Post-Severe-COVID-19 Cardiopulmonary Rehabilitation: A Comprehensive Study on Patient Features and Recovery Dynamics in Correlation with Workout Intensity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics
2.2. Patients’ Inclusion and Exclusion Criteria
2.3. Rehabilitation Protocols
2.4. Study Variables
2.5. Statistical Analysis
3. Results
3.1. Patients’ Background
3.2. Laboratory Data
3.3. Cardiopulmonary Measurements
3.4. Cardiopulmonary Exercise Testing
3.5. Final Comparisons
4. Discussion
4.1. Literature Findings
4.2. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manolescu, D.; Timar, B.; Bratosin, F.; Rosca, O.; Citu, C.; Oancea, C. Predictors for COVID-19 Complete Remission with HRCT Pattern Evolution: A Monocentric, Prospective Study. Diagnostics 2022, 12, 1397. [Google Scholar] [CrossRef]
- Raman, B.; Bluemke, D.A.; Lüscher, T.F.; Neubauer, S. Long COVID: Post-acute sequelae of COVID-19 with a cardiovascular focus. Eur. Heart J. 2022, 43, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Cerbu, B.; Grigoras, M.L.; Bratosin, F.; Bogdan, I.; Citu, C.; Bota, A.V.; Timircan, M.; Bratu, M.L.; Levai, M.C.; Marincu, I. Laboratory Profile of COVID-19 Patients with Hepatitis C-Related Liver Cirrhosis. J. Clin. Med. 2022, 11, 652. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.D.; Lavelle, M.; Boursiquot, B.C.; Wan, E.Y. Long-term complications of COVID-19. Am. J. Physiol. Cell Physiol. 2022, 322, C1–C11. [Google Scholar] [CrossRef] [PubMed]
- Pilut, C.N.; Citu, C.; Gorun, F.; Bratosin, F.; Gorun, O.M.; Burlea, B.; Citu, I.M.; Grigoras, M.L.; Manolescu, D.; Gluhovschi, A. The Utility of Laboratory Parameters for Cardiac Inflammation in Heart Failure Patients Hospitalized with SARS-CoV-2 Infection. Diagnostics 2022, 12, 824. [Google Scholar] [CrossRef] [PubMed]
- Marincu, I.; Citu, C.; Bratosin, F.; Bogdan, I.; Timircan, M.; Gurban, C.V.; Bota, A.V.; Braescu, L.; Grigoras, M.L. Clinical Characteristics and Outcomes of COVID-19 Hospitalized Patients: A Comparison between Complete mRNA Vaccination Profile and Natural Immunity. J. Pers. Med. 2022, 12, 259. [Google Scholar] [CrossRef]
- Visco, V.; Vitale, C.; Rispoli, A.; Izzo, C.; Virtuoso, N.; Ferruzzi, G.J.; Santopietro, M.; Melfi, A.; Rusciano, M.R.; Maglio, A.; et al. Post-COVID-19 Syndrome: Involvement and Interactions between Respiratory, Cardiovascular and Nervous Systems. J. Clin. Med. 2022, 11, 524. [Google Scholar] [CrossRef]
- Sherif, Z.A.; Gomez, C.R.; Connors, T.J.; Henrich, T.J.; Reeves, W.B.; RECOVER Mechanistic Pathway Task Force. Pathogenic mechanisms of post-acute sequelae of SARS-CoV-2 infection (PASC). Elife 2023, 12, e86002. [Google Scholar] [CrossRef]
- Scurati, R.; Papini, N.; Giussani, P.; Alberti, G.; Tringali, C. The Challenge of Long COVID-19 Management: From Disease Molecular Hallmarks to the Proposal of Exercise as Therapy. Int. J. Mol. Sci. 2022, 23, 12311. [Google Scholar] [CrossRef]
- Citu, C.; Burlea, B.; Gorun, F.; Motoc, A.; Gorun, O.M.; Malita, D.; Ratiu, A.; Margan, R.; Grigoras, M.L.; Bratosin, F.; et al. Predictive Value of Blood Coagulation Parameters in Poor Outcomes in COVID-19 Patients: A Retrospective Observational Study in Romania. J. Clin. Med. 2022, 11, 2831. [Google Scholar] [CrossRef]
- Tan, W.; Aboulhosn, J. The cardiovascular burden of coronavirus disease 2019 (COVID-19) with a focus on congenital heart disease. Int. J. Cardiol. 2020, 309, 70–77. [Google Scholar] [CrossRef]
