Microcyclophotocoagulation in Glaucoma Treatment: A Medium-Term Follow-Up Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IOP | Intraocular Pressure |
| MD | Mean Deviation |
| SS-OCT | Swept-Source Optical Coherence Tomography |
| TSCPC | Transscleral Cyclophotocoagulation |
| µCPC | Transscleral Microcyclophotocoagulation |
| UCP | Ultrasound Ciliary Plasty |
References
- Souissi, S.; Le Mer, Y.; Metge, F.; Portmann, A.; Baudouin, C.; Labbé, A.; Hamard, P. An update on continuous-wave cyclophotocoagulation (CW-CPC) and micropulse transscleral laser treatment (MP-TLT) for adult and paediatric refractory glaucoma. Acta Ophthalmol. 2021, 99, e621–e653. [Google Scholar] [CrossRef]
- Dastiridou, A.I.; Katsanos, A.; Denis, P.; Francis, B.A.; Mikropoulos, D.G.; Teus, M.A.; Konstas, A.-G. Cyclodestructive Procedures in Glaucoma: A Review of Current and Emerging Options. Adv. Ther. 2018, 35, 2103–2127. [Google Scholar] [CrossRef] [Green Version]
- Michelessi, M.; Bicket, A.K.; Lindsley, K. Cyclodestructive procedures for non-refractory glaucoma. Cochrane Database Syst. Rev. 2018, 4, CD009313. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.M.; Chockalingam, M.; Aquino, M.C.; Lim, Z.I.; See, J.L.; Chew, P.T. Micropulse transscleral diode laser cyclophotocoagulation in the treatment of refractory glaucoma. Clin. Exp. Ophthalmol. 2010, 38, 266–272. [Google Scholar] [CrossRef]
- Aquino, M.C.D.; Barton, K.; Tan, A.M.W.T.; Sng, C.; Li, X.; Loon, S.C.; Chew, P.T.K. Micropulse versus continuous wave transscleral diode cyclophotocoagulation in refractory glaucoma: A randomized exploratory study. Clin. Exp. Ophthalmol. 2015, 43, 40–46. [Google Scholar] [CrossRef]
