Acute Changes in Right Ventricular Function in Pediatric Patients with Pulmonary Valve Stenosis Undergoing Percutaneous Valvuloplasty: A Speckle-Tracking Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Demographic and Clinical Data
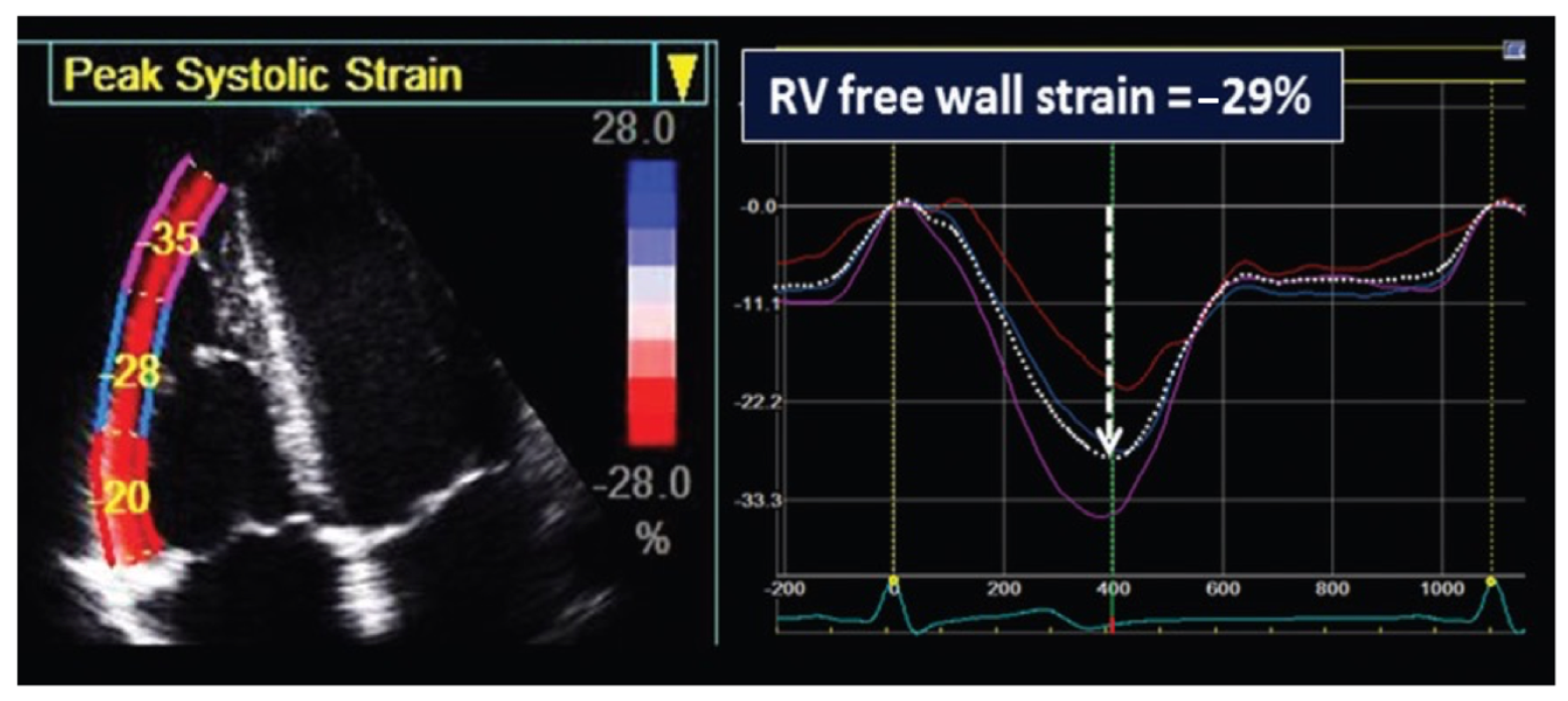
2.3. Standard and Speckle-Tracking Echocardiography
2.4. Interventional Procedure Data
2.5. Statistical Analysis
3. Results
3.1. Baseline Demographic and Echocardiographic Characteristics
3.2. Procedural Data and Efficacy of BPV
3.3. Echocardiographic Morphologic and Doppler Parameters
3.4. Echocardiographic Right Ventricle Functional Assessment
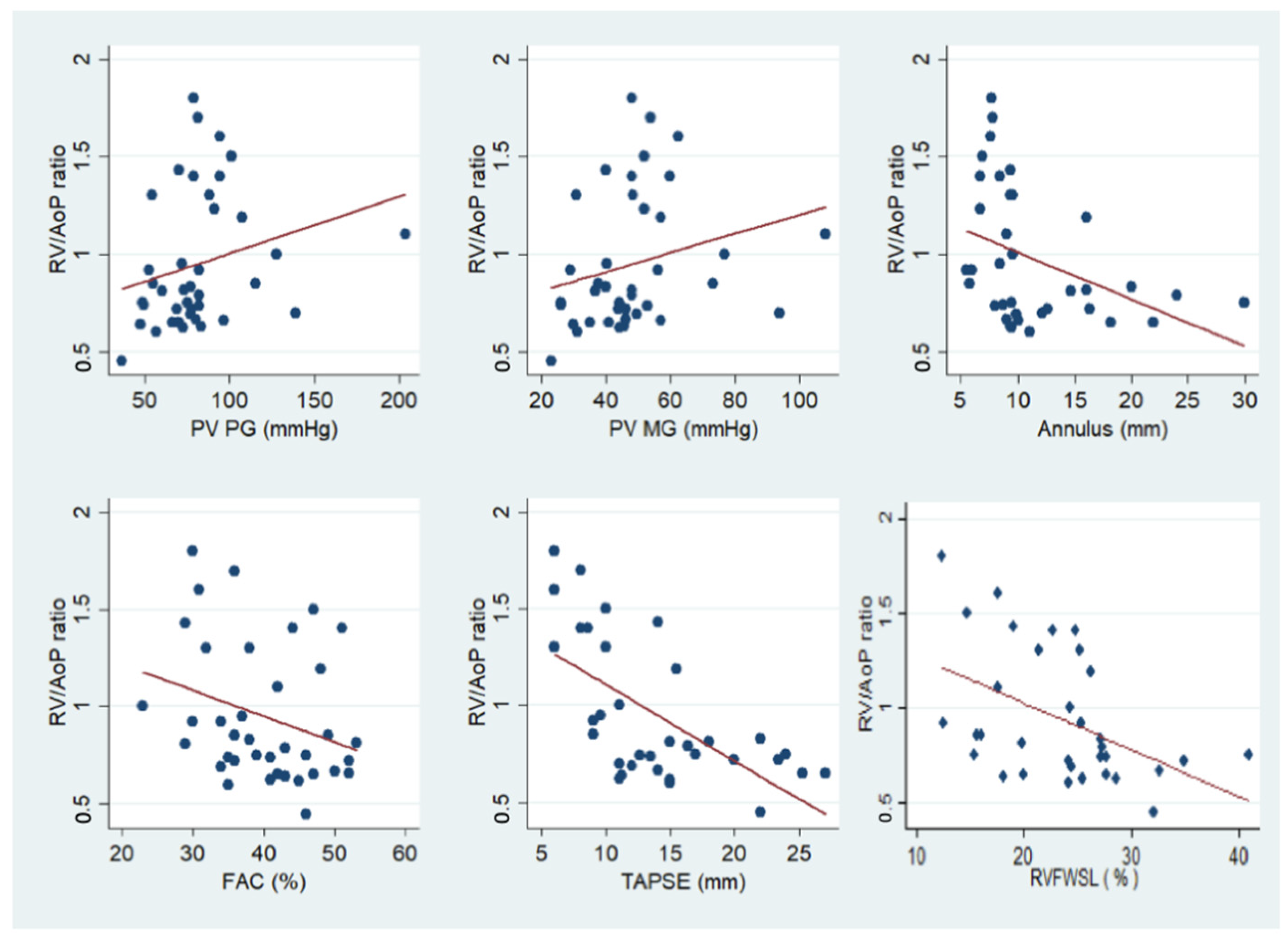
3.5. Correlation between Hemodynamic and Echocardiographic Parameters
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pansky, B. Review of Medical Embryology; Macmillan: New York, NY, USA, 1982. [Google Scholar]
- Moorman, A.; Webb, S.; Brown, N.A.; Lamers, W.; Anderson, R.H. Development of the heart: (1) formation of the cardiac chambers and arterial trunks. Heart 2003, 89, 806–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betts, J.G.; Young, K.A.; Wise, J.A.; Johnson, E.; Poe, B.; Kruse, D.H.; Korol, O.; Johnson, J.E.; Womble, M.; DeSaix, P. Anatomy and Physiology; Rice University: Houston, TX, USA, 2013. [Google Scholar]
- Gikonyo, B.M.; Lucas, R.V.; Edwards, J.E. Anatomic features of congenital pulmonary valvar stenosis. Pediatr. Cardiol. 1987, 8, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Kan, J.S.; White, R.I.; Mitchell, S.E.; Gardner, T.J. Percutaneous balloon valvuloplasty: A new method for treating congenital pulmonary-valve stenosis. N. Engl. J. Med. 1982, 307, 540–542. [Google Scholar] [CrossRef]
- Stanger, P.; Cassidy, S.C.; Girod, D.A.; Kan, J.S.; Lababidi, Z.; Shapiro, S.R. Balloon pulmonary valvuloplasty: Results of the Valvuloplasty and Angioplasty of Congenital Anomalies Registry. Am. J. Cardiol. 1990, 65, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Rey, C.; Marache, P.; Francart, C.; Dupuis, C. Percutaneous transluminal balloon valvuloplasty of congenital pulmonary valve stenosis, with a special report on infants and neonates. J. Am. Coll. Cardiol. 1988, 11, 815–820. [Google Scholar] [CrossRef] [Green Version]
