Risk Scoring Systems for Preterm Birth and Their Performance: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blencowe, H.; Cousens, S.; Oestergaard, M.Z.; Chou, D.; Moller, A.-B.; Narwal, R.; Adler, A.; Garcia, C.V.; Rohde, S.; Say, L.; et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: A systematic analysis and implications. Lancet 2012, 379, 2162–2172. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, R.L.; Gravett, M.G.; Iams, J.; Papageorghiou, A.T.; Waller, S.A.; Kramer, M.; Culhane, J.; Barros, F.; Conde-Agudelo, A.; Bhutta, Z.A.; et al. The preterm birth syndrome: Issues to consider in creating a classification system. Am. J. Obstet. Gynecol. 2011, 206, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Oza, S.; Hogan, D.; Chu, Y.; Perin, J.; Zhu, J.; Lawn, J.E.; Cousens, S.; Mathers, C.; Black, R.E. Global, regional, and national causes of under-5 mortality in 2000–15: An updated systematic analysis with implications for the Sustainable Development Goals. Lancet 2016, 388, 3027–3035. [Google Scholar] [CrossRef] [PubMed]
- Luu, T.M.; Mian, M.O.R.; Nuyt, A.M. Long-Term Impact of Preterm Birth: Neurodevelopmental and Physical Health Outcomes. Clin. Perinatol. 2017, 44, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Saigal, S.; Doyle, L.W. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2008, 371, 261–269. [Google Scholar] [CrossRef]
- Falsaperla, R.; Lombardo, F.; Filosco, F.; Romano, C.; Saporito, M.A.N.; Puglisi, F.; Piro, E.; Ruggieri, M.; Pavone, P. Oxidative Stress in Preterm Infants: Overview of Current Evidence and Future Prospects. Pharmaceuticals 2020, 13, 145. [Google Scholar] [CrossRef]
- Henderson, J.; Carson, C.; Redshaw, M. Impact of preterm birth on maternal well-being and women’s perceptions of their baby: A population-based survey. BMJ Open 2016, 6, e012676. [Google Scholar] [CrossRef]
- Bérard, A.; Le Tiec, M.; De Vera, M. Study of the costs and morbidities of late-preterm birth. Arch. Dis. Child.-Fetal Neonatal Ed. 2012, 97, F329–F334. [Google Scholar] [CrossRef]
- Cobo, T.; Kacerovsky, M.; Jacobsson, B. Risk factors for spontaneous preterm delivery. Int. J. Gynecol. Obstet. 2020, 150, 17–23. [Google Scholar] [CrossRef]
- Vogel, J.P.; Chawanpaiboon, S.; Moller, A.-B.; Watananirun, K.; Bonet, M.; Lumbiganon, P. The global epidemiology of preterm birth. Best Pr. Res. Clin. Obstet. Gynaecol. 2018, 52, 3–12. [Google Scholar] [CrossRef]
- Waldenström, U.; Aasheim, V.; Nilsen, A.B.V.; Rasmussen, S.; Pettersson, H.J.; Schytt, E. Adverse Pregnancy Outcomes Related to Advanced Maternal Age Compared with Smoking and Being Overweight. Obstet. Gynecol. 2014, 123, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Liem, S.M.; Mol, B.W.J.; Abu-Hanna, A.; Ravelli, A.C.; Schaaf, J.M. Ethnic and Racial Disparities in the Risk of Preterm Birth: A Systematic Review and Meta-Analysis. Am. J. Perinatol. 2012, 30, 433–450. [Google Scholar] [CrossRef] [PubMed]
- Faber, T.; Kumar, A.; Mackenbach, J.P.; Millett, C.; Basu, S.; Sheikh, A.; Been, J.V. Effect of tobacco control policies on perinatal and child health: A systematic review and meta-analysis. Lancet Public Health 2017, 2, e420–e437. [Google Scholar] [CrossRef]
- Ruiz, M.; Goldblatt, P.; Morrison, J.; Kukla, L.; Švancara, J.; Riitta-Järvelin, M.; Taanila, A.; Saurel-Cubizolles, M.-J.; Lioret, S.; Bakoula, C.; et al. Mother’s education and the risk of preterm and small for gestational age birth: A DRIVERS meta-analysis of 12 European cohorts. J. Epidemiol. Community Health 2015, 69, 826–833. [Google Scholar] [CrossRef]
- Wendt, A.; Gibbs, C.M.; Peters, S.; Hogue, C.J. Impact of Increasing Inter-pregnancy Interval on Maternal and Infant Health. Paediatr. Périnat. Epidemiol. 2012, 26 (Suppl. S1), 239–258. [Google Scholar] [CrossRef]
- Kazemier, B.; Buijs, P.; Mignini, L.; Limpens, J.; De Groot, C.; Mol, B.; Connect, E. Impact of obstetric history on the risk of spontaneous preterm birth in singleton and multiple pregnancies: A systematic review. BJOG Int. J. Obstet. Gynaecol. 2014, 121, 1197–1208. [Google Scholar] [CrossRef]
- Barros-Silva, J.; Pedrosa, A.C.; Matias, A. Sonographic measurement of cervical length as a predictor of preterm delivery: A systematic review. J. Périnat. Med. 2013, 42, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Morgan, T.K. Role of the Placenta in Preterm Birth: A Review. Am. J. Perinatol. 2016, 33, 258–266. [Google Scholar] [CrossRef]
- Ananth, C.V.; Berkowitz, G.S.; Savitz, D.A.; Lapinski, R.H. Placental Abruption and Adverse Perinatal Outcomes. JAMA 1999, 282, 1646–1651. [Google Scholar] [CrossRef]
- Fox, N.; Roman, A.; Stern, E.M.; Gerber, R.S.; Saltzman, D.H.; Rebarber, A. Type of congenital uterine anomaly and adverse pregnancy outcomes. J. Matern. Neonatal Med. 2013, 27, 949–953. [Google Scholar] [CrossRef]
- Brown, W.R. Association of Preterm Birth with Brain Malformations. Pediatr. Res. 2009, 65, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, F.; Senat, M.-V. Multiple gestations and preterm birth. Semin. Fetal Neonatal Med. 2016, 21, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Haahr, T.; Ersbøll, A.S.; Karlsen, M.A.; Svare, J.; Sneider, K.; Hee, L.; Weile, L.K.; Ziobrowska-Bech, A.; Østergaard, C.; Jensen, J.S.; et al. Treatment of bacterial vaginosis in pregnancy in order to reduce the risk of spontaneous preterm delivery-a clinical recommendation. Acta Obstet. Gynecol. Scand. 2016, 95, 850–860. [Google Scholar] [CrossRef]
- Galinsky, R.; Polglase, G.R.; Hooper, S.B.; Black, M.J.; Moss, T.J.M. The Consequences of Chorioamnionitis: Preterm Birth and Effects on Development. J. Pregnancy 2013, 2013, 412831. [Google Scholar] [CrossRef] [PubMed]