- Maslin, J.S.; Chen, P.P.; Sinard, J.; Nguyen, A.T.; Noecker, R. Histopathologic changes in cadaver eyes after MicroPulse and continuous wave transscleral cyclophotocoagulation. Can. J. Ophthalmol. 2020, 55, 330–335. [Google Scholar] [CrossRef]
- Moussa, K.; Feinstein, M.; Pekmezci, M.; Lee, J.H.; Bloomer, M.; Oldenburg, C.; Sun, Z.; Lee, R.K.; Ying, G.-S.; Han, Y. Histologic Changes following Continuous Wave and Micropulse Transscleral Cyclophotocoagulation: A Randomized Comparative Study. Transl. Vis. Sci. Technol. 2020, 9, 22. [Google Scholar] [CrossRef]
- Kotula, M.A.; Paust, K.; Wirdemann, A.; Msigomba, E.; Burusu, L. Glaucoma treatment by transscleral cyclophotocoagulation in micropulse technology in a low-income setting. Die Ophthalmol. 2022, 119, 1275–1279. [Google Scholar] [CrossRef] [PubMed]
- Shaarawy, T.M.; Sherwood, M.B.; Grehn, F. Guidelines on Design and Reporting of Surgical Trials—World Glaucoma Association; Kugler Publications: Amsterdam, The Netherlands, 2009; ISBN 978-90-6299-219-5. [Google Scholar]
- Sanchez, F.G.; Peirano-Bonomi, J.C.; Grippo, T.M. Micropulse Transscleral Cyclophotocoagulation: A Hypothesis for the Ideal Parameters. Med. Hypothesis Discov. Innov. Ophthalmol. 2018, 7, 94–100. [Google Scholar] [PubMed]
- Sanchez, F.G.; Peirano-Bonomi, J.C.; Barbosa, N.B.; Khoueir, Z.; Grippo, T.M. Update on Micropulse Transscleral Cyclophotocoagulation. J. Glaucoma 2020, 29, 598–603. [Google Scholar] [CrossRef]