- Kveselis, D.A.; Rocchini, A.P.; Snider, A.R.; Rosenthal, A.; Crowley, D.C.; Dick, M. Results of balloon valvuloplasty in the treatment of congenital valvar pulmonary stenosis in children. Am. J. Cardiol. 1985, 56, 527–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCrindle, B.W. Independent predictors of long-term results after balloon pulmonary valvuloplasty. Valvuloplasty and Angioplasty of Congenital Anomalies (VACA) Registry Investigators. Circulation 1994, 89, 1751–1759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarrar, M.; Betbout, F.; Farhat, M.B.; Maatouk, F.; Gamra, H.; Addad, F.; Hammami, S.; Hamda, K.B. Long-term invasive and noninvasive results of percutaneous balloon pulmonary valvuloplasty in children, adolescents, and adults. Am. Heart. J. 1999, 138, 950–954. [Google Scholar] [CrossRef]
- Masura, J.; Burch, M.; Deanfield, J.E.; Sullivan, I.D. Five-year follow-up after balloon pulmonary valvuloplasty. J. Am. Coll. Cardiol. 1993, 21, 132–136. [Google Scholar] [CrossRef] [Green Version]
- Gozar, L.; Iancu, M.; Gozar, H.; Sglimbea, A.; Cerghit Paler, A.; Gabor-Miklosi, D.; Toganel, R.; Făgărășan, A.; Iurian, D.R.; Toma, D. Assessment of Biventricular Myocardial Function with 2-Dimensional Strain and Conventional Echocardiographic Parameters: A Comparative Analysis in Healthy Infants and Patients with Severe and Critical Pulmonary Stenosis. J. Pers. Med. 2022, 12, 57. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. Available online: https://journals.scholarsportal.info/details/08947317/v28i0001/1_rfccqbteaoci.xml (accessed on 12 July 2022). [CrossRef] [PubMed] [Green Version]
- Zoghbi, W.A.; DiCarli, M.F.; Blankstein, R.; Choi, A.D.; Dilsizian, V.; Flachskampf, F.A.; Geske, J.B.; Grayburn, P.A.; Jaffer, F.A.; Kwong, R.Y.; et al. Multimodality Cardiovascular Imaging in the Midst of the COVID-19 Pandemic. JACC Cardiovasc. Imaging 2020, 13, 1615–1626. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1936878X20304745 (accessed on 29 January 2021). [CrossRef]