- Cunnington, M.; Kortsalioudaki, C.; Heath, P. Genitourinary pathogens and preterm birth. Curr. Opin. Infect. Dis. 2013, 26, 219–230. [Google Scholar] [CrossRef]
- Berghella, V.; Saccone, G. Fetal fibronectin testing for reducing the risk of preterm birth. Cochrane Database Syst. Rev. 2019, 7, CD006843. [Google Scholar] [CrossRef]
- Hornaday, K.K.; Wood, E.M.; Slater, D.M. Is there a maternal blood biomarker that can predict spontaneous preterm birth prior to labour onset? A systematic review. PLoS ONE 2022, 17, e0265853. [Google Scholar] [CrossRef]
- Kaplan, Z.A.O.; Ozgu-Erdinc, A.S. Prediction of Preterm Birth: Maternal Characteristics, Ultrasound Markers, and Biomarkers: An Updated Overview. J. Pregnancy 2018, 2018, 8367571. [Google Scholar] [CrossRef]
- Illarionov, R.A.; Pachuliia, O.V.; Vashukova, E.S.; Tkachenko, A.A.; Maltseva, A.R.; Postnikova, T.B.; Nasykhova, Y.A.; Bespalova, O.N.; Glotov, A.S. Plasma miRNA Profile in High Risk of Preterm Birth during Early and Mid-Pregnancy. Genes 2022, 13, 2018. [Google Scholar] [CrossRef]
- Keirse, M.J.N.C. An Evaluation of Formal Risk Scoring for Preterm Birth. Am. J. Perinatol. 1989, 6, 226–233. [Google Scholar] [CrossRef]
- Shiono, P.H.; Klebanoff, M.A. A Review of Risk Scoring for Preterm Birth. Clin. Perinatol. 1993, 20, 107–125. [Google Scholar] [CrossRef] [PubMed]
- Honest, H.; Bachmann, L.; Sundaram, R.; Gupta, J.; Kleijnen, J.; Khan, K. The accuracy of risk scores in predicting preterm birth—A systematic review. J. Obstet. Gynaecol. 2004, 24, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.-A.; Watson, L.; Rayner, J.A.; Rowlands, S. Risk-scoring systems for predicting preterm birth with the aim of reducing associated adverse outcomes. Cochrane Database Syst. Rev. 2015, 2015, CD004902. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Fedrick, J. Antenatal Identification of Women at High Risk of Spontaneous Pre-Term Birth. BJOG: Int. J. Obstet. Gynaecol. 1976, 83, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Creasy, R.K.; Gummer, A.B.; Liggins, G.C. System for predicting spontaneous preterm birth. Obstet. Gynecol. 1980, 55, 692–695. [Google Scholar]
- Guzick, D.S.; Daikoku, N.H.; Kaltreider, D.F. Predictability of pregnancy outcome in preterm delivery. Obstet. Gynecol. 1984, 63, 645–650. [Google Scholar] [PubMed]
- Neilson, J.P.; Verkuyl, A.D.; Crowther, A.C.; Bannerman, C. Preterm labor in twin pregnancies: Prediction by cervical assessment. Obstet. Gynecol. 1988, 72, 719–723. [Google Scholar]
- Holbrook, J.R.; Laros, J.R.; Creasy, R.K. Evaluation of a Risk-Scoring System for Prediction of Preterm Labor. Am. J. Perinatol. 1989, 6, 62–68. [Google Scholar] [CrossRef]
- Mueller-Heubach, E.; Guzick, D.S. Evaluation of risk scoring in a preterm birth prevention study of indigent patients. Am. J. Obstet. Gynecol. 1989, 160, 829–835. [Google Scholar] [CrossRef]
- Owen, J.; Goldenberg, R.L.; Davis, R.O.; Kirk, K.A.; Copper, R.L. Evaluation of a risk scoring system as a predictor of preterm birth in an indigent population. Am. J. Obstet. Gynecol. 1990, 163, 873–879. [Google Scholar] [CrossRef]
- Newman, R.B.; Godsey, R.K.; Ellings, J.M.; Campbell, B.A.; Eller, D.P.; Miller, C.M. Quantification of cervical change: Relationship to preterm delivery in the multifetal gestation. Am. J. Obstet. Gynecol. 1991, 165, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Guinn, D.; Wigton, T.R.; Owen, J.; Socol, M.L.; Frederiksen, M.C. Prediction of preterm birth in nulliparous patients. Am. J. Obstet. Gynecol. 1994, 171, 1111–1115. [Google Scholar] [CrossRef] [PubMed]
- Edenfield, S.M.; Thomas, S.D.; Thompson, O.W.; Marcotte, J.J. Validity of the Creasy risk appraisal instrument for prediction of preterm labor. Nurs. Res. 1995, 44, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Mercer, B.; Goldenberg, R.; Das, A.; Moawad, A.; Iams, J.; Meis, P.; Copper, R.; Johnson, F.; Thom, E.; McNellis, D.; et al. The preterm prediction study: A clinical risk assessment system. Am. J. Obstet. Gynecol. 1996, 174, 1885–1895. [Google Scholar] [CrossRef]
- Rizzo, G.; Capponi, A.; Arduini, D.; Lorido, C.; Romanini, C. The value of fetal fibronectin in cervical and vaginal secretions and of ultrasonographic examination of the uterine cervix in predicting premature delivery for patients with preterm labor and intact membranes. Am. J. Obstet. Gynecol. 1996, 175, 1146–1151. [Google Scholar] [CrossRef]
- Heine, R.; McGregor, J.A.; Dullien, V.K. Accuracy of salivary estriol testing compared to traditional risk factor assessment in predicting preterm birth. Am. J. Obstet. Gynecol. 1999, 180, S214–S218. [Google Scholar] [CrossRef]
- McLean, M.; Bisits, A.; Davies, J.; Walters, W.; Hackshaw, A.; De Voss, K.; Smith, R. Predicting risk of preterm delivery by second-trimester measurement of maternal plasma corticotropin-releasing hormone and α-fetoprotein concentrations. Am. J. Obstet. Gynecol. 1999, 181, 207–215. [Google Scholar] [CrossRef]
- Gudmundsson, S.; Korszun, P.; Olofsson, P.; Dubiel, M. New score indicating placental vascular resistance. Acta Obstet. Gynecol. Scand. 2003, 82, 807–812. [Google Scholar] [CrossRef]
- Tekesin, I.; Hellmeyer, L.; Heller, G.; Römer, A.; Kühnert, M.; Schmidt, S. Evaluation of quantitative ultrasound tissue characterization of the cervix and cervical length in the prediction of premature delivery for patients with spontaneous preterm labor. Am. J. Obstet. Gynecol. 2003, 189, 532–539. [Google Scholar] [CrossRef]