- Abdelmassih, Y.; Tomey, K.; Khoueir, Z. Micropulse Transscleral Cyclophotocoagulation. J. Curr. Glaucoma Pract. 2021, 15, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Emanuel, M.E.; Grover, D.S.; Fellman, R.L.; Godfrey, D.G.; Smith, O.; Butler, M.R.; Kornmann, H.L.; Feuer, W.J.; Goyal, S. Micropulse Cyclophotocoagulation: Initial Results in Refractory Glaucoma. J. Glaucoma 2017, 26, 726–729. [Google Scholar] [CrossRef]
- Williams, A.L.; Moster, M.R.; Rahmatnejad, K.; Resende, A.F.; Horan, T.; Reynolds, M.; Yung, E.; Abramowitz, B.; Kuchar, S.; Waisbourd, M. Clinical Efficacy and Safety Profile of Micropulse Transscleral Cyclophotocoagulation in Refractory Glaucoma. J. Glaucoma 2018, 27, 445–449. [Google Scholar] [CrossRef]
- Varikuti, V.N.; Shah, P.; Rai, O.; Chaves, A.C.; Miranda, A.; Lim, B.-A.; Dorairaj, S.K.; Sieminski, S.F. Outcomes of Micropulse Transscleral Cyclophotocoagulation in Eyes with Good Central Vision. J. Glaucoma 2019, 28, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Kass, M.A.; Heuer, D.K.; Higginbotham, E.J.; Johnson, C.; Keltner, J.L.; Miller, J.P.; Parrish, R.K.; Wilson, M.R.; Gordon, M.O. The Ocular Hypertension Treatment Study: A randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch. Ophthalmol. 2002, 120, 701–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heijl, A.; Leske, M.C.; Bengtsson, B.; Hyman, L.; Bengtsson, B.; Hussein, M. Reduction of Intraocular Pressure and Glaucoma Progression: Results from the Early Manifest Glaucoma Trial. Arch. Ophthalmol. 2002, 120, 1268–1279. [Google Scholar] [CrossRef]
- Frezzotti, P.; Mittica, V.; Martone, G.; Motolese, I.; Lomurno, L.; Peruzzi, S.; Motolese, E. Longterm follow-up of diode laser transscleral cyclophotocoagulation in the treatment of refractory glaucoma. Acta Ophthalmol. 2010, 88, 150–155. [Google Scholar] [CrossRef]
