The Effect of Terra-Cortril as Local Pain Medication on the Healing Process of a Fresh Extraction Socket: A Retrospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Selection
- (1)
- “Exposure cohort” if TC had been administered;
- (2)
- “Non-exposure cohort” if no TC had been administered.
- (1)
- Tooth extraction in other practice;
- (2)
- Other systemic antibiotics taken or topical antibiotics applied post-extraction;
- (3)
2.3. Healing Outcome
- -
- Deficient healing noted in the file by the operating dentist;
- -
- Confirmed after opening the flap and observing an open alveolus with black, tar-like substance, as seen in Figure 2;
- -
- Constrainedly placing the implant differently than planned due to a lack of primary stability or additional need of guided bone regeneration.
2.4. Explanatory Variables
- Prolonged healing time;
- Two-phase placement;
- Deviated angle of placement or position;
- Additional need and use of guided bone regeneration (GBR) before or during implant placement.
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohamed, H.B.; El-Hamid, B.N.A.; Fathalla, D.; Fouad, E.A. Current trends in pharmaceutical treatment of dry eye disease: A review. Eur. J. Pharm. Sci. 2022, 175, 106206. [Google Scholar] [CrossRef]
- Jaafar, N.; Nor, G.M. The prevalence of post-extraction complications in an outpatientdental clinic in Kuala Lumpur Malaysia–a retrospective survey. Singap. Dent. J. 2000, 23, 24–28. [Google Scholar]
- Bouloux, G.F.; Steed, M.B.; Perciaccante, V.J. Complications of third molar surgery. Oral Maxillofac. Surg. Clin. N. Am. 2007, 19, 117–128. [Google Scholar] [CrossRef]
- Bortoluzzi, M.C.; Guollo, A.; Capella, D.L. Pain levels after third molar surgical removal: An evaluation ofpredictive variables. J. Contemp. Dent. Pr. 2011, 12, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.B.; Chong, S.; Le, N.D. A survey of antibiotic use in dentistry. J. Am. Dent. Assoc. 2000, 131, 1600–1609. [Google Scholar] [CrossRef]
- Lodi, G.; Azzi, L.; Varoni, E.M.; Pentenero, M.; Del Fabbro, M.; Carrassi, A.; Sardella, A.; Manfredi, M. Antibiotics to prevent complications following tooth extractions. Cochrane Database Syst. Rev. 2021, 2021, CD003811. [Google Scholar] [CrossRef]
- Martin, A.; Perinetti, G.; Costantinides, F.; Maglione, M. Coronectomy as a surgical approach to impacted mandibular third molars: A systematic review. Head Face Med. 2015, 11, 9. [Google Scholar] [CrossRef] [Green Version]
- Cervera-Espert, J.; Pérez-Martínez, S.; Cervera-Ballester, J.; Peñarrocha-Oltra, D.; Peñarrocha-Diago, M. Coronectomy of impacted mandibular third molars: A meta-analysis and systematic review of the literature. Med. Oral Patol. Oral Y Cir. Bucal 2016, 21, e505–e513. [Google Scholar] [CrossRef]
- Cosola, S.; Kim, Y.S.; Park, Y.M.; Giammarinaro, E.; Covani, U. Coronectomy of mandibular third molar: Four years of follow-up of 130 cases. Medicina 2020, 56, 654. [Google Scholar] [CrossRef] [PubMed]