- Gurbuz, A.; Karateke, A.; Ozturkmen, M.; Kabaca, C. Human chorionic gonadotropin assay in cervical secretions for accurate diagnosis of preterm labor. Int. J. Gynecol. Obstet. 2003, 85, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Maslovitz, S.; Hartoov, J.; Wolman, I.; Jaffa, A.; Lessing, J.B.; Fait, G. Cervical Length in the Early Second Trimester for Detection of Triplet Pregnancies at Risk for Preterm Birth. J. Ultrasound Med. 2004, 23, 1187–1191. [Google Scholar] [CrossRef] [PubMed]
- Tekesin, I.; Eberhart, L.H.J.; Schaefer, V.; Wallwiener, D.; Schmidt, S. Evaluation and validation of a new risk score (CLEOPATRA score) to predict the probability of premature delivery for patients with threatened preterm labor. Ultrasound Obstet. Gynecol. 2005, 26, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Vayssière, C.; Favre, R.; Audibert, F.; Chauvet, M.P.; Gaucherand, P.; Tardif, D.; Grangé, G.; Novoa, A.; Descamps, P.; Perdu, M.; et al. Cervical assessment at 22 and 27 weeks for the prediction of spontaneous birth before 34 weeks in twin pregnancies: Is transvaginal sonography more accurate than digital examination? Ultrasound Obstet. Gynecol. 2005, 26, 707–712. [Google Scholar] [CrossRef]
- Ghosh, G.; Breborowicz, A.; Brązert, M.; Maczkiewicz, M.; Kobelski, M.; Dubiel, M.; Gudmundsson, S. Evaluation of third trimester uterine artery flow velocity indices in relationship to perinatal complications. J. Matern. Neonatal Med. 2006, 19, 551–555. [Google Scholar] [CrossRef]
- Grgic, O.; Matijevic, R.; Vasilj, O. Qualitative glandular cervical score as a potential new sonomorphological parameter in screening for preterm delivery. Ultrasound Med. Biol. 2006, 32, 333–338. [Google Scholar] [CrossRef]
- Matijevic, R.; Grgic, O.; Vasilj, O. Is sonographic assessment of cervical length better than digital examination in screening for preterm delivery in a low-risk population? Acta Obstet. Gynecol. Scand. 2006, 85, 1342–1347. [Google Scholar] [CrossRef]
- To, M.S.; Skentou, C.A.; Royston, P.; Yu, C.K.H.; Nicolaides, K.H. Prediction of patient-specific risk of early preterm delivery using maternal history and sonographic measurement of cervical length: A population-based prospective study. Ultrasound Obstet. Gynecol. 2006, 27, 362–367. [Google Scholar] [CrossRef]
- Tan, H.; Wen, S.W.; Chen, X.K.; Demissie, K.; Walker, M. Early prediction of preterm birth for singleton, twin, and triplet pregnancies. Eur. J. Obstet. Gynecol. Reprod. Biol. 2007, 131, 132–137. [Google Scholar] [CrossRef]
- Celik, E.; To, M.; Gajewska, K.; Smith, G.C.S.; Nicolaides, K.H. Cervical length and obstetric history predict spontaneous preterm birth: Development and validation of a model to provide individualized risk assessment. Ultrasound Obstet. Gynecol. 2008, 31, 549–554. [Google Scholar] [CrossRef]
- Allouche, M.; Huissoud, C.; Guyard-Boileau, B.; Rouzier, R.; Parant, O. Development and validation of nomograms for predicting preterm delivery. Am. J. Obstet. Gynecol. 2011, 204, 242.e1–242.e8. [Google Scholar] [CrossRef]
- Beta, J.; Akolekar, R.; Ventura, W.; Syngelaki, A.; Nicolaides, K.H. Prediction of spontaneous preterm delivery from maternal factors, obstetric history and placental perfusion and function at 11–13 weeks. Prenat. Diagn. 2011, 31, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Bastek, J.A.; Sammel, M.D.; Srinivas, S.K.; McShea, M.A.; Foreman, M.N.; Elovitz, M.A.; Metlay, J.P. Clinical Prediction Rules for Preterm Birth in Patients Presenting with Preterm Labor. Obstet. Gynecol. 2012, 119, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, F.; Senat, M.-V.; Fernandez, H.; Gervaise, A.; Frydman, R.; Bouyer, J. Predictive score for early preterm birth in decisions about emergency cervical cerclage in singleton pregnancies. Acta Obstet. Gynecol. Scand. 2012, 91, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, J.M.; Ravelli, A.C.; Mol, B.W.J.; Abu-Hanna, A. Development of a prognostic model for predicting spontaneous singleton preterm birth. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 164, 150–155. [Google Scholar] [CrossRef]
- Kahyaoglu, S.; Kahyaoglu, I.; Kaymak, O.; Sagnic, S.; Mollamahmutoglu, L.; Danişman, N. Can transvaginal ultrasonographic evaluation of the endocervical glandular area predict preterm labor among patients who received tocolytic therapy for threatened labor: A cross-sectional study. J. Matern. Neonatal Med. 2013, 26, 920–925. [Google Scholar] [CrossRef]
- Sananes, N.; Meyer, N.; Gaudineau, A.; Aissi, G.; Boudier, E.; Fritz, G.; Viville, B.; Nisand, I.; Langer, B.; Favre, R. Prediction of spontaneous preterm delivery in the first trimester of pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 171, 18–22. [Google Scholar] [CrossRef]
- Alleman, B.W.; Smith, A.R.; Byers, H.M.; Bedell, B.; Ryckman, K.K.; Murray, J.C.; Borowski, K.S. A proposed method to predict preterm birth using clinical data, standard maternal serum screening, and cholesterol. Am. J. Obstet. Gynecol. 2013, 208, 472.e1–472.e11. [Google Scholar] [CrossRef]
- Abbott, D.S.; Hezelgrave, N.L.; Seed, P.T.; Norman, J.E.; David, A.L.; Bennett, P.R.; Girling, J.C.; Chandirimani, M.; Stock, S.J.; Carter, J.; et al. Quantitative Fetal Fibronectin to Predict Preterm Birth in Asymptomatic Women at High Risk. Obstet. Gynecol. 2015, 125, 1168–1176. [Google Scholar] [CrossRef]
- Chaiworapongsa, T.; Romero, R.; Whitten, A.E.; Korzeniewski, S.J.; Chaemsaithong, P.; Hernandez-Andrade, E.; Yeo, L.; Hassan, S.S. The use of angiogenic biomarkers in maternal blood to identify which SGA fetuses will require a preterm delivery and mothers who will develop pre-eclampsia. J. Matern. Neonatal Med. 2015, 29, 1214–1228. [Google Scholar] [CrossRef]