- Sirico, D.; Castaldi, B.; Ciliberti, P.; Sabatino, J.; Cazzoli, I.; Secinaro, A.; Calcaterra, G.; Oreto, L.; Calabrò, M.P.; Chessa, M.; et al. Cardiac imaging in congenital heart disease during the coronavirus disease-2019 pandemic: Recommendations from the Working Group on Congenital Heart Disease of the Italian Society of Cardiology. J. Cardiovasc. Med. 2020, 21, 467–471. Available online: https://journals.lww.com/10.2459/JCM.0000000000000990 (accessed on 29 January 2021). [CrossRef] [PubMed]
- Benson, M.J.; Silverton, N.; Morrissey, C.; Zimmerman, J. Strain Imaging: An Everyday Tool for the Perioperative Echocardiographer. J. Cardiothorac. Vasc. Anesth. 2020, 34, 2707–2717. [Google Scholar] [CrossRef] [PubMed]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Industry representatives, Reviewers: This document was reviewed by members of the 2016–2018 EACVI Scientific Documents Committee. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar]
- Rao, P.S. Percutaneous balloon pulmonary valvuloplasty: State of the art. Catheter. Cardiovasc. Interv. 2007, 69, 747–763. [Google Scholar] [CrossRef]
- Holzer, R.J.; Gauvreau, K.; Kreutzer, J.; Trucco, S.M.; Torres, A.; Shahanavaz, S.; Bergersen, L. Safety and efficacy of balloon pulmonary valvuloplasty: A multicenter experience. Catheter. Cardiovasc. Interv. 2012, 80, 663–672. [Google Scholar] [CrossRef]
- Vanderpool, R.R.; Pinsky, M.R.; Naeije, R.; Deible, C.; Kosaraju, V.; Bunner, C.; Mathier, M.A.; Lacomis, J.; Champion, H.C.; Simon, M.A. RV-pulmonary arterial coupling predicts outcome in patients referred for pulmonary hypertension. Heart 2015, 101, 37–43. Available online: https://heart.bmj.com/content/101/1/37 (accessed on 24 March 2023). [CrossRef] [Green Version]
- Vitarelli, A.; Sardella, G.; Roma, A.D.; Capotosto, L.; De Curtis, G.; D’Orazio, S.; Cicconetti, P.; Battaglia, D.; Caranci, F.; De Maio, M.; et al. Assessment of right ventricular function by three-dimensional echocardiography and myocardial strain imaging in adult atrial septal defect before and after percutaneous closure. Int. J. Cardiovasc. Imaging 2012, 28, 1905–1916. [Google Scholar] [CrossRef]
- Schubert, U.; Müller, M.; Norman, M.; Abdul-Khaliq, H. Transition from fetal to neonatal life: Changes in cardiac function assessed by speckle-tracking echocardiography. Early Hum. Dev. 2013, 89, 803–808. Available online: https://www.sciencedirect.com/science/article/pii/S0378378213001473 (accessed on 28 September 2022). [CrossRef] [Green Version]