- Blum, I.R. Contemporary views on dry socket (alveolar osteitis): A clinical appraisal of standardization, aetiopathogenesis and management: A critical review. Int. J. Oral Maxillofac. Surg. 2002, 31, 309–317. [Google Scholar] [CrossRef] [Green Version]
- Fridrich, K.L.; Olson, R.A. Alveolar osteitis following surgical removal of mandibular third molars. Anesth. Prog. 1990, 37, 32–41. [Google Scholar] [PubMed]
- Julius, L.L.; Hungerford, R.W.; Nelson, W.J.; McKercher, T.C.; Zellhoefer, R.W. Prevention of dry socket with local application of Terra-Cortril in gelfoam. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 1982, 40, 285–286. [Google Scholar] [CrossRef] [PubMed]
- Otake, H.; Sato, Y.; Nakatani, E.; Hawke, P.; Takei, S.; Ogino, A.; Asai, H.; Abe, A.; Fukuta, K.; Adachi, M. Oxytetracycline-hydrocortisone ointment reduces the occurrence of both dry socket and post-extraction pain after third molar extraction: An observational study. PLoS ONE 2021, 7, e0254221. [Google Scholar] [CrossRef]
- Zuniga, J.R.; Leist, J.C. Topical tetracycline-induced neuritis: A case report. J. Oral Maxillofac. Surg. 1995, 53, 196–199. [Google Scholar] [CrossRef]
- Bosco, J.M.D.; de Oliveira, S.R.; Bosco, A.F.; Schweitzer, C.M.; Júnior, E.G.J. Influence of local tetracycline on the microbiota of alveolarosteitis in rats. Braz Dent J. 2008, 19, 119–123. [Google Scholar] [CrossRef] [Green Version]
- Fisher, S.C.; Horning, G.M.; Hellstein, J.W. Myospherulosis complicating cortical block grafting: A case report. J. Periodontol. 2001, 72, 1755–1759. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, P.; Ghannoum, J.E. Myospherulosis of the Mandible Presenting as a Multilocular Lesion: A Case Report and Review of the Literature. Head Neck Pathol. 2016, 10, 221–224. [Google Scholar] [CrossRef]
- Lynch, D.P.; Newland, J.R.; McClendon, J.L. Myospherulosis of the oral hard and soft tissues. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 1984, 42, 349–355. [Google Scholar] [CrossRef]
- Moore, J.W.; Brekke, J.H. Foreign body giant cell reaction related to placement of tetracycline-treated polylactic acid: Report of 18 cases. J. Oral Maxillofac. Surg. 1990, 48, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Belfiglio, E.J.; Wonderlich, S.T.; Fox, L.J. Myospherulosis of the alveolus secondary to the use of Terra-Cortril and Gelfoam. Report of a case. Oral Surg. Oral Med. Oral Pathol. 1986, 61, 12–14. [Google Scholar] [CrossRef]
- Eslami, A.; Van Swol, R.L.; Sadeghi, E.M. Connective tissue reactions to 3% tetracycline ointment in rat skin. J. Oral Maxillofac. Surg. 1987, 45, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Rosai, J. The nature of myospherulosis of the upper respiratory tract. Am. J. Clin. Pathol. 1978, 69, 475–481. [Google Scholar] [CrossRef] [Green Version]
- Cardaropoli, G.; Araújo, M.; Hayacibara, R.; Sukekava, F.; Lindhe, J. Healing of extraction sockets and surgically produced-Augmented and non-augmented-Defects in the alveolar ridge. An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 435–440. [Google Scholar] [CrossRef]