- Saade, G.R.; Boggess, K.A.; Sullivan, S.A.; Markenson, G.; Iams, J.D.; Coonrod, D.V.; Pereira, L.M.; Esplin, M.S.; Cousins, L.M.; Lam, G.K.; et al. Development and validation of a spontaneous preterm delivery predictor in asymptomatic women. Am. J. Obstet. Gynecol. 2016, 214, 633.e1–633.e24. [Google Scholar] [CrossRef] [PubMed]
- Kuhrt, K.; Smout, E.; Hezelgrave, N.; Seed, P.T.; Carter, J.; Shennan, A.H. Development and validation of a tool incorporating cervical length and quantitative fetal fibronectin to predict spontaneous preterm birth in asymptomatic high-risk women. Ultrasound Obstet. Gynecol. 2016, 47, 104–109. [Google Scholar] [CrossRef]
- Sepúlveda-Martínez, A.; Díaz, F.; Muñoz, H.; Valdés, E.; Parra-Cordero, M. Second-Trimester Anterior Cervical Angle in a Low-Risk Population as a Marker for Spontaneous Preterm Delivery. Fetal Diagn. Ther. 2016, 41, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Winger, E.E.; Reed, J.L.; Ji, X. Early first trimester peripheral blood cell microRNA predicts risk of preterm delivery in pregnant women: Proof of concept. PLoS ONE 2017, 12, e0180124. [Google Scholar] [CrossRef] [PubMed]
- Baer, R.J.; McLemore, M.R.; Adler, N.; Oltman, S.P.; Chambers, B.D.; Kuppermann, M.; Pantell, M.S.; Rogers, E.E.; Ryckman, K.K.; Sirota, M.; et al. Pre-pregnancy or first-trimester risk scoring to identify women at high risk of preterm birth. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 231, 235–240. [Google Scholar] [CrossRef]
- Baños, N.; Perez-Moreno, A.; Julià, C.; Murillo-Bravo, C.; Coronado, D.; Gratacós, E.; Deprest, J.; Palacio, M. Quantitative analysis of cervical texture by ultrasound in mid-pregnancy and association with spontaneous preterm birth. Ultrasound Obstet. Gynecol. 2018, 51, 637–643. [Google Scholar] [CrossRef]
- Tsikouras, P.; Anastasopoulos, G.; Maroulis, V.; Bothou, A.; Chalkidou, A.; Deuteraiou, D.; Anthoulaki, X.; Tsatsaris, G.; Bourazan, A.H.; Iatrakis, G.; et al. Comparative Evaluation of Arabin Pessary and Cervical Cerclage for the Prevention of Preterm Labor in Asymptomatic Women with High Risk Factors. Int. J. Environ. Res. Public Health 2018, 15, 791. [Google Scholar] [CrossRef]
- Vandewiele, G.; Dehaene, I.; Janssens, O.; Ongenae, F.; De Backere, F.; De Turck, F.; Roelens, K.; Van Hoecke, S.; Demeester, T. Time-to-Birth Prediction Models and the Influence of Expert Opinions; Springer: Berlin/Heidelberg, Germany, 2019; pp. 286–291. [Google Scholar]
- Gesthuysen, A.; Hammer, K.; Möllers, M.; Braun, J.; de Murcia, K.O.; Falkenberg, M.K.; Köster, H.A.; Möllmann, U.; Fruscalzo, A.; Bormann, E.; et al. Evaluation of Cervical Elastography Strain Pattern to Predict Preterm Birth. Ultraschall Der Med.-Eur. J. Ultrasound 2019, 41, 397–403. [Google Scholar] [CrossRef]
- Guszczynska-Losy, M.; Wirstlein, P.K.; Wender-Ozegowska, E.; Kedzia, M. Evaluation of predictive value of biochemical markers for adverse obstetrics outcomes in pregnancies complicated by cholestasis. Ginekol. Polska 2020, 91, 269–276. [Google Scholar] [CrossRef]
- Leneuve-Dorilas, M.; Buekens, P.; Favre, A.; Carles, G.; Louis, A.; Breart, G.; Nacher, M. Predictive factors of preterm delivery in French Guiana for singleton pregnancies: Definition and validation of a predictive score. J. Matern. Neonatal Med. 2018, 33, 1709–1716. [Google Scholar] [CrossRef]
- Maia, M.C.; Nomura, R.; Mendonça, F.; Rios, L.; Moron, A. Is cervical length evaluated by transvaginal ultrasonography helpful in detecting true preterm labor? J. Matern. Neonatal Med. 2019, 33, 2902–2908. [Google Scholar] [CrossRef] [PubMed]
- Markenson, G.R.; Saade, G.R.; Laurent, L.C.; Heyborne, K.D.; Coonrod, D.V.; Schoen, C.N.; Baxter, J.K.; Haas, D.M.; Longo, S.; Grobman, W.A.; et al. Performance of a proteomic preterm delivery predictor in a large independent prospective cohort. Am. J. Obstet. Gynecol. MFM 2020, 2, 100140. [Google Scholar] [CrossRef] [PubMed]
- Winger, E.E.; Reed, J.L.; Ji, X.; Gomez-Lopez, N.; Pacora, P.; Romero, R. MicroRNAs isolated from peripheral blood in the first trimester predict spontaneous preterm birth. PLoS ONE 2020, 15, e0236805. [Google Scholar] [CrossRef]
- Patil, A.S.; Grotegut, C.A.; Gaikwad, N.W.; Dowden, S.D.; Haas, D.M. Prediction of neonatal morbidity and very preterm delivery using maternal steroid biomarkers in early gestation. PLoS ONE 2021, 16, e0243585. [Google Scholar] [CrossRef]
- Shields, L.B.; Weymouth, C.; Bramer, K.L.; Robinson, S.; McGee, D.; Richards, L.; Ogle, C.; Shields, C.B. Risk assessment of preterm birth through identification and stratification of pregnancies using a real-time scoring algorithm. SAGE Open Med. 2021, 9, 2050312120986729. [Google Scholar] [CrossRef]
- Zhang, J.; Pan, M.; Zhan, W.; Zheng, L.; Jiang, X.; Xue, X. Two-stage nomogram models in mid-gestation for predicting the risk of spontaneous preterm birth in twin pregnancy. Arch. Gynecol. Obstet. 2020, 303, 1439–1449. [Google Scholar] [CrossRef] [PubMed]
- Belaghi, R.A.; Beyene, J.; McDonald, S.D. Prediction of preterm birth in nulliparous women using logistic regression and machine learning. PLoS ONE 2021, 16, e0252025. [Google Scholar] [CrossRef]
- Merlo, I.; Cantarutti, A.; Allotta, A.; Tavormina, E.E.; Iommi, M.; Pompili, M.; Rea, F.; Agodi, A.; Locatelli, A.; Zanini, R.; et al. Development and Validation of a Novel Pre-Pregnancy Score Predictive of Preterm Birth in Nulliparous Women Using Data from Italian Healthcare Utilization Databases. Healthcare 2022, 10, 1443. [Google Scholar] [CrossRef]