- Qu, Y.-Y.; Li, H.; Rottbauer, W.; Ma, G.-S.; Buckert, D.; Rasche, V. Right ventricular free wall longitudinal strain and strain rate quantification with cardiovascular magnetic resonance based tissue tracking. In Int. J. Cardiovasc. Imaging; 2020; 36, pp. 1985–1996. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7497525/ (accessed on 28 September 2022). [CrossRef] [PubMed]
- Monti, C.B.; Secchi, F.; Capra, D.; Guarnieri, G.; Lastella, G.; Barbaro, U.; Carminati, M.; Sardanelli, F. Right ventricular strain in repaired Tetralogy of Fallot with regards to pulmonary valve replacement. Eur. J. Radiol. 2020, 131, 109235. [Google Scholar] [CrossRef]
- Costantini, P.; Perone, F.; Siani, A.; Groenhoff, L.; Muscogiuri, G.; Sironi, S.; Marra, P.; Carriero, S.; Pavon, A.G.; Guglielmo, M. Multimodality Imaging of the Neglected Valve: Role of Echocardiography, Cardiac Magnetic Resonance and Cardiac Computed Tomography in Pulmonary Stenosis and Regurgitation. J. Imaging 2022, 8, 278. Available online: https://www.mdpi.com/2313-433X/8/10/278 (accessed on 9 June 2023). [CrossRef] [PubMed]
- Kawakubo, M.; Yamasaki, Y.; Toyomura, D.; Yamamura, K.; Sakamoto, I.; Moriyama, T.; Yabuuchi, H.; Ishigami, K. Unchanged right ventricular strain in repaired tetralogy of Fallot after pulmonary valve replacement with radial long-axis cine magnetic resonance images. In Sci. Rep.; 2021; 11, p. 18879. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8460644/ (accessed on 27 December 2022). [CrossRef] [PubMed]


| Demographic Data | Value * |
|---|---|
| Age (years) | 0.5 (0.1–4.0) |
| Age groups | |
| Neonates (%) | 9 (21) |
| Infants (%) | 18 (42) |
| Children (%) | 11 (25) |
| Adolescents (%) | 5 (12) |
| Female gender (%) | 24 (56) |
| Weight (kg) | 7.6 (4.8–15.7) |
| BSA (m2) | 0.2 (0.1–0.6) |
| Comorbidities | |
| Noonan syndrome | 3 (7) |
| PFO/small ASD | 7 (16) |
| Small PDA | 6 (14) |
| Tricuspid dysplasia | 1 (2) |
| Pre Valvuloplasty | Post Valvuloplasty | p-Value | |
|---|---|---|---|