- Fericean, R.M.; Citu, C.; Manolescu, D.; Rosca, O.; Bratosin, F.; Tudorache, E.; Oancea, C. Characterization and Outcomes of SARS-CoV-2 Infection in Overweight and Obese Patients: A Dynamic Comparison of COVID-19 Pandemic Waves. J. Clin. Med. 2022, 11, 2916. [Google Scholar] [CrossRef]
- Budea, C.M.; Pricop, M.; Mot, I.C.; Horhat, F.G.; Hemaswini, K.; Akshay, R.; Negrean, R.A.; Oprisoni, A.L.; Citu, C.; Bumbu, B.A.; et al. The Assessment of Antimicrobial Resistance in Gram-Negative and Gram-Positive Infective Endocarditis: A Multicentric Retrospective Analysis. Medicina 2023, 59, 457. [Google Scholar] [CrossRef]
- Momsen, A.H.; Ørtenblad, L.; Maribo, T. Effective rehabilitation interventions and participation among people with multiple sclerosis: An overview of reviews. Ann. Phys. Rehabil. Med. 2022, 65, 101529. [Google Scholar] [CrossRef]
- Papava, I.; Dehelean, L.; Romosan, R.S.; Bondrescu, M.; Dimeny, C.Z.; Domuta, E.M.; Bratosin, F.; Bogdan, I.; Grigoras, M.L.; Tigmeanu, C.V.; et al. The Impact of Hyper-Acute Inflammatory Response on Stress Adaptation and Psychological Symptoms of COVID-19 Patients. Int. J. Environ. Res. Public Health 2022, 19, 6501. [Google Scholar] [CrossRef] [PubMed]
- Dehelean, L.; Papava, I.; Musat, M.I.; Bondrescu, M.; Bratosin, F.; Bucatos, B.O.; Bortun, A.-M.C.; Mager, D.V.; Romosan, R.S.; Romosan, A.-M.; et al. Coping Strategies and Stress Related Disorders in Patients with COVID-19. Brain Sci. 2021, 11, 1287. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, V.A.; Allan, L.; Bethel, A.; Cowley, A.; Cross, J.L.; Day, J.; Drummond, A.; Hall, A.J.; Howard, M.; Morley, N.; et al. Rehabilitation to enable recovery from COVID-19: A rapid systematic review. Physiotherapy 2021, 111, 4–22. [Google Scholar] [CrossRef]
- De Biase, S.; Cook, L.; Skelton, D.A.; Witham, M.; Ten Hove, R. The COVID-19 rehabilitation pandemic. Age Ageing 2020, 49, 696–700. [Google Scholar] [CrossRef]
- Li, X.; Zhong, X.; Wang, Y.; Zeng, X.; Luo, T.; Liu, Q. Clinical determinants of the severity of COVID-19: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0250602. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, I.; Gadela, T.; Bratosin, F.; Dumitru, C.; Popescu, A.; Horhat, F.G.; Negrean, R.A.; Horhat, R.M.; Mot, I.C.; Bota, A.V.; et al. The Assessment of Multiplex PCR in Identifying Bacterial Infections in Patients Hospitalized with SARS-CoV-2 Infection: A Systematic Review. Antibiotics 2023, 12, 465. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. ICD-10: International Statistical Classification of Diseases and Related Health Problems: Tenth Revision, 2nd ed.; World Health Organization: Geneva, Switzerland, 2004; Available online: https://apps.who.int/iris/handle/10665/42980 (accessed on 7 April 2023).
- ARDS Definition Task Force; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef] [PubMed]
- The Agency for Health Technology Assessment and Tariff System. Recommendation on Medical Technologies, Health Policy Programs and the Conditions for the Implementation of These Programs Regarding the Therapeutic Rehabilitation for People after COVID-19 Disease. Available online: https://bipold.aotm.gov.pl/assets/files/ppz/2021/RPT/RAPORT_rekom_art_48aa_Covid_rehabilitacja.pdf (accessed on 7 May 2022).