- Zhou, G.; Holzman, C.; Heng, Y.J.; Kibschull, M.; Lye, S.J. Maternal blood EBF1-based microRNA transcripts as biomarkers for detecting risk of spontaneous preterm birth: A nested case-control study. J. Matern. Neonatal Med. 2020, 35, 1239–1247. [Google Scholar] [CrossRef]
- Coutinho, C.M.; Sotiriadis, A.; Odibo, A.; Khalil, A.; D’Antonio, F.; Feltovich, H.; Salomon, L.J.; Sheehan, P.; Napolitano, R.; Berghella, V.; et al. ISUOGPractice Guidelines: Role of ultrasound in the prediction of spontaneous preterm birth. Ultrasound Obstet. Gynecol. 2022, 60, 435–456. [Google Scholar] [CrossRef]

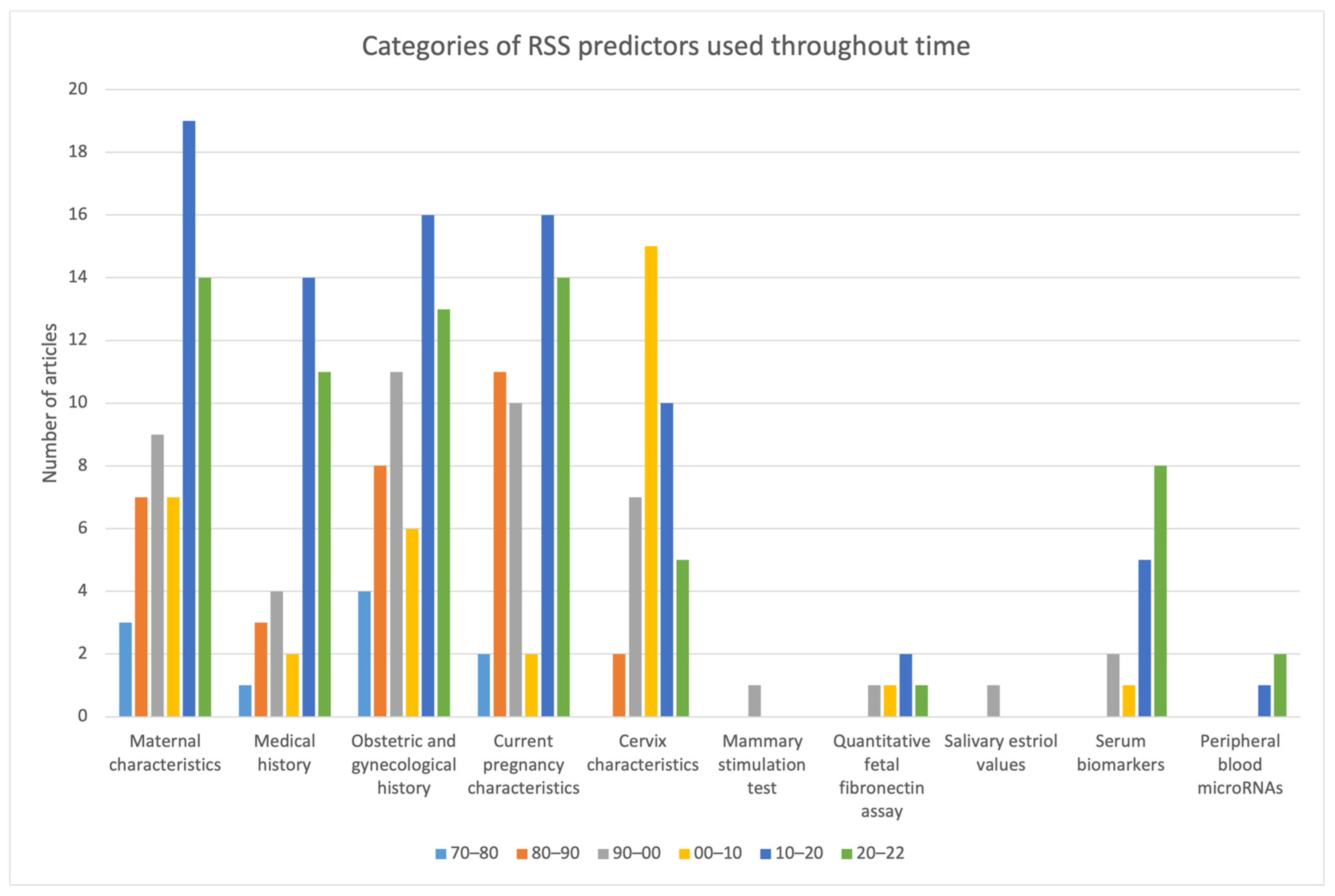
| 76–80 | 80–90 | 90–00 | 00–10 | 10–20 | 20–22 | Total | |
|---|---|---|---|---|---|---|---|
| Maternal characteristics: | |||||||
| Maternal age | 1 | 1 | 2 | 2 | 5 | 4 | 15 |
| Social class | 1 | - | 1 | - | 1 | 1 | 4 |
| Location of mother’s birth | - | - | - | - | 1 | - | 1 |
| Race/ethnicity | - | 1 | 1 | 2 | 4 | 2 | 10 |
| Marital status | - | 2 | 1 | 1 | - | - | 4 |
| Education grade | - | - | 1 | 1 | 2 | 2 | 6 |
| Height | - | 1 | 1 | - | 2 | 1 | 5 |
| Weight/Body mass index | 1 | 2 | 2 | 1 | 4 | 4 | 14 |
| Medical history: | |||||||
| Preexisting diabetes | - | - | - | - | 3 | 3 | 6 |
| Preexisting hypertension | - | 1 | 1 | - | 1 | 2 | 5 |
| Smoking | 1 | 2 | 3 | 2 | 5 | 2 | 15 |
| Drug/alcohol abuse | - | - | - | - | 3 | 3 | 6 |
| Mental illness | - | - | - | - | 2 | 1 | 3 |
| Obstetric and gynecological history: | |||||||
| Previous abortions | 1 | 2 | 3 | - | - | 4 | 10 |
| Previous preterm births | 1 | 3 | 3 | 3 | 9 | 2 | 21 |
| Previous livebirths < 2500 g | 1 | - | - | - | - | 1 | 2 |
| Previous large (4000 g+) infants | 1 | - | - | - | - | - | 1 |
| Previous cervical interventions | - | 1 | 2 | 1 | 3 | 1 | 8 |
| Parity | - | - | - | 2 | 3 | 3 | 8 |
| Interpregnancy interval | - | 1 | 1 | - | 1 | - | 3 |
| Uterine anomaly | - | 1 | 2 | - | - | 2 | 5 |
| Current pregnancy characteristics: | |||||||
| Number of fetuses | - | 2 | 2 | - | 3 | 4 | 11 |
| Method of conception | - | - | - | - | 2 | 2 | 4 |
| Threatened abortion | 1 | - | - | - | - | - | 1 |
| Vaginal bleeding | 1 | 1 | 2 | - | 2 | 1 | 7 |
| Abruptio placentae | - | 1 | - | - | - | - | 1 |
| Placenta previa | - | 2 | 1 | - | - | 1 | 4 |
| Preeclampsia | - | 1 | - | - | - | 1 | 2 |
| Breech | - | 1 | - | - | - | - | 1 |
| Preterm premature rupture of membranes | - | 1 | - | - | 2 | 1 | 4 |
| Bacteriuria | - | 1 | 3 | - | 1 | 2 | 7 |
| Prenatal care visit initiation | - | - | - | 1 | 3 | 2 | 6 |
| Uterine contractions | - | - | 1 | - | 1 | - | 2 |
| Presence of membranes bulging into the vagina | - | - | - | - | 1 | - | 1 |
| Need and GA at transfer to a tertiary care center | - | - | - | - | 1 | - | 1 |
| Maternal weight gain per week | - | 1 | 1 | 1 | - | - | 3 |
| Cervix characteristics: | |||||||
| Bishop score—clinical digital examination | - | 1 | 3 | 2 | 2 | - | 8 |
| Cervical assessment (cervical dilation—cervical length) | - | 1 | 1 | 1 | - | - | 3 |
| Cervical length | - | - | 2 | 6 | 4 | 3 | 15 |
| Cervical funneling | - | - | 1 | 1 | 2 | ||
| Mean gray value—Two-dimensional transvaginal ultrasound measurement of cervical length | - | - | - | 1 | - | - | 1 |
| Qualitative glandular cervical score | - | - | - | 1 | - | - | 1 |
| Endocervical glandular area | - | - | - | - | 1 | - | 1 |
| Anterior cervical angle | - | - | - | - | 1 | - | 1 |
| Elastography index | - | - | - | - | - | 1 | 1 |
| Strain pattern score | - | - | - | - | - | 1 | 1 |
| Blood velocity in the umbilical artery waveform | - | - | - | 2 | - | - | 2 |
| Blood velocity in the uterine artery waveform | - | - | - | 2 | 1 | - | 3 |
| Mammary stimulation test | - | - | 1 | - | - | - | 1 |
| Quantitative fetal fibronectin assay | - | - | 1 | 1 | 2 | 1 | 5 |
| Salivary estriol values | - | - | 1 | - | - | - | 1 |
| Serum biomarkers: | |||||||
| Corticotropin-releasing hormone concentration. | - | - | 1 | - | - | - | 1 |
| Alpha-fetoprotein concentration | - | - | 1 | - | 1 | 1 | 3 |
| Human chorionic gonadotropin | - | - | - | 1 | - | 1 | 2 |
| Inhibin A | - | - | - | - | 1 | 1 | 2 |
| Total cholesterol | - | - | - | - | 1 | - | 1 |
| Insulin-like growth factor-binding protein | - | - | - | - | 1 | 1 | 2 |
| Sex hormone-binding globulin | - | - | - | - | 1 | 1 | 2 |
| Total bile acids | - | - | - | - | - | 1 | 1 |
| 11-deoxycorticosterone | - | - | - | - | - | 1 | 1 |
| 16-alpha hydroxyprogesterone | - | - | - | - | - | 1 | 1 |
| Peripheral blood microRNAs | - | - | - | - | 1 | 2 | 3 |
| Article and Year | PTB GA | PTB Type | Model Used | Model Output and Outcomes | Variables Included | Performance Analysis | Evaluation Method | GA at Testing |
|---|---|---|---|---|---|---|---|---|
| 1976 [35] | Not reported | Spontaneous | Product of relative risks | Low (<5) and high risk (≥5) | Maternal age, social class, weight, smoking, threatened abortion, previous abortions. Parous women only: previous PTB, previous livebirths < 2500 g, previous large (4000 g+) infants, previous antepartum hemorrhage | Multiparous: Sens. 25.3%; PPV 34.7% | Whole sample | Not reported |
| 1980 [36] | <37 w | Spontaneous | Sum of points | Low (0–5), medium (6–9) and high risk (≥10) | Predictors related to socioeconomic status, medical history, daily habits, and current pregnancy | High/med. risk: Sens. 80% Spec. 72%; PPV 15%; NPV 98% | Whole sample | First prenatal visit, updated at 26–28 w |
| 1984 [37] | <37 w | Not reported | Logistic regression | Low (<10%) and high risk (≥10%) | presence or absence of previous premature delivery, previous spontaneous abortion, abruptio placentae, placenta previa, severe preeclampsia, breech, smoking (no. cigarettes), PPROM, multiple pregnancy | Sens. 62.2%; Spec. 79.4%; PPV 22.7% | Whole sample | Not reported |