- Araújo, M.G.; Lindhe, J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Al Yafi, F.; Alchawaf, B.; Nelson, K. What is the Optimum for Alveolar Ridge Preservation? Dent. Clin. N. Am. 2019, 63, 399–418. [Google Scholar] [CrossRef]
- Garg, A. Preservation, augmentation, and reconstruction of the alveolar ridge. Dent. Implantol. Update 2001, 12, 81–85. [Google Scholar] [PubMed]
- Albrektsson, T.; Brånemark, P.I.; Hansson, H.A.; Lindström, J. Osseointegrated titanium implants: Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop. 1981, 52, 155–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mardas, N.; Trullenque-Eriksson, A.; MacBeth, N.; Petrie, A.; Donos, N. Does ridge preservation following tooth extraction improve implant treatment outcomes: A systematic review: Group 4: Therapeutic concepts & methods Does ridge preservation following tooth extraction improve implant treatment outcomes: A systematic review: Group 4: Therapeutic concepts & methods Mardas et al. Clin. Oral Implant. Res. 2015, 26, 180–201. [Google Scholar] [CrossRef]
- Saez-Alcaide, L.M.; Fernandez-Tresguerres, F.G.; Brinkmann, J.C.B.; Segura-Mori, L.; Iglesias-Velazquez, O.; Perez-Gonzalez, F.; Lopez-Pintor, R.M.; Garcia-Denche, J.T. Socket shield technique: A systematic review of human studies. Ann. Anat. 2021, 238, 151779. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Chen, Z.; Pan, W.-L.; Wang, H.-L. Assessment of a Novel Alveolar Ridge Preservation Concept in Dehiscence Sockets: A Pilot Study. Int. J. Periodontics Restor. Dent. 2022, 42, e75–e83. [Google Scholar] [CrossRef]
- Atieh, M.A.; Alfardan, L.; Alsabeeha, N.H.M. Flapped versus flapless alveolar ridge preservation: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2022, 51, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Avila-Ortiz, G.; Gubler, M.; Romero-Bustillos, M.; Nicholas, C.L.; Zimmerman, M.B.; Barwacz, C.A. Efficacy of Alveolar Ridge Preservation: A Randomized Controlled Trial. J. Dent. Res. 2020, 99, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Sanchis, J.M.; Sáez, U.; Peñarrocha, M.; Gay, C. Tetracycline compound placement to prevent dry socket: A postoperative study of 200 impacted mandibular third molars. J. Oral Maxillofac. Surg. 2004, 62, 587–591. [Google Scholar] [CrossRef]
- Ford, P.J.; Rich, A.M. Tobacco Use and Oral Health. Addiction 2021, 116, 3531–3540. [Google Scholar] [CrossRef]
- Kalra, S.; Jain, V. Dental complications and management of patients on bisphosphonate therapy: A review article. J. Oral Biol. Craniofacial Res. 2013, 3, 25–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seyssens, L.; Eghbali, A.; Christiaens, V.; De Bruyckere, T.; Doornewaard, R.; Cosyn, J. A one-year prospective study on alveolar ridge preservation using collagen-enriched deproteinized bovine bone mineral and saddle connective tissue graft: A cone beam computed tomography analysis. Clin. Implant. Dent. Relat. Res. 2019, 21, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Bouckaert, E.; De Bruyckere, T.; Eghbali, A.; Younes, F.; Wessels, R.; Cosyn, J. A randomized controlled trial comparing guided bone regeneration to connective tissue graft to re-establish buccal convexity at dental implant sites: Three-year results. Clin. Oral Implant. Res. 2022, 33, 461–471. [Google Scholar] [CrossRef]