- Zampogna, E.; Paneroni, M.; Belli, S.; Aliani, M.; Gandolfo, A.; Visca, D.; Bellanti, M.T.; Ambrosino, N.; Vitacca, M. Pulmonary rehabilitation in patients recovering from COVID-19. Respiration 2021, 100, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhang, W.; Yang, Y.; Zhang, J.; Li, Y.; Chen, Y. Respiratory rehabilitation in elderly patients with COVID-19: A randomized controlled study. Complement. Ther. Clin. Pract. 2020, 39, 101166. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.C.; Limbach, M.; Schuler, M.; Merkl, S.; Schwarzl, G.; Jakab, K.; Nowak, D.; Schultz, K. Effectiveness of a Three-Week Inpatient Pulmonary Rehabilitation Program for Patients after COVID-19: A Prospective Observational Study. Int. J. Environ. Res. Public Health 2021, 18, 9001. [Google Scholar] [CrossRef]
- Gloeckl, R.; Leitl, D.; Jarosch, I.; Schneeberger, T.; Nell, C.; Stenzel, N.; Vogelmeier, C.F.; Kenn, K.; Koczulla, A.R. Benefits of pulmonary rehabilitation in COVID-19: A prospective observational cohort study. ERJ Open Res. 2021, 7, 00108–2021. [Google Scholar] [CrossRef]
- Łoboda, D.; Gibiński, M.; Wilczek, J.; Paradowska-Nowakowska, E.; Ekiert, K.; Rybicka, E.; Sarecka-Hujar, B.; Szołtysek-Bołdys, I.; Zielińska-Danch, W.; Gołba, K.S. Effectiveness of cardiopulmonary rehabilitation after COVID-19 in Poland. Pol. Arch. Intern. Med. 2023, 133, 16341. [Google Scholar] [CrossRef] [PubMed]
- Spielmanns, M.; Buelow, M.M.; Pekacka-Egli, A.M.; Cecon, M.; Spielmanns, S.; Windisch, W.; Hermann, M. Clinical and functional predictors of response to a comprehensive pulmonary rehabilitation in severe post-COVID-19 patients. Microorganisms 2021, 9, 2452. [Google Scholar] [CrossRef]
- Świątkiewicz, I.; Di Somma, S.; De Fazio, L.; Mazzilli, V.; Taub, P.R. Effectiveness of Intensive Cardiac Rehabilitation in High-Risk Patients with Cardiovascular Disease in Real-World Practice. Nutrients 2021, 13, 3883. [Google Scholar] [CrossRef]
- Calabrese, M.; Garofano, M.; Palumbo, R.; Di Pietro, P.; Izzo, C.; Damato, A.; Venturini, E.; Iesu, S.; Virtuoso, N.; Strianese, A.; et al. Exercise Training and Cardiac Rehabilitation in COVID-19 Patients with Cardiovascular Complications: State of Art. Life 2021, 11, 259. [Google Scholar] [CrossRef]