| 1988 [38] | <37 w | Spontaneous | Cervical score (length minus dilatation) | Low (≥−1) and high risk (<−1) | Cervical assessment (subtracting dilatation from length) | PPV 76% | Whole sample | 27–34 w |
| 1989 [39] | <37 w | Both | Sum of points (based on Creasy [36]) | Low (<10) and high risk (≥10) | Socioeconomic factors, previous medical history, daily habits and current pregnancy problems (18 variables) | Sens. 41.0%; PPV 24.6%; NPV 94.2% | Whole sample | First prenatal visit |
| 1989 [40] | <37 w | Both | Logistic regression | Low (<1.83) and high risk (≥1.83) | pre-pregnancy weight <45.5 kg, black race, single marital status, history of PTB | Sens. 28.8%; Spec. 91.0%; PPV 21.9% | Whole sample | First prenatal visit, <28 w |
| 1990 [41] | <37 w and <32 w (VPTB) | Spontaneous | Sum of points (based on Creasy [36]) | Low (<10) and high risk (≥10) | Socioeconomic factors, previous medical history, daily habits and current pregnancy problems | Sens. 29%; Spec. 85%; PPV 16%; NPV 93% | Whole sample | First prenatal visit, <30 w |
| 1991 [42] | <37 w and <34 w | Spontaneous | Cervical score (length minus dilatation) | For PTB < 34 w: Low (>0) and high risk (≤0) | Cervical assessment (subtracting dilatation from length) | Sens. 88%; Spec. 62%; PPV 75%; NPV 81% | Whole sample | <34 w |
| 1994 [43] | <37 w | Spontaneous | Discriminant model | Low and high risk (no cutoff reported) | Positive mammary stimulation test result, soft cervix at 26 to 28 w, bacteriuria at the 1st prenatal visit, smoking during pregnancy, history of spontaneous abortion | Sens. 34.6%; Spec. 95.6%; PPV 47.4%, NPV 92.9% | Whole sample | 26–28 w |
| 1995 [44] | <37 w | Not reported | Sum of points (Creasy [36]) | low (0–5), medium (6–9) and high risk (≥10) | Predictors related to socioeconomic status, past history, daily habits, and current pregnancy | Sens. 30.5%; Spec. 83.9%; PPV 44.3%; NPV 74.2% | Whole sample | First prenatal visit |
| 1996 [45] | <37 w | Spontaneous | Logistic regression | Low (<20%) and high risk (≥20%) | Race, poor social environment, paying job during pregnancy, prior SPTD, acute or chronic lung disease, vaginal bleeding, contractions, BMI < 19.8, Bishop score | Multiparous: Sens. 24.2%; Spec. 92.1%; PPV 30.8%; NPV 89.4 | Cross-validation (85% for training and 15% for testing) | 23–24 w |
| 1996 [46] | <37 w | Not reported | Simple cutoff | Low (<60 ng/mL) and high risk (≥60 ng/mL) | Fetal fibronectin assay of cervical and vaginal secretions, cervical length, presence of funneling, cervical index | Sens. 80.9%; Spec. 83.6%; PPV 79.2%; NPV 85.0% | Whole sample | 24–36 w |
| 1999 [47] | PTB within 72 h before 37 w | Spontaneous | Simple cutoff | Low (<2.1 ng/mL) and high risk (≥2.1 ng/mL) | Salivary estriol values | PPV 91% | Whole sample | ≥21 w |
| 1999 [48] | <37 w | Both | Based on likelihood ratios | Low and high risk (no cutoff reported) | Predictors related to socioeconomic status, past history, daily habits, and current pregnancy, corticotropin releasing-hormone and alpha-fetoprotein concentrations | Sens. 37%; Spec. 95%, | Whole sample | ≥12 w |
| 2003 [49] | <37 w | Both | Sum of scores | Numerical model (0–7) without specified categories | Blood velocity in the umbilical artery waveform (4 categories) and uterine artery blood flow velocity waveforms (5 categories) | AUC 0.71 | Whole sample | Not reported |
| 2003 [50] | <37 w | Spontaneous | Simple cutoff | Low (≤6.54) and high risk (>6.54) | Two-dimensional transvaginal ultrasound measurement of cervical length—Mean gray value | AUC 0.80; Sens. 82.1%; Spec. 72.5%; PPV 67.6%; NPV 85.3% | Whole sample | 20–35 w |
| 2004 [51] | <37 w and <35 w | Not reported | Simple cutoff | For PTB < 37 w: Low (<27) and high risk (≥27) | Human chorionic gonadotropin | For PTB < 37 w: Sens. 76%; Spec. 50%; PPV 85%; NPV 37%; Acc. 71% | Whole sample | 25–35 w |
| 2004 [52] | <32 w | Both | Simple cutoff | Low (>25 mm) and high risk (≤25 mm) | Cervical length | Sens. 75%; Spec. 90%; PPV 83%; NPV 81% | Whole sample | 14–20 w |
| 2005 [53] | <37 w | Spontaneous | Logistic regression | Low and high risk (threshold not reported) | CLEOPATRA I: cervical length and previous PTB; CLEOPATRA II: fetal fibronectin and previous PTB | CLEOPATRA I: AUC 0.69 CLEOPATRA II: AUC 0.81 | Cross-validation (50% for training and 50% for testing) | 24–35 w |
| 2005 [54] | <34 w | Spontaneous | Simple cutoff | Cervical index: Low (<0.04) and high risk (≥0.04) | Ultrasound cervical assessment (cervical index and cervical score) and digital examination (Bishop score and cervical score) | Cervical index: AUC 0.85; Sens. 92%; Spec. 74%; PPV 26%; NPV 99% | Whole sample | 27 w |
| 2006 [55] | <37 w | Not reported | Simple cutoff | Low (<1.5) and high risk (≥1.5) | Umbilical artery pulsatility index | AUC 0.796 | Whole sample | Not reported |
| 2006 [56] | <34 w and 34–37 w | Spontaneous | Sum of the cervical mucus area and glandular invasion score | PTB 34–37 w: Low (>1) and high risk (≤1) | Cervical length and qualitative glandular cervical score | PTB 34–37 w: Sens. 50%; Spec. 96%; PPV 28%; NPV 98% | Whole sample | 16–23 w |
| 2006 [57] | <37 w | Not reported | Simple cutoff | Low (>24 mm) and high risk (≤24 mm) | Cervical length | Sens. 57.1%; Spec. 98.4%; PPV 66.7%; NPV 97.7% | Whole sample | 16–23 w |
| 2006 [58] | <37 w and <32 w | Both | Logistic regression | Low and high risk (no cutoff reported) | Maternal age, ethnicity, BMI, smoking status, obstetric history, previous cervical surgery, cervical length | For PTB < 37 w: AUC 0.667 | Whole sample | 22–25 w |