- Grueb, M.; Rohrbach, J.M.; Bartz-Schmidt, K.U.; Schlote, T. Transscleral diode laser cyclophotocoagulation as primary and secondary surgical treatment in primary open-angle and pseudoexfoliatve glaucoma. Graefe’s Arch. Clin. Exp. Ophthalmol. 2006, 244, 1293–1299. [Google Scholar] [CrossRef]
- Jankowska-Szmul, J.; Dobrowolski, D.; Wylegala, E. CO2 laser-assisted sclerectomy surgery compared with trabeculectomy in primary open-angle glaucoma and exfoliative glaucoma. A 1-year follow-up. Acta Ophthalmol. 2018, 96, e582–e591. [Google Scholar] [CrossRef] [Green Version]
- Vernon, S.A.; Koppens, J.M.; Menon, G.J.; Negi, A.K. Diode laser cycloablation in adult glaucoma: Long-term results of a standard protocol and review of current literature. Clin. Exp. Ophthalmol. 2006, 34, 411–420. [Google Scholar] [CrossRef]
- Walland, M.J. Diode laser cyclophotocoagulation: Longer term follow up of a standardized treatment protocol. Clin. Exp. Ophthalmol. 2000, 28, 263–267. [Google Scholar] [CrossRef]
- Pucci, V.; Tappainer, F.; Borin, S.; Bellucci, R. Long-Term Follow-Up after Transscleral Diode Laser Photocoagulation in Refractory Glaucoma. Ophthalmologica 2003, 217, 279–283. [Google Scholar] [CrossRef]
- Pantcheva, M.B.; Kahook, M.Y.; Schuman, J.S.; Noecker, R.J. Comparison of acute structural and histopathological changes in human autopsy eyes after endoscopic cyclophotocoagulation and trans-scleral cyclophotocoagulation. Br. J. Ophthalmol. 2007, 91, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.C.; Chen, M.J.; Lin, M.S.; Howes, E.; Stamper, R.L. Vascular effects on ciliary tissue from endoscopic versus trans-scleral cyclophotocoagulation. Br. J. Ophthalmol. 2006, 90, 496–500. [Google Scholar] [CrossRef] [Green Version]
