in response to ezetimibe treatment. Genomics 2005, 86, 648–656. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dujovne, C.A.; Ettinger, M.P.; McNeer, J.F.; Lipka, L.J.; LeBeaut, A.P.; Suresh, R.; Yang, B.; Veltri, E.P. Efficacy and safety of a potent new selective cholesterol absorption inhibitor, ezetimibe, in patients with primary hypercholesterolemia. Am. J. Cardiol. 2002, 90, 1092–1097. [Google Scholar] [CrossRef]
- Cannon, C.P.; Blazing, M.A.; Giugliano, R.P.; McCagg, A.; White, J.A.; Theroux, P.; Darius, H.; Lewis, B.S.; Ophuis, T.O.; Jukema, J.W.; et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N. Engl. J. Med. 2015, 372, 2387–2397. [Google Scholar] [CrossRef]
- Baigent, C.; Landray, M.J.; Reith, C.; Emberson, J.; Wheeler, D.C.; Tomson, C.; Wanner, C.; Krane, V.; Cass, A.; Craig, J.; et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): A randomised placebo-controlled trial. Lancet 2011, 377, 2181–2192. [Google Scholar] [CrossRef]
- Rossebø, A.B.; Pedersen, T.R.; Boman, K.; Brudi, P.; Chambers, J.B.; Egstrup, K.; Gerdts, E.; Gohlke-Bärwolf, C.; Holme, I.; Kesäniemi, Y.A.; et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N. Engl. J. Med. 2008, 359, 1343–1356. [Google Scholar] [CrossRef]
- Sharp Collaborative, G. Study of Heart and Renal Protection (SHARP): Randomized trial to assess the effects of lowering low-density lipoprotein cholesterol among 9438 patients with chronic kidney disease. Am. Heart J. 2010, 160, 785–794.e10. [Google Scholar] [CrossRef]
- Kim, B.K.; Hong, S.J.; Lee, Y.J.; Hong, S.J.; Yun, K.H.; Hong, B.K.; Heo, J.H.; Rha, S.W.; Cho, Y.H.; Lee, S.J.; et al. Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): A randomised, open-label, non-inferiority trial. Lancet 2022, 400, 380–390. [Google Scholar] [CrossRef]
- Abifadel, M.; Varret, M.; Rabès, J.P.; Allard, D.; Ouguerram, K.; Devillers, M.; Cruaud, C.; Benjannet, S.; Wickham, L.; Erlich, D.; et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 2003, 34, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Norata, G.D.; Tibolla, G.; Catapano, A.L. Targeting PCSK9 for hypercholesterolemia. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 273–293. [Google Scholar] [CrossRef] [PubMed]
- Cho, L.; Rocco, M.; Colquhoun, D.; Sullivan, D.; Rosenson, R.S.; Dent, R.; Xue, A.; Scott, R.; Wasserman, S.M.; Stroes, E. Clinical Profile of Statin Intolerance in the Phase 3 GAUSS-2 Study. Cardiovasc. Drugs Ther. 2016, 30, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.F.; Carter, J.L.; Pearce, L.S.; Wilkins, J.T.; Overington, J.P.; Hingorani, A.D.; Casas, J.P. PCSK9 monoclonal antibodies for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2020, 10, Cd011748. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.G.; Farnier, M.; Krempf, M.; Bergeron, J.; Luc, G.; Averna, M.; Stroes, E.S.; Langslet, G.; Raal, F.J.; El Shahawy, M.; et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 2015, 372, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.A.; Turner, T.A. Are the PCSK9 inhibitors the panacea of atherosclerosis treatment? Expert Rev. Cardiovasc. Ther. 2017, 15, 491–494. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Steg, P.G.; Szarek, M.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; Hanotin, C.; Harrington, R.A.; et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N. Engl. J. Med. 2018, 379, 2097–2107. [Google Scholar] [CrossRef]