- Divine, K.A. Multilocular Myospherulosis of the Mandible: A Case Report. J. Endod. 2022, 48, 961–964. [Google Scholar] [CrossRef]
- Coulier, B.; Desgain, O.; Gielen, I. Sinonasal myospherulosis and paraffin retention cysts suggested by CT: Report of a case. Head Neck Pathol. 2012, 6, 270–274. [Google Scholar] [CrossRef] [Green Version]
- Carlson, E.R.; Jackson, B. Myospherulosis. Complicating wound healing. J. Am. Dent. Assoc. 1991, 122, 65–66. [Google Scholar] [CrossRef]
- Navas, R.M.A.; Mendoza, M.G.M. Case report: Late complication of a dry socket treatment. Int. J. Dent. 2010, 2010, 479306. [Google Scholar] [CrossRef]
- Barootchi, S.; Tavelli, L.; Majzoub, J.; Stefanini, M.; Wang, H.L.; Avila-Ortiz, G. Alveolar ridge preservation: Complications and cost-effectiveness. Periodontology 2000 2022. [Google Scholar] [CrossRef] [PubMed]
- Wessels, R.; Vervaeke, S.; Seyssens, L.; Eghbali, A.; Cosyn, J. A 5-year cohort study on early implant placement with guided bone regeneration or alveolar ridge preservation with connective tissue graft. Clin. Implant. Dent. Relat. Res. 2020, 22, 697–705. [Google Scholar] [CrossRef]
- Sculean, A.; Gruber, R.; Bosshardt, D.D. Soft tissue wound healing around teeth and dental implants. J. Clin. Periodontol. 2014, 41, S6–S22. [Google Scholar] [CrossRef] [Green Version]
- McClatchie, S.; Warambo, M.W.; Bremner, A.D. Myospherulosis: A previously unreported disease? Am. J. Clin. Pathol. 1969, 51, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Kyriakos, M. Myospherulosis of the paranasal sinuses, nose and middle ear. A possible iatrogenic disease. Am. J. Clin. Pathol. 1977, 67, 118–130. [Google Scholar] [CrossRef]
- De Schryver-Kecskemeti, K.; Kyriakos, M. Myospherulosis. An electron-microscopic study of a human case. Am. J. Clin. Pathol. 1977, 67, 555–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyne, P.J.; Kruger, G.O. Fluorescence microscopy of alveolar bone repair. Oral Surg. Oral Med. Oral Pathol. 1962, 15, 265–281. [Google Scholar] [CrossRef]
- MacBeth, N.; Trullenque-Eriksson, A.; Donos, N.; Mardas, N. Hard and soft tissue changes following alveolar ridge preservation: A systematic review. Clin. Oral Implant. Res. 2017, 28, 982–1004. [Google Scholar] [CrossRef]
- Froum, S.; Cho, S.-C.; Rosenberg, E.; Rohrer, M.; Tarnow, D. Histological Comparison of Healing Extraction Sockets Implanted With Bioactive Glass or Demineralized Freeze-Dried Bone Allograft: A Pilot Study. J. Periodontol. 2002, 73, 94–102. [Google Scholar] [CrossRef]
- Mardas, N.; Chadha, V.; Donos, N. Alveolar ridge preservation with guided bone regeneration and a synthetic bone substitute or a bovine-derived xenograft: A randomized, controlled clinical trial. Clin. Oral Implant. Res. 2010, 21, 688–698. [Google Scholar] [CrossRef]