- Berke, S.; Cohen, A.; Sturm, R.; Caronia, R.; Nelson, D. Endoscopic cyclophotocoagulation (Ecp) and phacoemulsification in the treatment of medically controlled primary open-angle glaucoma. J. Glaucoma 2000, 9, 129. [Google Scholar] [CrossRef]
- Siegel, M.J.; Boling, W.S.; Faridi, O.S.; Gupta, C.K.; Kim, C.; Boling, R.C.; Citron, M.E.; Siegel, M.J.; Siegel, L.I. Combined endoscopic cyclophotocoagulation and phacoemulsification versus phacoemulsification alone in the treatment of mild to moderate glaucoma. Clin. Exp. Ophthalmol. 2015, 43, 531–539. [Google Scholar] [CrossRef]
- Noecker, R.J.; Kahook, M.Y.; Berke, S.J.M.; Nichamin, L.D.; Weston, J.-M.; Mackool, R.; Tyson, F.; Lima, F.; Kleinfeldt, N. Uncontrolled Intraocular Pressure after Endoscopic Cyclophotocoagulation. J. Glaucoma 2008, 17, 250–251. [Google Scholar] [CrossRef] [PubMed]
- Noecker, R.J.; ECS Group. Complications of Endoscopic Cyclophotocoagulation. In Proceedings of the ASCRS Symposium on Cataract, IOL and Refractive Surgery, San Diego, CA, USA, 27 April–2 May 2007. [Google Scholar]
- Schubert, H.D.; Agarwala, A. Quantitative CW Nd:YAG Pars Plana Transscleral Photocoagulation in Postmortem Eyes. Ophthalmic Surg. 1990, 21, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.-J.; Mizukawa, A.; Okisaka, S. Mechanism of Intraocular Pressure Decrease after Contact Transscleral Continuous-Wave Nd:YAG Laser Cyclophotocoagulation. Ophthalmic Res. 1994, 26, 65–79. [Google Scholar] [CrossRef]
- Barac, R.; Vuzitas, M.; Balta, F. Choroidal thickness increase after micropulse transscleral cyclophotocoagulation. Rom. J. Ophthalmol. 2018, 61, 144–148. [Google Scholar] [CrossRef]