- Kaliterna Lipovčan, L.; Prizmić-Larsen, Z.; Franc, R. Differences between COVID-19-vaccinated and unvaccinated participants from Croatia. Croat. Med. J. 2022, 63, 508–514. [Google Scholar] [CrossRef]
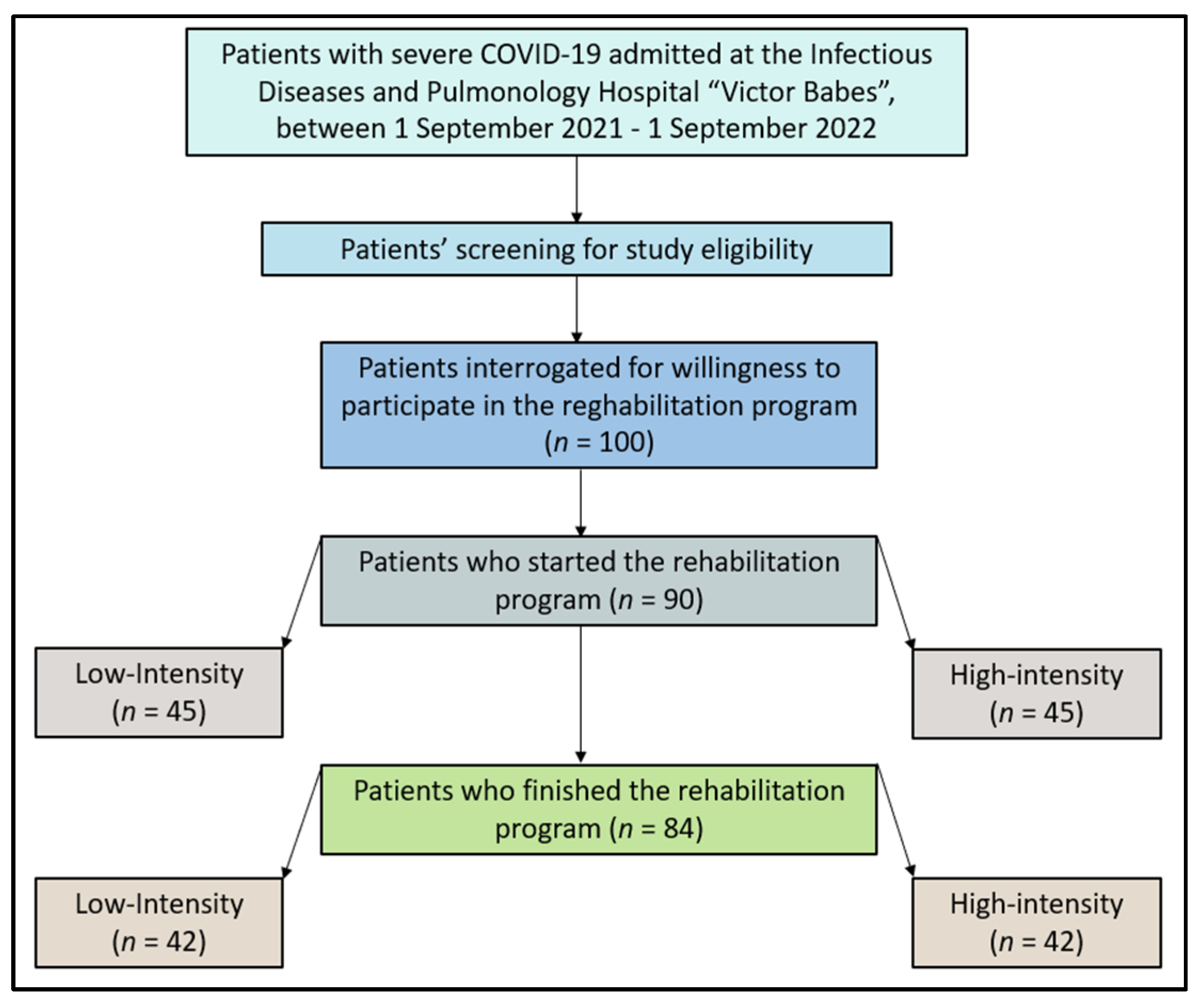

| Variables * | Low Intensity (n = 42) | High Intensity (n = 42) | p-Value |
|---|---|---|---|
| Age (mean ± SD) | 56.3 ± 7.3 | 53.1 ± 10.8 | 0.115 |
| Age (range) | 42–67 | 35–72 | - |
| Sex | 0.659 | ||
| Men (n, %) | 23 (54.8%) | 25 (59.5%) | |
| Women (n, %) | 19 (45.2%) | 17 (40.5%) | |
| BMI | 0.669 | ||
| Normal weight (18.5–24.9 kg/m2) | 14 (33.3%) | 12 (28.6%) | |
| Overweight (>24.9 kg/m2) | 20 (47.6%) | 24 (57.1%) | |
| Obese (>29.9 kg/m2) | 8 (19.0%) | 6 (14.3%) | |
| COVID-19 vaccination status | 0.629 | ||
| Vaccinated (2 doses) | 11 (26.2%) | 13 (31.0%) | |
| Unvaccinated | 31 (73.8%) | 29 (69.0%) | |
| Level of physical activity | 0.512 | ||
| Sedentary | 26 (61.9%) | 22 (52.4%) | |
| Active | 12 (28.6%) | 17 (40.5%) | |
| Very active | 4 (9.5%) | 3 (7.1%) | |
| Duration of hospitalization (mean ± SD) | 11.6 ± 7.2 | 12.2 ± 6.8 | 0.695 |
| Comorbidities (n, %) | 0.708 | ||
| Cardiovascular ** | 16 (38.1%) | 13 (31.0%) | |
| Metabolic disease | 10 (23.8%) | 14 (33.3%) | |
| Digestive | 6 (14.3%) | 9 (21.4%) | |
| Others | 8 (19.0%) | 10 (23.8%) | |
| Antiviral medication requirement * | 0.266 | ||
| Yes | 32 (76.2%) | 36 (85.7%) | |
| No | 10 (23.8%) | 6 (14.3%) |
| Variables | Normal Range | Low Intensity (n = 42) | Median (IQR) | High Intensity (n = 42) | Median (IQR) | p-Value |
|---|---|---|---|---|---|---|
| WBC (1000/mm3) | 4.5–11.0 | 14 (33.3%) | 11.4 (4.6) | 16 (38.1%) | 10.6 (4.8) | 0.649 |
| Lymphocytes (1000/mm3) | 1.0–4.8 | 15 (35.7%) | 6.1 (2.3) | 11 (26.2%) | 6.4 (2.5) | 0.345 |
| Hemoglobin (g/dL) | 13.0–17.0 | 4 (9.5%) | 14.1 (5.0) | 3 (7.1%) | 14.5 (5.2) | 0.693 |
| AST (U/L) | 10–40 | 12 (28.6%) | 33 (7) | 13 (31.0%) | 37 (8) | 0.811 |