| Peak-to-peak PG (mmHg) | 49.1 ± 13.7 | 18.3 ± 10.7 | <0.001 |
| RV/AoP ratio | 1.0 ± 0.4 | 0.5 ± 0.2 | <0.001 |
| 2D TTE | Pre-Procedure | Post-Procedure | p-Value |
|---|---|---|---|
| Pulmonary regurgitation | |||
| Mild (%) | 3(8) | 11 (29) | 0.007 |
| Moderate (%) | 3(8) | 4 (11) | 0.15 |
| Severe (%) | 0 (0) | 0 (0) | 1 |
| PV peak velocity (m/s) | 4.4 ± 0.8 | 2.9 ± 0.7 | <0.001 |
| PV Peak PG (mmHg) | 81.1 ± 29.5 | 35.5 ± 17.3 | <0.001 |
| PV Mean PG (mmHg) | 48.2 ± 17.3 | 20.1 ± 9.9 | <0.001 |
| RVSP (mmHg) | 83.0 ± 30.8 | 37.3 ± 17.0 | <0.001 |
| RV length (mm) | 38.7 ± 13.7 | 38.7 ± 13.6 | 0.59 |
| RV basal diameter (mm) | 22.6 ± 8.0 | 22.3 ± 7.7 | 0.97 |
| 2D TTE | Pre-Procedure | Post-Procedure | p-Value |
|---|---|---|---|
| FAC (%) | 39.5 ± 7.7 | 44.5 ± 9.6 | 0.034 |
| TAPSE (mm) | 13.9 ± 6.0 | 13.4 ± 7.2 | 0.79 |
| 2D STE | |||
| RVFWSL (%) | −22.8 ± 5.5 | −25.3 ± 6.3 | 0.18 |
| Basal (%) | −23.0 ± 6.5 | −23.1 ± 6.2 | 0.95 |
| Mid (%) | −24.0 ± 6.7 | −26.3 ± 8.0 | 0.33 |
| Apical (%) | −21.4 ± 7.5 | −26.6 ± 7.5 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirico, D.; Spigariol, G.; Mahmoud, H.T.; Basso, A.; Cuppini, E.; Avesani, M.; Sabatino, J.; Castaldi, B.; Di Salvo, G. Acute Changes in Right Ventricular Function in Pediatric Patients with Pulmonary Valve Stenosis Undergoing Percutaneous Valvuloplasty: A Speckle-Tracking Study. J. Clin. Med. 2023, 12, 4344. https://doi.org/10.3390/jcm12134344
Sirico D, Spigariol G, Mahmoud HT, Basso A, Cuppini E, Avesani M, Sabatino J, Castaldi B, Di Salvo G. Acute Changes in Right Ventricular Function in Pediatric Patients with Pulmonary Valve Stenosis Undergoing Percutaneous Valvuloplasty: A Speckle-Tracking Study. Journal of Clinical Medicine. 2023; 12(13):4344. https://doi.org/10.3390/jcm12134344
Chicago/Turabian StyleSirico, Domenico, Giulia Spigariol, Heba Talat Mahmoud, Alessia Basso, Elena Cuppini, Martina Avesani, Jolanda Sabatino, Biagio Castaldi, and Giovanni Di Salvo. 2023. "Acute Changes in Right Ventricular Function in Pediatric Patients with Pulmonary Valve Stenosis Undergoing Percutaneous Valvuloplasty: A Speckle-Tracking Study" Journal of Clinical Medicine 12, no. 13: 4344. https://doi.org/10.3390/jcm12134344