- Johnstone, M.A.; Song, S.; Padilla, S.; Wen, K.; Xin, C.; Wen, J.C.; Martin, E.; Wang, R.K. Microscope Real-Time Video (MRTV), High-Resolution OCT (HROCT) & Histopathology (HP) to Assess How Transcleral Micropulse Laser (TML) Affects the Sclera, Ciliary Body (CB), Muscle (CM), Secretory Epithelium (CBSE), Suprachoroidal Space (SCS) & Aqueous Outflow System. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2825. [Google Scholar]
- Quigley, H.A. Improved Outcomes for Transscleral Cyclophotocoagulation Through Optimized Treatment Parameters. J. Glaucoma 2018, 27, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.; Maslin, J.; Noecker, R.J. Early results of micropulse transscleral cyclophotocoagulation for the treatment of glaucoma. Eur. J. Ophthalmol. 2020, 30, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Shi, Y.; Amoozgar, B.; Aderman, C.; Campomanes, A.D.A.; Lin, S.; Han, Y. Outcome of Micropulse Laser Transscleral Cyclophotocoagulation on Pediatric versus Adult Glaucoma Patients. J. Glaucoma 2017, 26, 936–939. [Google Scholar] [CrossRef]
- Sarrafpour, S.; Saleh, D.; Ayoub, S.; Radcliffe, N.M. Micropulse Transscleral Cyclophotocoagulation: A Look at Long-Term Effectiveness and Outcomes. Ophthalmol. Glaucoma 2019, 2, 167–171. [Google Scholar] [CrossRef]
- Abdelrahman, A.M.; El Sayed, Y.M. Micropulse versus Continuous Wave Transscleral Cyclophotocoagulation in Refractory Pediatric Glaucoma. J. Glaucoma 2018, 27, 900–905. [Google Scholar] [CrossRef]
- Subramaniam, K.; Price, M.O.; Feng, M.T.; Price, F.W. Micropulse Transscleral Cyclophotocoagulation in Keratoplasty Eyes. Cornea 2019, 38, 542–545. [Google Scholar] [CrossRef]