| ALT (U/L) | 7–35 | 13 (31.0%) | 34 (10) | 10 (23.8%) | 36 (11) | 0.463 |
| Creatinine (µmol/L) | 0.74–1.35 | 15 (35.7%) | 1.09 (0.71) | 18 (42.9%) | 1.42 (0.76) | 0.502 |
| CRP (mg/dL) | 0–10 | 18 (42.9%) | 24 (16) | 24 (57.1%) | 28 (14) | 0.190 |
| IL-6 (pg/mL) | 0.8–6.4 | 19 (45.2%) | 9.8 (4.7) | 21 (50.0%) | 12.9 (5.8) | 0.662 |
| Procalcitonin (μg/L) | 0–0.25 | 3 (7.1%) | 0.10 (0.04) | 5 (11.9%) | 0.12 (0.06) | 0.457 |
| D-dimers (ng/mL) | <250 | 10 (23.8%) | 231 (96) | 12 (28.6%) | 244 (101) | 0.619 |
| Ferritin (ng/mL) | 20–250 | 14 (33.3%) | 206 (48) | 10 (23.8%) | 225 (62) | 0.334 |
| Variables | Normal Range | Low Intensity (n = 42) | Median (IQR) | High Intensity (n = 42) | Median (IQR) | p-Value |
|---|---|---|---|---|---|---|
| Lung injury (%) | <5% | 16 (38.1%) | 21 (17–33) | 18 (42.9%) | 23 (16–32) | 0.652 |
| Oxygen need (%) | <21% | 20 (47.6%) | 30 (25–35) | 22 (52.4%) | 28 (23–33) | 0.844 |
| EF | >50% | 10 (23.8%) | 82 (78–86) | 12 (28.6%) | 84 (80–88) | 0.670 |
| SPAP | <25 mmHg | 24 (57.1%) | 30 (28–32) | 26 (61.9%) | 29 (27–31) | 0.713 |
| MVV | 80–120 L/min | 18 (42.9%) | 75 (71–82) | 20 (47.6%) | 78 (73–83) | 0.699 |
| FVC | 80–120% predicted | 19 (45.2%) | 73 (70–79) | 21 (50.0%) | 76 (72–81) | 0.527 |
| FEV1 | 80–120% predicted | 20 (47.6%) | 75 (70–80) | 22 (52.4%) | 74 (70–80) | 0.735 |
| FEV1/FVC ratio | 70–80% | 12 (28.6%) | 68 (65–71) | 14 (33.3%) | 70 (67–73) | 0.680 |
| PEF | 80–100% predicted | 18 (42.9%) | 76 (72–80) | 20 (47.6%) | 79 (75–83) | 0.704 |
| FEF 25–75 | 80–120% predicted | 18 (42.9%) | 78 (74–82) | 20 (47.6%) | 81 (77–85) | 0.316 |
| Variables | Normal Range | Low Intensity (n = 42) | Median (IQR) | High Intensity (n = 42) | Median (IQR) | p-Value |
|---|---|---|---|---|---|---|
| Lung injury (%) | <5% | 8 (19.0%) | 14 (8–20) | 6 (14.3%) | 12 (10–17) | 0.504 |
| Oxygen need (%) | <21% | 10 (23.8%) | 20 (18–22) | 8 (19.0%) | 19 (17–21) | 0.441 |
| EF | >50% | 4 (9.5%) | 85 (82–88) | 3 (7.1%) | 87 (85–89) | 0.496 |
| SPAP | <25 mmHg | 12 (28.6%) | 25 (23–27) | 10 (23.8%) | 24 (22–26) | 0.210 |
| MVV | 80–120 L/min | 10 (23.8%) | 85 (82–89) | 8 (19.0%) | 87 (84–89) | 0.385 |