- Chan, H.-L.; Lin, G.-H.; Fu, J.-H.; Wang, H.-L. Alterations in Bone Quality After Socket Preservation with Grafting Materials: A Systematic Review. Int. J. Oral Maxillofac. Implant. 2013, 28, 710–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horváth, A.; Mardas, N.; Mezzomo, L.A.; Needleman, I.G.; Donos, N. Alveolar ridge preservation. A systematic review. Clin. Oral Investig. 2013, 17, 341–363. [Google Scholar] [CrossRef] [PubMed]
- Becker, W.; Becker, B.E.; Caffesse, R. A Comparison of Demineralized Freeze-Dried Bone and Autologous Bone to Induce Bone Formation in Human Extraction Sockets. J. Periodontol. 1994, 65, 1128–1133. [Google Scholar] [CrossRef] [PubMed]
- Becker, W.; Urist, M.; Becker, B.E.; Jackson, W.; Party, D.A.; Bartold, M.; Vincenzzi, G.; De Georges, D.; Niederwanger, M. Clinical and Histologie Observations of Sites Implanted With Intraoral. J. Periodontol. 1996, 67, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Del Fabbro, M.; Khijmatgar, S.; Panda, S.; Ravidà, A.; Tommasato, G.; Sculean, A.; Pesce, P. Dimensional and histomorphometric evaluation of biomaterials used for alveolar ridge preservation: A systematic review and network meta-analysis. Clin. Oral Investig. 2022, 26, 141–158. [Google Scholar] [CrossRef]
- Swanson, A.E. A double-blind study on the effectiveness of tetracycline in reducing the incidence of fibrinolytic alveolitis. J. oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 1989, 47, 165–167. [Google Scholar] [CrossRef]
- López-Jornet, P.; Camacho-Alonso, F.; Martinez-Canovas, A.; Sidrach-Cardona, M. Topical 1% oxytetracycline hydrochloride versus placebo in oral mucosa biopsy. Dermatol. Surg. 2012, 38, 1054–1058. [Google Scholar] [CrossRef]
- Sari, O.; Temiz, C.; Golcur, M.; Aydogan, U.; Tanoglu, A.; Ezgu, M.C.; Tehli, O. Pain perception differences between patients and physicians: A pain severity study in patients with low back pain. Turk. Neurosurg. 2015, 25, 461–468. [Google Scholar] [CrossRef] [Green Version]
- Sukumar, S.; Martin, F.E.; Hughes, T.E.; Adler, C.J. Think before you prescribe: How dentistry contributes to antibiotic resistance. Aust. Dent. J. 2020, 65, 21–29. [Google Scholar] [CrossRef]
- Sologova, D.; Diachkova, E.; Gor, I.; Sologova, S.; Grigorevskikh, E.; Arazashvili, L.; Petruk, P.; Tarasenko, S. Antibiotics Efficiency in the Infection Complications Prevention after Third Molar Extraction: A Systematic Review. Dent. J. 2022, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Rakhshan, V. Common risk factors of dry socket (alveolitis osteitis) following dental extraction: A brief narrative review. J. Stomatol. Oral Maxillofac. Surg. 2018, 119, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Tarakji, B.; Saleh, L.A.; Umair, A.; Azzeghaiby, S.N.; Hanouneh, S. Systemic review of dry socket: Aetiology, treatment, and prevention. J. Clin. Diagn. Res. 2015, 9, ZE10-3. [Google Scholar] [CrossRef] [PubMed]