- Magacho, L.; Lima, F.E.; Ávila, M.P. Double-Session Micropulse Transscleral Laser (CYCLO G6) as a Primary Surgical Procedure for Glaucoma. J. Glaucoma 2020, 29, 205–210. [Google Scholar] [CrossRef]
- Aquino, M.C.D.; Chew, P.T. Long-Term Efficacy of MicroPulse Diode Transscleral Cyclophotocoagulation in the Treatment of Refractory Glaucoma. Malay 2016, 3, 22. [Google Scholar]
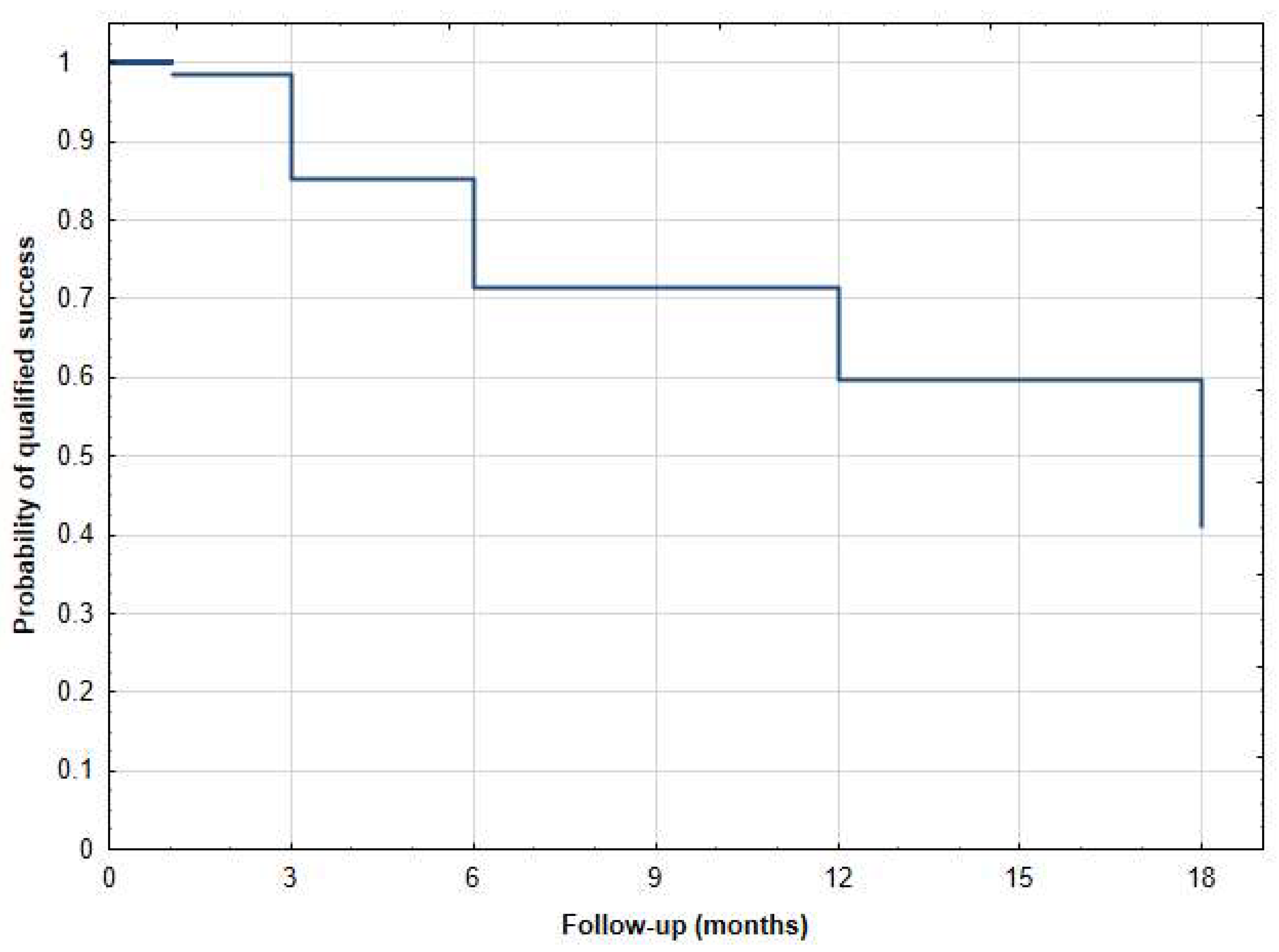
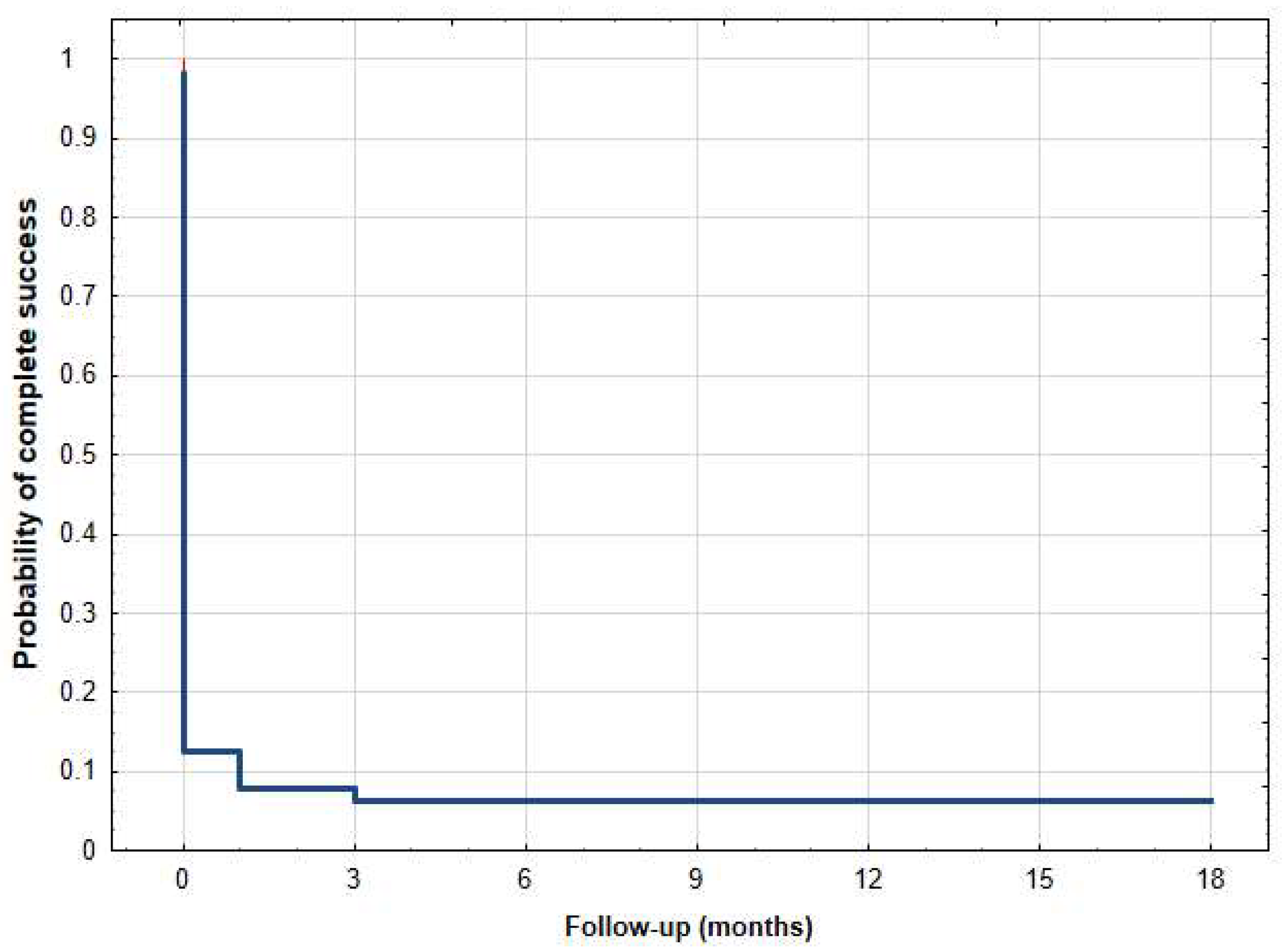
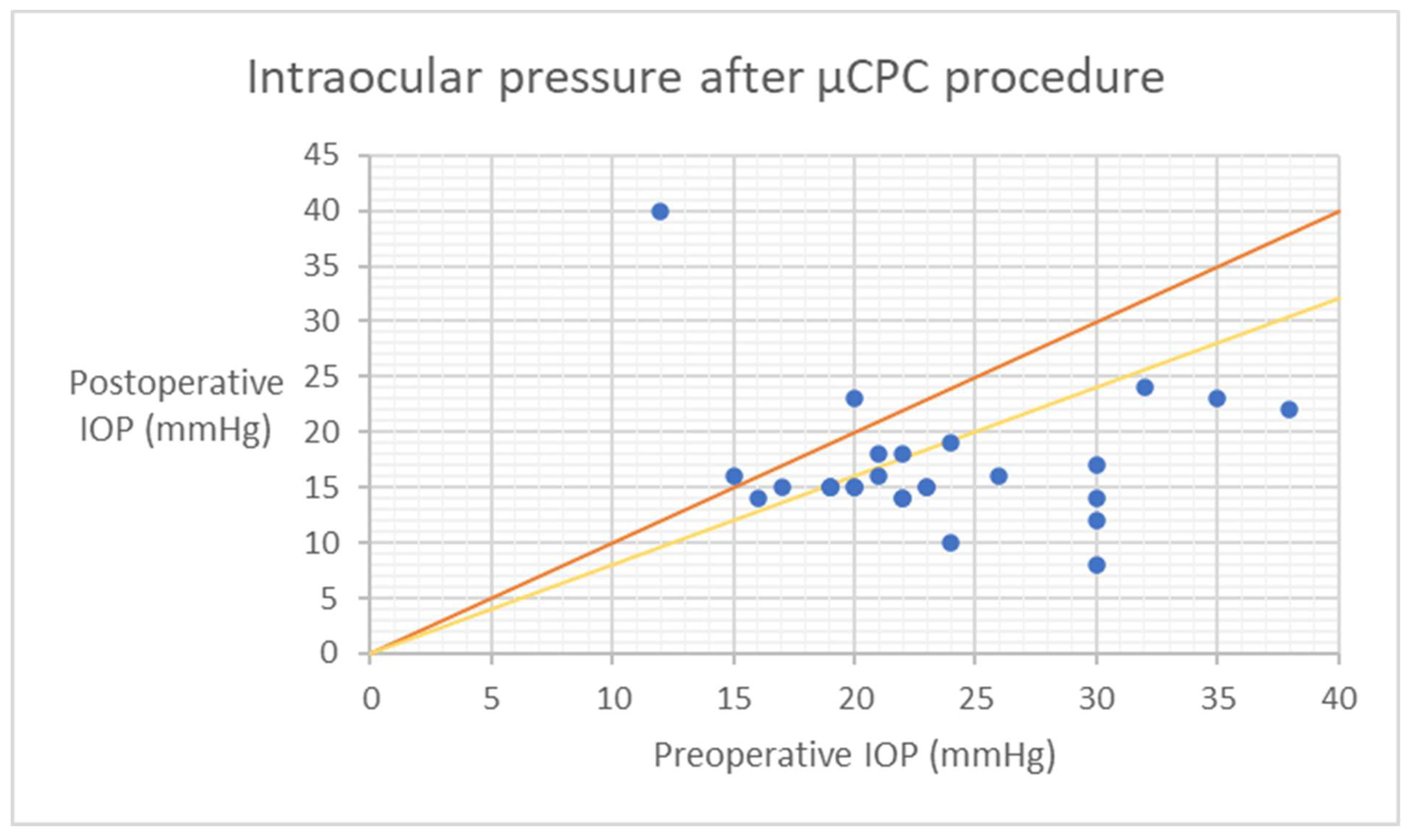
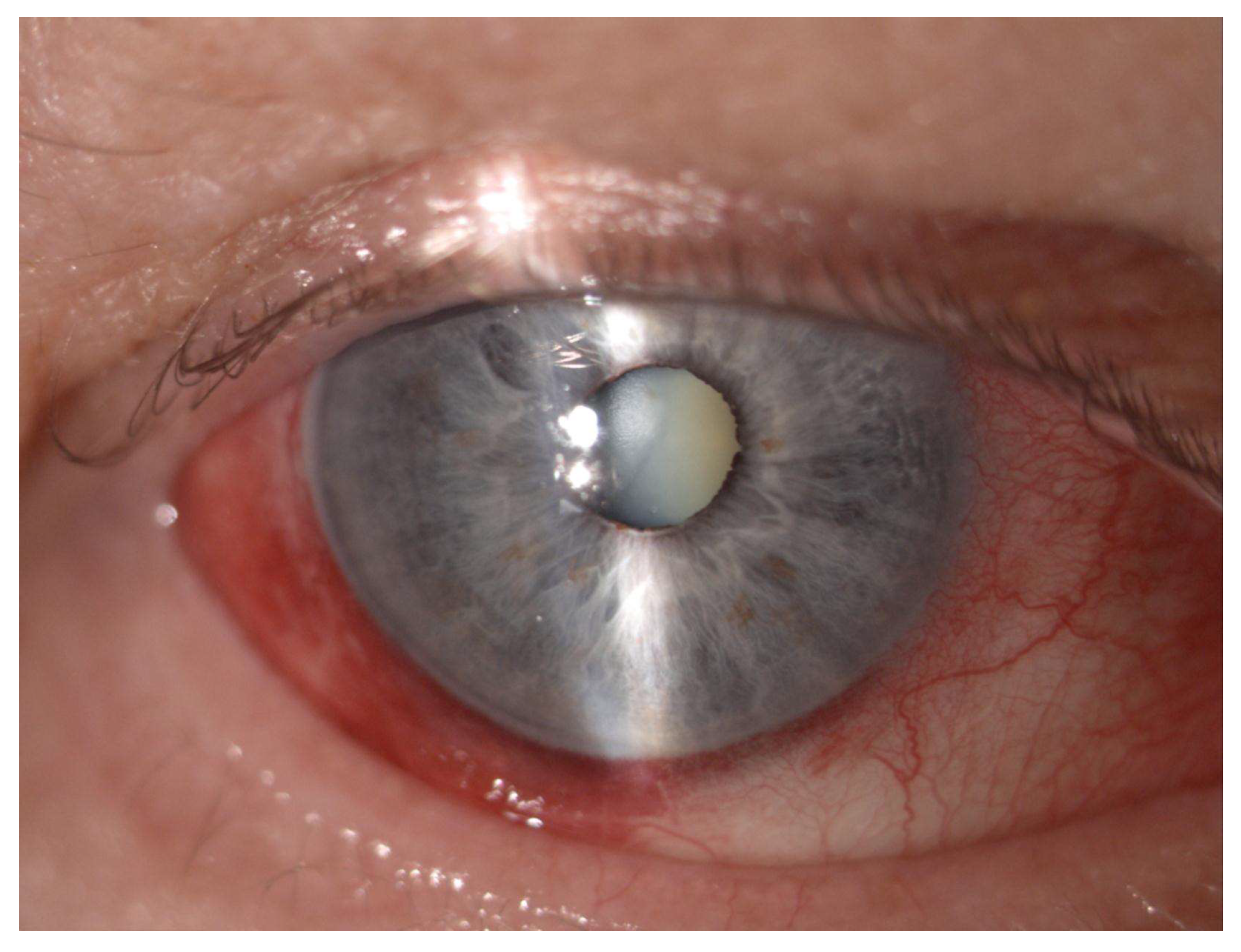

| Age (Years), Mean (SD) [Range] | 69.00 ± 14.22 [30–92] |
|---|---|
| Gender (male/female) | 29/35 |
| Type of glaucoma: | |
| Primary open-angle glaucoma | 37 |
| Secondary open-angle glaucoma | |
| Post-penetrating keratoplasty glaucoma | 13 |
| Exfoliative | 0 |
| Pigmentary | 0 |
| Uveitic glaucoma | 2 |
| Neovascular glaucoma | 3 |
| Other | 2 |
| Primary angle-closure glaucoma | 4 |
| Secondary angle-closure glaucoma | 1 |
| Aniridic glaucoma | 2 |
| Prior glaucoma surgeries: | |
| Trabeculectomy | 9 |
| Deep sclerectomy | 1 |
| Surgery (tube/stents) | 2 |
| Transscleral cyclophotocoagulation | 16 |
| Ultrasound Cyclo Plasty | 5 |
| Lens status: | |
| Phakic | 35 |
| Pseudophakic | 29 |
| Aphakic | 0 |
| Mean IOP ± SD | p-Value | Number of Hypotensive Medications ± SD | p-Value | % IOP Reduction | No. Patietns | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Preop | 25.1 | ± | 8.4 | 4.2 | ± | 1 | - | 64 | ||
| 1 day | 17.3 | ± | 4.5 | p < 0.001 | 2.4 | ± | 1.1 | p < 0.001 | 31.2 | 60 |