| FVC | 80–120% predicted | 6 (14.3%) | 85 (81–88) | 7 (16.7%) | 85 (80–88) | 0.730 |
| FEV1 | 80–120% predicted | 10 (23.8%) | 83 (82–86) | 8 (19.0%) | 87 (85–91) | 0.708 |
| FEV1/FVC ratio | 70–80% | 6 (14.3%) | 75 (72–78) | 5 (11.9%) | 76 (74–78) | 0.662 |
| PEF | 80–100% predicted | 8 (19.0%) | 81 (78–85) | 6 (14.3%) | 81 (79–85) | 0.317 |
| FEF 25–75 | 80–120% predicted | 5 (11.9%) | 84 (82–90) | 6 (14.3%) | 83 (81–87) | 0.301 |
| Variables | Normal Range | Low Intensity (n = 42) | Median (IQR) | High Intensity (n = 42) | Median (IQR) | p-Value |
|---|---|---|---|---|---|---|
| Work intensity (W) | 10–60 | 20 (47.6%) | 27 (22–29) | 22 (52.4%) | 43 (41–46) | <0.001 |
| VO2 (peak) | >20 mL/kg/min | 22 (52.4%) | 18 (17–19) | 24 (57.1%) | 19 (18–20) | 0.294 |
| VO2 (from predicted) | 80–120% | 24 (57.1%) | 75 (70–80) | 26 (61.9%) | 78 (73–83) | 0.708 |
| VO2 at AT | >11 mL/kg/min | 20 (47.6%) | 10 (9–11) | 22 (52.4%) | 11 (10–12) | 0.460 |
| RER | 0.8–1.15 | 16 (38.1%) | 1.2 (1.15–1.25) | 18 (42.9%) | 1.3 (1.25–1.35) | 0.692 |
| HR (from max.) | 60–90% | 20 (47.6%) | 60 (55–65) | 22 (52.4%) | 65 (60–70) | 0.347 |
| ΔHR/ΔVO2 | 1–1.5 | 18 (42.9%) | 1.6 (1.5–1.7) | 21 (50.0%) | 1.7 (1.6–1.8) | 0.422 |
| ΔVO2/W | 10–15 mL/min/W | 20 (47.6%) | 8 (7–9) | 19 (45.2%) | 9 (8–10) | 0.783 |
| ΔVO2/HR | 10–15 mL/beat | 20 (47.6%) | 9 (8–10) | 22 (52.4%) | 10 (9–11) | 0.419 |
| BR | 20–40% | 22 (52.4%) | 15 (12–18) | 24 (57.1%) | 18 (15–21) | 0.301 |
| Variables | Normal Range | Low Intensity (n = 42) | Median (IQR) | High Intensity (n = 42) | Median (IQR) | p-Value |
|---|---|---|---|---|---|---|
| Work intensity (W) | 10–60 | 15 (35.7%) | 38 (36–44) | 12 (28.6%) | 47 (44–51) | 0.094 |
| VO2 (peak) | >20 mL/kg/min | 12 (28.6%) | 21 (20–22) | 10 (23.8%) | 23 (22–24) | 0.331 |
| VO2 (from predicted) | 80–120% | 13 (31.0%) | 85 (80–90) | 11 (26.2%) | 90 (85–95) | 0.416 |
| VO2 at AT | >11 mL/kg/min | 9 (21.4%) | 12 (11–13) | 7 (16.7%) | 13 (12–14) | 0.208 |
| RER | 0.8–1.15 | 14 (33.3%) | 0.95 (0.90–1.00) | 12 (28.6%) | 1.00 (0.95–1.05) | 0.190 |