- Chow, O.; Wang, R.; Ku, D.; Huang, W. Alveolar Osteitis: A Review of Current Concepts. J. Oral Maxillofac. Surg. 2020, 78, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Lynham, A.J.; Hsu, E. Postoperative interventions to reduce inflammatory complications after third molar surgery: Review of the current evidence. Aust. Dent. J. 2017, 62, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Brancaccio, Y.; Antonelli, A.; Barone, S.; Bennardo, F.; Fortunato, L.; Giudice, A. Evaluation of local hemostatic efficacy after dental extractions in patients taking antiplatelet drugs: A randomized clinical trial. Clin. Oral Investig. 2021, 25, 1159–1167. [Google Scholar] [CrossRef]
- Ustaoğlu, G.; Bulut, D.G.; Gümüş, K. Evaluation of different platelet-rich concentrates effects on early soft tissue healing and socket preservation after tooth extraction. J. Stomatol. Oral Maxillofac. Surg. 2020, 121, 539–544. [Google Scholar] [CrossRef]
- de Almeida Barros Mourão, C.F.; de Mello-Machado, R.C.; Javid, K.; Moraschini, V. The use of leukocyte- and platelet-rich fibrin in the management of soft tissue healing and pain in post-extraction sockets: A randomized clinical trial. J. Cranio-Maxillofacial. Surg. 2020, 48, 452–457. [Google Scholar] [CrossRef]
- Ghanaati, S.; Śmieszek-Wilczewska, J.; Al-Maawi, S.; Neff, P.; Zadeh, H.H.; Sader, R.; Heselich, A.; Rutkowski, J.L. Solid PRF Serves as Basis for Guided Open Wound Healing of the Ridge after Tooth Extraction by Accelerating the Wound Healing Time Course—A Prospective Parallel Arm Randomized Controlled Single Blind Trial. Bioengineering 2022, 9, 661. [Google Scholar] [CrossRef]
- Miron, R.J.; Zucchelli, G.; Pikos, M.A.; Salama, M.; Lee, S.; Guillemette, V.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Wang, H.L.; et al. Use of platelet-rich fibrin in regenerative dentistry: A systematic review. Clin. Oral Investig. 2017, 21, 1913–1927. [Google Scholar] [CrossRef]



| Healing | |||||||
|---|---|---|---|---|---|---|---|
| Successful | Unsuccessful | OR | p-Value * | ||||
| Variable | n | % | n | % | |||
| Gender | |||||||
| Male | 26 | 53.06 | 23 | 46.94 | 0.75 | 0.486 | |
| Female | 30 | 60.00 | 20 | 40.00 | |||
| Treatment group | |||||||
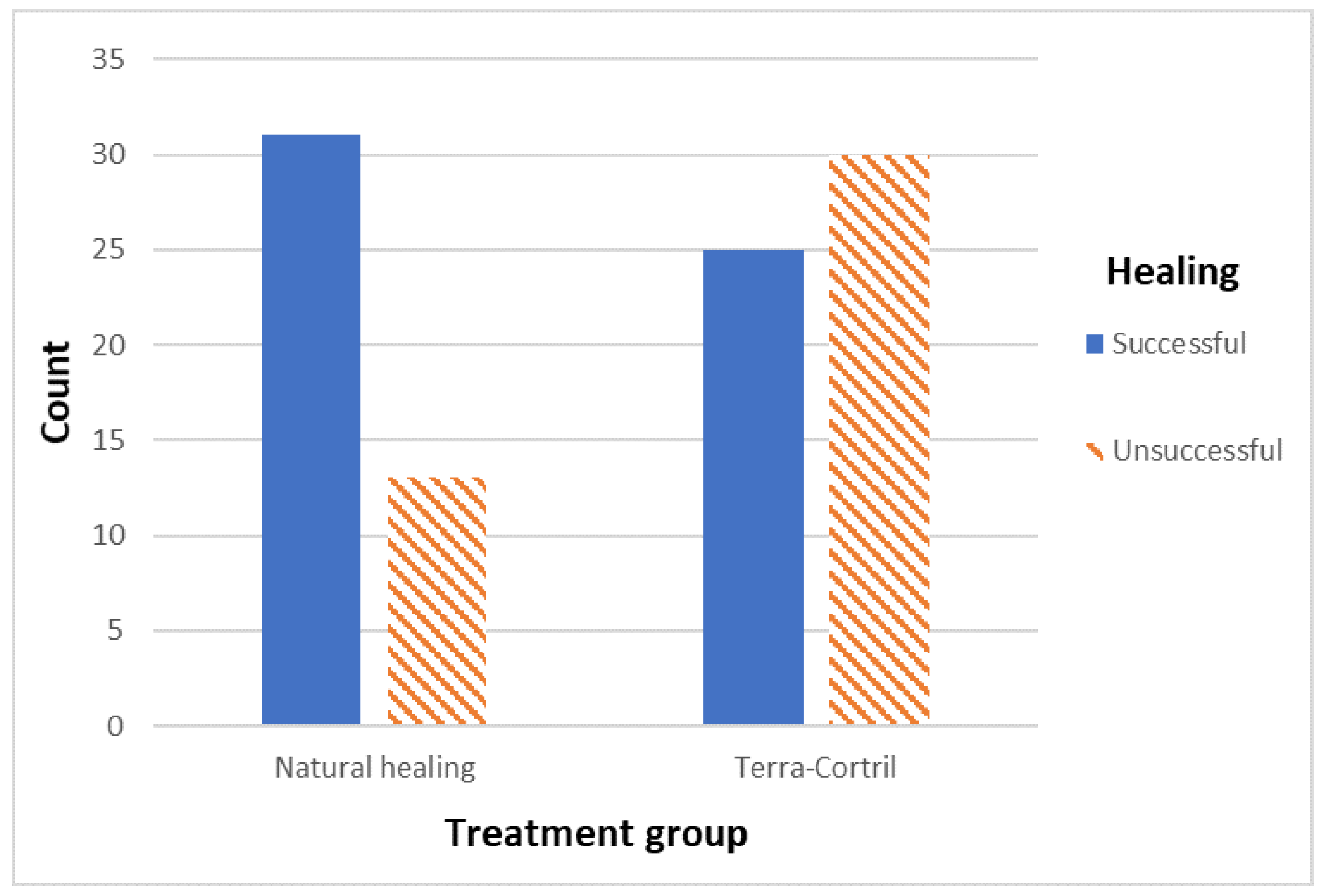
| Natural healing | 31 | 70.45 | 13 | 29.55 | 2.86 | 0.013 | |
| TC | 25 | 45.45 | 30 | 54.55 | |||
| Jaw | |||||||
| Maxilla | 35 | 67.31 | 17 | 32.69 | 2.55 | 0.023 | |
| Mandible | 21 | 44.68 | 26 | 55.32 | |||
| Apical radiolucency | |||||||
| Yes | 37 | 52.11 | 34 | 47.89 | 0.44 | 0.117 | |
| No | 15 | 71.43 | 6 | 28.57 | |||
| Tooth location | |||||||
| Anterior | 16 | 61.54 | 10 | 38.46 | 0.98 ** | 0.303 | |
| Molar | 26 | 61.90 | 16 | 38.10 | 1.94 *** | ||
| Premolar | 14 | 45.16 | 17 | 54.84 | |||
| Successful | Unsuccessful | B | df | p-value **** | |||
| Variable | mean | 95% CI | mean | 95% CI | |||
| Age | |||||||
| 60.22 | 56.67–63.77 | 56.02 | 51.85–60.18 | −0.023 | 1 | 0.136 | |
| L1 | L3 | L5 | L7 | Vmax | Hmax | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (%) | 95% C.I. | p | Mean (%) | 95% C.I. | p | Mean (%) | 95% C.I. | p | Mean (%) | 95% C.I. | p | Mean (mm) | 95% C.I. | p | Mean (mm) | 95% C.I. | p | |
| Treatment group | ||||||||||||||||||