| 1 week | 16.5 | ± | 6.1 | p < 0.001 | 2.6 | ± | 1.1 | p < 0.001 | 34.4 | 59 |
| 1 month | 20.5 | ± | 8.3 | p < 0.001 | 2.7 | ± | 1.1 | p < 0.001 | 18.6 | 55 |
| 3 months | 17.1 | ± | 6.2 | p < 0.001 | 3 | ± | 1.2 | p < 0.001 | 32.1 | 46 |
| 6 months | 18 | ± | 7.1 | p < 0.001 | 3 | ± | 1.1 | p < 0.001 | 28.4 | 35 |
| 12 months | 15.8 | ± | 3.2 | p < 0.001 | 3.3 | ± | 1 | p < 0.001 | 37 | 30 |
| 18 months | 17 | ± | 5.9 | p < 0.001 | 3.3 | ± | 1.1 | p < 0.001 | 32.5 | 27 |
| Complications | |
|---|---|
| Intraoperative: | |
| Pain | 15/64 (23.4%) |
| Corneal thermal injury | 0/64 (0%) |
| Subconjunctival hemorrhage | 38/64 (59.4%) |
| Postoperative: | |
| Conjunctival hyperemia | 44/64 (68.8%) |
| Epithelial defects | 0/64 (0%) |
| Corneal edema | 0/64 (0%) |
| Subconjunctival hemorrhage | 0/64 (0%) |
| Hypotony, choroid detachment | 1/64 (1.6%) |
| Retinal detachment | 0/64 (0%) |
| Cataract | 0/64 (0%) |
| Phthisis bulbi | 0/64 (0%) |
| Scleral mark | 0/64 (0%) |
| Uveitis | 2/64 (3.1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolek, B.; Wylęgała, A.; Wylęgała, E. Microcyclophotocoagulation in Glaucoma Treatment: A Medium-Term Follow-Up Study. J. Clin. Med. 2023, 12, 4342. https://doi.org/10.3390/jcm12134342
Bolek B, Wylęgała A, Wylęgała E. Microcyclophotocoagulation in Glaucoma Treatment: A Medium-Term Follow-Up Study. Journal of Clinical Medicine. 2023; 12(13):4342. https://doi.org/10.3390/jcm12134342
Chicago/Turabian StyleBolek, Bartłomiej, Adam Wylęgała, and Edward Wylęgała. 2023. "Microcyclophotocoagulation in Glaucoma Treatment: A Medium-Term Follow-Up Study" Journal of Clinical Medicine 12, no. 13: 4342. https://doi.org/10.3390/jcm12134342