| 2007 [59] | <32 w | Spontaneous | Logistic regression | Low and high risk (singletons > 0.04; twins > 0.10; triplets > 0.34) | Maternal age, maternal race, maternal education, marital status, parity, prenatal care visit initiation, maternal smoking, maternal weight gain per week, medical complications | Singletons: AUC 0.73; Sens. 24.6%; Spec. 93.5%; PPV 5.9%; NPV 98.7%; | Holdout (80% training and 20% testing) | Not reported |
| 2008 [60] | <28 w, 28–30 w, 31–33 w, 34–36 w | Spontaneous | Logistic regression | Low and high risk (no cutoff reported) | Cervical length, obstetric history (parity and GA of previous delivery) | <28: AUC 0.92 28–30: AUC 0.84 31–33: AUC 0.82 34–36: AUC 0.65 | Holdout | 20–25 w |
| 2011 [61] | <32 w | Not reported | Sum of points | Low and high risk (no cutoff reported) | PPROM, sonographic cervical length, gestational age at transfer, uterine contractions requiring tocolysis, multiple pregnancies, and vaginal bleeding | Training: AUC 0.79 Validation: AUC 0.72 | Holdout (737 training and 169 validation) | 22–32 w |
| 2011 [62] | <34 w | Spontaneous | Logistic regression | Low and high risk (no cutoff reported) | Maternal age, height, racial origin, smoking status, method of conception and obstetric history | AUC 0.668; Sens. (FPR 10) 27.5% | Whole sample | 11–14 w |
| 2012 [63] | <37 w | Not reported | Logistic regression | Low (<2) and high risk (≥2) | Initial cervical dilation, obstetric history (parity and previous PTB), tobacco use | AUC 0.73; Sens. 79%; Spec. 50%; PPV 46%; NPV 82% | Internal validation with bootstrapping | 22–34 w |
| 2012 [64] | <32 w | Not reported | Logistic regression | Low and high risk (no cutoff reported) | Cervical dilation, obstetric history, presence of membranes bulging into the vagina and infection | AUC 0.88 | Whole sample | 15–24 w |
| 2012 [65] | <37 w | Spontaneous | Logistic regression | Low (<0.1) and high risk (≥0.1) | Maternal characteristics (maternal age, maternal ethnicity, socioeconomic status, living in a deprived area), obstetric history (parity, pre-existent diabetes mellitus, previous PTB, history of cervical surgery, psychiatric disorder, drug abuse), current pregnancy (booking visit ≥18 w of gestation, vaginal bleeding <20 w of gestation, male fetal sex) | Sens. 4.2%; Spec. 99.3%; PPV 19.4%; NPV 96.3% | Internal validation with bootstrapping | Around 20 w |
| 2013 [66] | <37 w | Spontaneous | Combined cutoff and categoric variable | High risk for short cervix (<20 mm) + echogenicity | Cervical length and endocervical glandular area | Sens. 34.4%; Spec. 41.5%; PPV 64.7%; NPV 77.8% | Whole sample | 24–34 w |
| 2013 [67] | <37 w | Spontaneous | Logistic regression | Low and high risk (no cutoff reported) | Maternal age, body mass index, smoking status, history of late miscarriage and/or preterm delivery, and previous delivery to term | AUC 0.618; Sens (FPR 10) 23.3% PPV 7.4%; NPV 97.2% | External validation | 1st trimester |
| 2013 [68] | <37 w | Both | Logistic regression | Low and high risk (no cutoff reported) | Maternal characteristics (maternal degree, prepregancy diabetes, previous PTB, previous live birth, and maternal BMI), routine serum analytes (AFP and inhibin A), cholesterol (first-trimester TC and TC change between trimesters [second TC trimester—first trimester TC]) | AUC 0.70; Sens.31.2%; Spec. 90.6%; PPV 21.3%; NPV 94.2% | Whole sample | 1st and 2nd trimesters |
| 2015 [69] | <37 w, <34 w, <30 w | Spontaneous | Simple cutoff | Low (<200 ng/mL) and high risk (≥200 ng/mL) | Quantitative fetal fibronectin | <30: AUC 0.82 <34: AUC 0.74 <37: AUC 0.67 | Whole sample | 18–28 w |
| 2016 [70] | <34 w | “Indicated” | Simple cutoff | Low and high risk (cutoff corresponding to 85% spec.) | Uterine artery pulsatility index | AUC 0.93; Sens. 87% | Whole sample | 24–34 w |
| 2016 [71] | <37 w | Spontaneous | Simple cutoff | Low (>−1.37) and high risk (≤−1.37) | Insulin-like growth factor-binding protein and sex hormone-binding globulin | AUC 0.75; Sens. 75%; Spec. 0.74% | Holdout (discovery, verification and validation subsets) | 17–29 w |
| 2016 [72] | <37 w | Spontaneous | Parametric survival model | High risk for individual probability > 10% | Cervicovaginal fluid quantitative fetal fibronectin, cervical length, previous PTB or PPROM | AUC 0.77; Sens. 74.5%; Spec. 63.5%; PPV 26.5%; NPV 93.4%. | Holdout (50% training and 50% validation) | 22–30 w |
| 2017 [73] | <34 w | Spontaneous | Logistic regression | Low and high risk (no cutoff reported) | Anterior cervical angle, cervical length and maternal characteristics (maternal age and previous history of PTB) | Sens. 37.6%; Spec. 90% | Whole sample | 20–25 w |
| 2017 [74] | <34 w, 34–38 w | Spontaneous | Sum of categorical variables | Low and high risk (no cutoff reported) | Peripheral blood mononuclear cell microRNA (miR-148a, -301a, -671, -181a, -210, -1267, -223, and -340) | PTB 34–38 w: AUC 0.92; Sens. 86%; Spec. 84% | Holdout (50% training and 50% validation) | 4–13 w |
| 2018 [75] | <37 w | Spontaneous | Logistic regression | Low and high risk (no cutoff reported) | Race or ethnicity, age at delivery, education, payment for prenatal care, parity, location of mother’s birth, body mass index, preexisting diabetes, preexisting hypertension, reported smoking, reported drug/alcohol abuse, mental illness, sickle cell anemia, previous cesarean delivery, previous PTB, interpregnancy interval | AUC 0.591 | Holdout (2/3 training and 1/3 testing) | 1st trimester |