- O’Donoghue, M.L.; Giugliano, R.P.; Wiviott, S.D.; Atar, D.; Keech, A.; Kuder, J.F.; Im, K.; Murphy, S.A.; Flores-Arredondo, J.H.; López, J.A.G.; et al. Long-Term Evolocumab in Patients with Established Atherosclerotic Cardiovascular Disease. Circulation 2022, 146, 1109–1119. [Google Scholar] [CrossRef]
- Fitzgerald, K.; White, S.; Borodovsky, A.; Bettencourt, B.R.; Strahs, A.; Clausen, V.; Wijngaard, P.; Horton, J.D.; Taubel, J.; Brooks, A.; et al. A Highly Durable RNAi Therapeutic Inhibitor of PCSK9. N. Engl. J. Med. 2017, 376, 41–51. [Google Scholar] [CrossRef]
- Ray, K.K.; Landmesser, U.; Leiter, L.A.; Kallend, D.; Dufour, R.; Karakas, M.; Hall, T.; Troquay, R.P.T.; Turner, T.; Visseren, F.L.J.; et al. Inclisiran in Patients at High Cardiovascular Risk with Elevated LDL Cholesterol. N. Engl. J. Med. 2017, 376, 1430–1440. [Google Scholar] [CrossRef]
- Ray, K.K.; Wright, R.S.; Kallend, D.; Koenig, W.; Leiter, L.A.; Raal, F.J.; Bisch, J.A.; Richardson, T.; Jaros, M.; Wijngaard, P.L.J.; et al. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. N. Engl. J. Med. 2020, 382, 1507–1519. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.K.; Raal, F.J.; Kallend, D.G.; Jaros, M.J.; Koenig, W.; Leiter, L.A.; Landmesser, U.; Schwartz, G.G.; Lawrence, D.; Friedman, A.; et al. Inclisiran and cardiovascular events: A patient-level analysis of phase III trials. Eur. Heart J. 2023, 44, 129–138. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Ditmarsch, M.; Kastelein, J.J.; Rigby, S.P.; Kling, D.; Curcio, D.L.; Alp, N.J.; Davidson, M.H. Lipid lowering effects of the CETP inhibitor obicetrapib in combination with high-intensity statins: A randomized phase 2 trial. Nat. Med. 2022, 28, 1672–1678. [Google Scholar] [CrossRef] [PubMed]
- Samson, S.L.; Vellanki, P.; Blonde, L.; Christofides, E.A.; Galindo, R.J.; Hirsch, I.B.; Isaacs, S.D.; Izuora, K.E.; Low Wang, C.C.; Twining, C.L.; et al. American Association of Clinical Endocrinology Consensus Statement: Comprehensive Type 2 Diabetes Management Algorithm—2023 Update. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2023, 29, 305–340. [Google Scholar] [CrossRef] [PubMed]
| Men 20 years of age or older |
| Women 20 years of age or older |
| Regardless of age, all patients with any of the following conditions: |
| Clinical evidence of atherosclerosis |
| Abdominal aortic aneurysm |
| DM |
| Current smoker |
| Stigmata of dyslipidemia 1 |
| Family history of premature cardiovascular disease 2 |
| Family history of dyslipidemia |
| Chronic kidney disease 3 |
| Obesity 4 |
| Inflammatory diseases 5 |
| HIV infection |
| Erectile dysfunction |
| Chronic obstructive pulmonary disease |
| History of hypertensive disorder of pregnancy |
| Extremely high risk | Individuals with a history of ACS who have any of the following conditions:
|
| Very high risk | Individuals who have any of the following conditions:
|
| High risk | Individuals who have any of the following conditions:
|
| Moderate risk | Individuals who have any of the following conditions:
|
| Low risk | Based on SCORE2 and SCORE2-OP results (see Table 4) |
| Less Than 50 Years | Between 50 and 69 Years | 70 Years or Older | |
|---|---|---|---|
| Low–moderate risk | <2.5% | <5% | <7.5% |
| High risk | 2.5%–<7.5% | 5%–<10% | 7.5%–<15% |
| Very high risk | ≥7.5% | ≥10% | ≥15% |
| TC | Optimal < 200 mg/dL Borderline 201–240 mg/dL Elevated > 240 mg/dL |
| TG | Optimal < 150 mg/dL |
| HDL-C | Men > 40 mg/dL Women > 45 mg/dL |
| LDL-C | Extremely high risk: < 40 mg/dL Very high risk: < 55 mg/dL High risk: < 70 mg/dL Moderate risk: < 100 mg/dL Low risk: < 116 mg/dL |