| Natural healing | 21.99 | 11.20–32.79 | 0.011 | 17.52 | 7.89–27.16 | 0.009 | 14.00 | 5.41–22.59 | 0.006 | 12.22 | 4.19–20.25 | 0.072 | 3.16 | 1.68–4.65 | 0.030 | 2.01 | 1.16–2.86 | 0.005 |
| TC | 45.83 | 35.86–55.79 | 39.40 | 30.58–48.22 | 30.74 | 22.67–38.81 | 18.64 | 11.75–25.52 | 4.61 | 3.52–5.70 | 3.85 | 2.99–4.70 | ||||||
| Jaw | ||||||||||||||||||
| Lower jaw | 39.25 | 28.27–50.22 | 0.245 | 35.00 | 24.90–45.09 | 0.073 | 30.86 | 21.95–39.76 | 0.011 | 21.63 | 13.40–29.87 | 0.015 | 5.02 | 3.68–6.35 | 0.022 | 3.59 | 2.67–4.51 | 0.098 |
| Upper jaw | 32.63 | 21.78–43.48 | 25.85 | 16.54–35.17 | 17.31 | 9.21–25.42 | 10.90 | 4.48–17.31 | 3.02 | 1.85–4.19 | 2.53 | 1.67–3.38 | ||||||
| Gender | ||||||||||||||||||
| Male | 35.46 | 24.17–46.75 | 0.982 | 29.51 | 19.76–39.25 | 0.899 | 23.93 | 15.36–32.51 | 0.796 | 17.42 | 9.12–25.72 | 0.673 | 4.14 | 2.88–5.40 | 0.604 | 3.36 | 2.39–4.32 | 0.354 |
| Female | 35.95 | 25.28–46.63 | 30.67 | 20.91–40.43 | 23.39 | 14.60–32.18 | 14.60 | 7.92–21.28 | 3.80 | 2.50–5.10 | 2.71 | 1.89–3.53 | ||||||
| Apical radio-translucency | ||||||||||||||||||
| Yes | 37.68 | 29.17–46.19 | 0.439 | 30.98 | 23.52–38.44 | 0.591 | 24.18 | 17.44–30.93 | 0.662 | 16.39 | 10.39–22.39 | 0.822 | 4.19 | 3.11–5.28 | 0.193 | 3.20 | 2.45–3.94 | 0.172 |
| No | 29.11 | 11.15–47.07 | 27.24 | 10.28–44.20 | 21.82 | 7.19–36.45 | 14.31 | 3.35–25.26 | 2.85 | 0.93–4.77 | 2.18 | 0.79–3.58 | ||||||
| Tooth location | ||||||||||||||||||
| Anterior | 27.81 | 11.92–43.70 | 0.314 | 25.69 | 11.72–39.65 | 0.571 | 20.17 | 7.36–32.99 | 0.328 | 13.85 | 2.80–24.90 | 0.618 | 2.53 | 1.00–4.07 | 0.137 | 2.30 | 0.93–3.68 | 0.105 |
| Pre-molar | 37.80 | 25.56–50.05 | 30.70 | 19.72–41.68 | 23.39 | 13.82–32.95 | 14.64 | 5.69–23.59 | 4.65 | 3.07–6.23 | 3.83 | 2.67–5.00 | ||||||
| Molar | 39.89 | 26.77–53.00 | 33.29 | 21.31–45.27 | 27.10 | 16.37–37.84 | 18.15 | 9.88–26.43 | 4.25 | 2.82–5.69 | 2.89 | 2.00–3.78 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vuylsteke, F.; Cosyn, J.; Tytgat, M.; Eghbali, A. The Effect of Terra-Cortril as Local Pain Medication on the Healing Process of a Fresh Extraction Socket: A Retrospective Cohort Study. J. Clin. Med. 2023, 12, 4372. https://doi.org/10.3390/jcm12134372
Vuylsteke F, Cosyn J, Tytgat M, Eghbali A. The Effect of Terra-Cortril as Local Pain Medication on the Healing Process of a Fresh Extraction Socket: A Retrospective Cohort Study. Journal of Clinical Medicine. 2023; 12(13):4372. https://doi.org/10.3390/jcm12134372
Chicago/Turabian StyleVuylsteke, Fauve, Jan Cosyn, Manon Tytgat, and Aryan Eghbali. 2023. "The Effect of Terra-Cortril as Local Pain Medication on the Healing Process of a Fresh Extraction Socket: A Retrospective Cohort Study" Journal of Clinical Medicine 12, no. 13: 4372. https://doi.org/10.3390/jcm12134372
APA StyleVuylsteke, F., Cosyn, J., Tytgat, M., & Eghbali, A. (2023). The Effect of Terra-Cortril as Local Pain Medication on the Healing Process of a Fresh Extraction Socket: A Retrospective Cohort Study. Journal of Clinical Medicine, 12(13), 4372. https://doi.org/10.3390/jcm12134372