| 2018 [76] | <37 w | Spontaneous | Simple cutoff (Cervical texture based score) | Low (>−0.68) and high risk (≤−0.68) | Cervical texture features | Sens. 70.4%; Spec. 77.4% | Leave-one-out cross validation | 19–25 w |
| 2018 [77] | <33 w | Not reported | Simple binary model | Low (no funneling) and high risk (funneling) | Cervical funneling | Sens. 51%; Spec. 61% | Whole sample | 10–28 w |
| 2019 [78] | <37 w within 48 h and 7 days | Not reported | Logistic regression | High-risk for probability > 0.5 | Number of fetuses, age (mother), gravidity, parity, length (mother), weight (mother), BMI, gestational age at admission, duration ruptured membranes, method of conception, smoking history, alcohol usage, drug usage, history of cesarean section, race (mother), and admission indications | Within 7 days: AUC 0.83; Acc. 80%; Sens. 60%; Spec. 90% | 5-fold cross validation | 24–37 w |
| 2020 [79] | <37 w | Spontaneous | Logistic regression | High risk for cervical length > 41.1 mm, elastography index > 1.325 and strain pattern = 2 | Elastography index, Strain pattern score, Cervical length | AUC 0.90; Sens. 52%; Spec. 96% | Whole sample | 20–34 w |
| 2020 [80] | <37 w | Both | Simple cutoff | Low (<32.1 umol/L) and high risk (≥32.1 umol/L) | Total bile acids | AUC 0.62; Sens. 55.6%; Spec. 72.6%; PPV 59.5%; NPV 69.2% | Whole sample | Not reported |
| 2020 [81] | <37 w | Not reported | Logistic regression | Low and high risk (no cutoff reported) | Age, family situation, health coverage, gestity, parity, scarred uterus, prenatal interview | Validation dataset: AUC 0.63 | External validation (prospective validation dataset) | 1st trimester |
| 2020 [82] | <37 w | Spontaneous | Simple cutoff | Low (>25 mm) and high risk (≤25 mm) | Cervical length | Sens. 70% | Whole sample | 25–35 w |
| 2020 [83] | <32 w | Both | Simple cutoff | Low and high risk (no cutoff reported) | Insulin-like growth factor-binding protein 4 and sex hormone-binding globulin | AUC 0.71 | Whole sample | 17–22 w |
| 2020 [84] | <35 w | Spontaneous | Sum of 12 dichotomized variables | Low (<2) and high risk (≥2) | Peripheral blood microRNA (miR-181a-3p, miR-221-3p, miR-33a-5p, miR-6752-3p, miR-1244, miR-148a-3p, miR-1-3p, miR-1267, miR-223-5p, miR-199b-5p, miR-133b and miR-144-3p) | AUC 0.80; Sens. 89%; Spec. 71%; PPV 23%; NPV 99% | Holdout (50% training and 50% validation) | 6–13 w |
| 2021 [85] | <32 w | Both | Logistic regression | Low and high risk (no cutoff reported) | Progesterone metabolites 11-deoxycorticosterone, 16-alpha hydroxyprogesterone, parity, age, race, BMI, prior preterm deliveries, prior miscarriages | AUC 0.94; Sens. 91%; Spec. 87%; PPV 63%; NPV 98% | Whole sample | Late 1st/Early 2nd trimester |
| 2021 [86] | <37 w | Not reported | Relative risk weight converted into 0–100 score | For the 3rd trimester Low (<2) and high risk (≥2) | 71 risk factors that constituted six groups: anatomical, behavioral, demographic, disease, historical, and environmental | For the 3rd trimester: AUC 0.73; Sens. 53.1%; Spec. 82.4%; PPV 16.8%; NPV 96.4% | Holdout (80% training and 20% test) | All pregnancy |
| 2021 [87] | <34 w | Spontaneous | Sum of points | For 26–28 w: Low (<100) and high risk (≥100) | 22–24 w: primiparity, monochorionicity, prepregnancy BMI, previous premature or late abortion, and cervical length. 26–28 w: primiparity, monochorionicity, history of premature or late abortion, cervical length and cervical length shortening rate. | AUC 0.88; Sens. 69.4%; Spec. 88.6%; PPV 63.2%; NPV 91.1% | Holdout (70% training and 30% validation) | 22–24 w and 26–28 w |
| 2021 [88] | <37 w | Both | Logistic regression and machine learning (artificial neural networks) | Low and high risk (no cutoff reported) | 23 possible predictors in the 1st trimester, 35 possible predictors in the 2nd trimester | Artificial neural networks 2nd trimester AUC 0.80; Sens. 62.7%; Spec. 84.6%; PPV 23.2%; NPV 97.0% | Holdout (2/3 for training and 1/3 for validation) | 1st and 2nd trimesters |
| 2022 [89] | <37 w | Both | Least absolute shrinkage and selection operator via logistic regression | Levels 0, 1, 2, and 3 (lower to higher risk) | 26 variables grouped as drugs, hospital diagnosis, inpatient procedures, exemptions, outpatient services, socio-demographic conditions, and use of assisted medical conception techniques | AUC 0.61 | holdout (70% training and 30% testing) | Pre-pregnancy |
| 2022 [90] | <37 w | Spontaneous | Linear combination | Low (<13.89) and high risk (≥13.89) | Maternal blood early B cell factor gene-based microRNA transcripts (MIR4266, MIR1251, MIR601 and MIR3612) | AUC 0.82; Sens. 81%; Spec. 72% | Cross-validation | 27–33 w |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, A.; Bernardes, J.; Gonçalves, H. Risk Scoring Systems for Preterm Birth and Their Performance: A Systematic Review. J. Clin. Med. 2023, 12, 4360. https://doi.org/10.3390/jcm12134360
Ferreira A, Bernardes J, Gonçalves H. Risk Scoring Systems for Preterm Birth and Their Performance: A Systematic Review. Journal of Clinical Medicine. 2023; 12(13):4360. https://doi.org/10.3390/jcm12134360
Chicago/Turabian StyleFerreira, Amaro, João Bernardes, and Hernâni Gonçalves. 2023. "Risk Scoring Systems for Preterm Birth and Their Performance: A Systematic Review" Journal of Clinical Medicine 12, no. 13: 4360. https://doi.org/10.3390/jcm12134360
APA StyleFerreira, A., Bernardes, J., & Gonçalves, H. (2023). Risk Scoring Systems for Preterm Birth and Their Performance: A Systematic Review. Journal of Clinical Medicine, 12(13), 4360. https://doi.org/10.3390/jcm12134360