| HR (% from max.) | 60–90% | 16 (38.1%) | 75 (70–80) | 13 (31.0%) | 82 (78–86) | 0.106 |
| ΔHR/ΔVO2 | 1–1.5 | 11 (26.2%) | 1.2 (1.1–1.3) | 9 (21.4%) | 1.3 (1.2–1.4) | 0.325 |
| ΔVO2/W | 10–15 mL/min/W | 12 (28.6%) | 11 (10–12) | 10 (23.8%) | 14 (10–15) | 0.282 |
| ΔVO2/HR | 10–15 mL/beat | 14 (33.3%) | 12 (11–13) | 11 (26.2%) | 13 (12–14) | 0.440 |
| BR | 20–40% | 13 (31.0%) | 24 (22–26) | 10 (23.8%) | 29 (27–31) | 0.273 |
| Variables | Low Intensity Initial (n = 42) | Low Intensity at 3 Months (n = 42) | p-Value | High Intensity Initial (n = 42) | High Intensity at 3 Months (n = 42) | p-Value |
|---|---|---|---|---|---|---|
| Lung injury (%) | 21 (17–33) | 14 (8–20) | 0.005 | 23 (16–32) | 12 (10–17) | 0.001 |
| Oxygen need (%) | 30 (25–35) | 20 (18–22) | 0.002 | 28 (23–33) | 19 (17–21) | 0.003 |
| FE | 82 (78–86) | 85 (82–88) | 0.010 | 84 (80–88) | 87 (85–89) | 0.020 |
| SPAP | 30 (28–32) | 25 (23–27) | 0.015 | 29 (27–31) | 24 (22–26) | 0.011 |
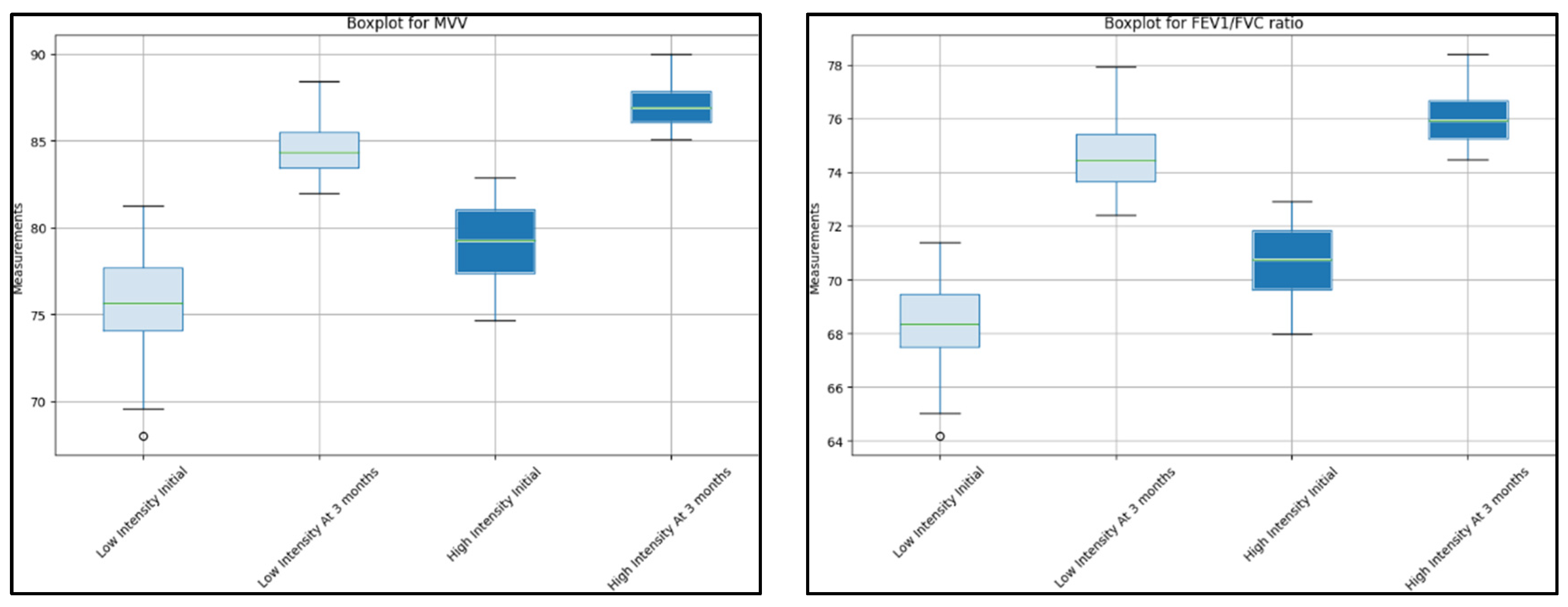
| MVV | 75 (71–82) | 85 (82–89) | <0.001 | 78 (73–83) | 87 (84–89) | 0.008 |
| FVC | 73 (70–79) | 85 (81–88) | 0.004 | 76 (72–81) | 85 (80–88) | 0.007 |
| FEV1 | 75 (70–80) | 83 (82–86) | 0.009 | 74 (70–80) | 87 (85–91) | <0.001 |
| FEV1/FVC ratio | 68 (65–71) | 75 (72–78) | <0.001 | 70 (67–73) | 76 (74–78) | 0.010 |
| PEF | 76 (72–80) | 81 (78–85) | 0.012 | 79 (75–83) | 81 (79–85) | 0.020 |
| FEF 25–75 | 78 (74–82) | 84 (82–90) | 0.005 | 81 (77–85) | 83 (81–87) | 0.022 |
| Variables | Low Intensity Initial (n = 42) | Low Intensity at 3 Months (n = 42) | p-Value | High Intensity Initial (n = 42) | High Intensity at 3 Months (n = 42) | p-Value |
|---|---|---|---|---|---|---|
| Work intensity (W) | 27 (22–29) | 38 (36–44) | <0.001 | 43 (41–46) | 47 (44–51) | 0.135 |
| VO2 (peak) | 18 (17–19) | 21 (20–22) | 0.010 | 19 (18–20) | 23 (22–24) | 0.002 |
| VO2 (from predicted) | 75 (70–80) | 85 (81–90) | 0.003 | 78 (73–83) | 90 (85–95) | <0.001 |
| VO2 at AT | 10 (9–11) | 12 (11–13) | 0.004 | 11 (10–12) | 13 (12–14) | 0.004 |
| RER | 1.20 (1.15–1.25) | 0.95 (0.90–1.00) | 0.005 | 1.3 (1.25–1.35) | 1.00 (0.95–1.05) | 0.005 |