| Decrease body weight and improve physical activity |
| Decrease intake of trans fats |
| Increase consumption of dietary fiber (fruit, vegetables, legumes, barley, and oats) |
| Maintain consumption of added sugars as less than 10% of the total intake |
| Avoid excessive alcohol intake |
| Smoking cessation |
| Rational dietary supplements such as monacolin, red yeast rice, phytosterols, dietary fiber, soy, policosanol, berberine, and n-3 fatty acids should be considered by a health professional |
| High Intensity | Moderate Intensity | Low Intensity | |
|---|---|---|---|
| Percentage of LDL-C reduction | ≥50% | 30–49% | <30% |
| Statin | Atorvastatin (40–80 mg) Rosuvastatin (20–40 mg) | Atorvastatin (10–20 mg) Rosuvastatin (5–10 mg) Simvastatin (20–40 mg) Pravastatin (40–80 mg) Fluvastatin XL (80 mg) Pitavastatin (1–4 mg) | Simvastatin (10 mg) Pravastatin (20 mg) Fluvastatin (20–40 mg) |
| Medication | Effect | Status |
|---|---|---|
| Fibrates | Lowers TG by 25–50% and elevates HDL-C by 10–25% | Mainly used to prevent TG-induced pancreatitis |
| Bile acids sequestrants (resins) | Lowers LDL-C by 18–25% without substantial effects on HDL-C levels | Induces significant reductions in cardiovascular events in patients with dyslipidemia |
| Omega 3 fatty acids | Lowers TG levels by 45% | Mainly used to reduce high serum TG levels In patients at high risk with mild-to-moderate hypertriglyceridemia, the use of a high dose of Icosapent ethyl resulted in a notable decrease in ASCVD (REDUCE-IT) |
| Bempedoic acid | Lowers LDL-C levels by approximately 30% Combined with ezetimibe, may lower LDL-C levels by 50% | Treatment with Bempedoic acid significantly reduced LDL-C, non-HDL-C, TC, Apo B, and hs-CRP The use of Bempedoic acid reduced the risk of ASCVD in high-risk patients with statin intolerance (CLEAR-OUTCOMES) |
| Obicetrapib | Lowers non-HDL-C by approximately 44% and increases HDL levels by 165% | Phase 3 clinical trial underway (BROADWAY) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Mousa, E.; Al-Azzam, S.; Araydah, M.; Karasneh, R.; Ghnaimat, M.; Al-Makhamreh, H.; Al Khawaldeh, A.; Ali Abu Al-Samen, M.; Haddad, J.; Al Najjar, S.; et al. A Jordanian Multidisciplinary Consensus Statement on the Management of Dyslipidemia. J. Clin. Med. 2023, 12, 4312. https://doi.org/10.3390/jcm12134312
Al Mousa E, Al-Azzam S, Araydah M, Karasneh R, Ghnaimat M, Al-Makhamreh H, Al Khawaldeh A, Ali Abu Al-Samen M, Haddad J, Al Najjar S, et al. A Jordanian Multidisciplinary Consensus Statement on the Management of Dyslipidemia. Journal of Clinical Medicine. 2023; 12(13):4312. https://doi.org/10.3390/jcm12134312
Chicago/Turabian StyleAl Mousa, Eyas, Sayer Al-Azzam, Mohammad Araydah, Reema Karasneh, Mohammad Ghnaimat, Hanna Al-Makhamreh, Abdelkarim Al Khawaldeh, Muneer Ali Abu Al-Samen, Jihad Haddad, Said Al Najjar, and et al. 2023. "A Jordanian Multidisciplinary Consensus Statement on the Management of Dyslipidemia" Journal of Clinical Medicine 12, no. 13: 4312. https://doi.org/10.3390/jcm12134312
APA StyleAl Mousa, E., Al-Azzam, S., Araydah, M., Karasneh, R., Ghnaimat, M., Al-Makhamreh, H., Al Khawaldeh, A., Ali Abu Al-Samen, M., Haddad, J., Al Najjar, S., Alsalaheen Abbadi, H., & Hammoudeh, A. J. (2023). A Jordanian Multidisciplinary Consensus Statement on the Management of Dyslipidemia. Journal of Clinical Medicine, 12(13), 4312. https://doi.org/10.3390/jcm12134312