| HR (% from max.) | 60 (55–65) | 75 (70–80) | 0.020 | 65 (60–70) | 82 (78–86) | 0.006 |
| ΔHR/ΔVO2 | 1.6 (1.5–1.7) | 1.2 (1.1–1.3) | 0.007 | 1.7 (1.6–1.8) | 1.3 (1.2–1.4) | 0.001 |
| ΔVO2/W | 8 (7–9) | 11 (10–12) | 0.030 | 9 (8–10) | 14 (10–15) | 0.008 |
| ΔVO2/HR | 9 (8–10) | 12 (11–13) | 0.009 | 10 (9–11) | 13 (12–14) | 0.045 |
| BR | 15 (12–18) | 24 (22–26) | <0.001 | 18 (15–21) | 29 (27–31) | 0.010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumitrescu, A.; Doros, G.; Lazureanu, V.E.; Septimiu-Radu, S.; Bratosin, F.; Rosca, O.; Patel, H.; Porosnicu, T.M.; Vitcu, G.M.; Mirea, A.; et al. Post-Severe-COVID-19 Cardiopulmonary Rehabilitation: A Comprehensive Study on Patient Features and Recovery Dynamics in Correlation with Workout Intensity. J. Clin. Med. 2023, 12, 4390. https://doi.org/10.3390/jcm12134390
Dumitrescu A, Doros G, Lazureanu VE, Septimiu-Radu S, Bratosin F, Rosca O, Patel H, Porosnicu TM, Vitcu GM, Mirea A, et al. Post-Severe-COVID-19 Cardiopulmonary Rehabilitation: A Comprehensive Study on Patient Features and Recovery Dynamics in Correlation with Workout Intensity. Journal of Clinical Medicine. 2023; 12(13):4390. https://doi.org/10.3390/jcm12134390
Chicago/Turabian StyleDumitrescu, Andreea, Gabriela Doros, Voichita Elena Lazureanu, Susa Septimiu-Radu, Felix Bratosin, Ovidiu Rosca, Harshkumar Patel, Tamara Mirela Porosnicu, Gabriela Mut Vitcu, Andrei Mirea, and et al. 2023. "Post-Severe-COVID-19 Cardiopulmonary Rehabilitation: A Comprehensive Study on Patient Features and Recovery Dynamics in Correlation with Workout Intensity" Journal of Clinical Medicine 12, no. 13: 4390. https://doi.org/10.3390/jcm12134390
APA StyleDumitrescu, A., Doros, G., Lazureanu, V. E., Septimiu-Radu, S., Bratosin, F., Rosca, O., Patel, H., Porosnicu, T. M., Vitcu, G. M., Mirea, A., Oancea, C., Mihaicuta, S., Stoicescu, E. R., & Barata, P. I. (2023). Post-Severe-COVID-19 Cardiopulmonary Rehabilitation: A Comprehensive Study on Patient Features and Recovery Dynamics in Correlation with Workout Intensity. Journal of Clinical Medicine, 12(13), 4390. https://doi.org/10.3390/jcm12134390







