Untreated Injuries to the Anterolateral Capsular Structures Do Not Affect Outcomes and Kinematics after Anatomic Anterior Cruciate Ligament Reconstruction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Surgical Procedure and Rehabilitation
2.2. In Vivo Joint Kinematics
2.3. MRI Analysis and Patient-Reported Outcome Measures
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kittl, C.; El-Daou, H.; Athwal, K.K.; Gupte, C.M.; Weiler, A.; Williams, A.; Amis, A.A. The Role of the Anterolateral Structures and the ACL in Controlling Laxity of the Intact and ACL-Deficient Knee. Am. J. Sports Med. 2016, 44, 345–354. [Google Scholar] [CrossRef]
- Musahl, V.; Rahnemai-Azar, A.A.; Costello, J.; Arner, J.W.; Fu, F.H.; Hoshino, Y.; Lopomo, N.; Samuelsson, K.; Irrgang, J.J. The Influence of Meniscal and Anterolateral Capsular Injury on Knee Laxity in Patients with Anterior Cruciate Ligament Injuries. Am. J. Sports Med. 2016, 44, 3126–3131. [Google Scholar] [CrossRef]
- Guenther, D.; Rahnemai-Azar, A.A.; Bell, K.; Irarrázaval, S.; Fu, F.H.; Musahl, V.; Debski, R.E. The Anterolateral Capsule of the Knee Behaves Like a Sheet of Fibrous Tissue. Am. J. Sports Med. 2017, 45, 849–855. [Google Scholar] [CrossRef]
- Getgood, A.; ALC Consensus Group; Brown, C.; Lording, T.; Amis, A.; Claes, S.; Geeslin, A.; Musahl, V. The anterolateral complex of the knee: Results from the International ALC Consensus Group Meeting. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 166–176. [Google Scholar] [CrossRef] [Green Version]
- Getgood, A.M.; Bryant, D.M.; Litchfield, R.; Heard, M.; McCormack, R.G.; Rezansoff, A.; Peterson, D.; Bardana, D.; Macdonald, P.B.; Verdonk, P.C.; et al. Lateral Extra-articular Tenodesis Reduces Failure of Hamstring Tendon Autograft Anterior Cruciate Ligament Reconstruction: 2-Year Outcomes from the STABILITY Study Randomized Clinical Trial. Am. J. Sports Med. 2020, 48, 285–297. [Google Scholar] [CrossRef]
- Hussein, M.; Van Eck, C.F.; Cretnik, A.; Dinevski, D.; Fu, F.H. Individualized anterior cruciate ligament surgery: A prospective study comparing anatomic single- and double-bundle reconstruction. Am. J. Sports Med. 2012, 40, 1781–1788. [Google Scholar] [CrossRef]
- Ayeni, O.R.; Chahal, M.; Tran, M.N.; Sprague, S. Pivot shift as an outcome measure for ACL reconstruction: A systematic review. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 767–777. [Google Scholar] [CrossRef]
- Hussein, M.; van Eck, C.F.; Cretnik, A.; Dinevski, D.; Fu, F.H. Prospective randomized clinical evaluation of conventional single-bundle, anatomic single-bundle, and anatomic double-bundle anterior cruciate ligament reconstruction: 281 cases with 3- to 5-year follow-up. Am. J. Sports Med. 2012, 40, 512–520. [Google Scholar] [CrossRef]
- Sonnery-Cottet, B.; Thaunat, M.; Freychet, B.; Pupim, B.H.; Murphy, C.G.; Claes, S. Outcome of a Combined Anterior Cruciate Ligament and Anterolateral Ligament Reconstruction Technique with a Minimum 2-Year Follow-up. Am. J. Sports Med. 2015, 43, 1598–1605. [Google Scholar] [CrossRef]
- Sundemo, D.; Sernert, N.; Kartus, J.; Senorski, E.H.; Svantesson, E.; Karlsson, J.; Samuelsson, K. Increased Postoperative Manual Knee Laxity at 2 Years Results in Inferior Long-term Subjective Outcome after Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 2018, 46, 2632–2645. [Google Scholar] [CrossRef]
- Magnussen, R.A.; Reinke, E.; Huston, L.; Hewett, T.E.; Spindler, K.P.; Andrish, J.T.; Jones, M.; Parker, R.D.; McCarty, E.C.; Vidal, A.F.; et al. Factors Associated with High-Grade Lachman, Pivot Shift, and Anterior Drawer at the Time of Anterior Cruciate Ligament Reconstruction. Arthroscopy 2016, 32, 1080–1085. [Google Scholar] [CrossRef] [Green Version]
- Musahl, V.; Ayeni, O.R.; Citak, M.; Irrgang, J.J.; Pearle, A.D.; Wickiewicz, T.L. The influence of bony morphology on the magnitude of the pivot shift. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 1232–1238. [Google Scholar] [CrossRef]
- Rahnemai-Azar, A.A.; Abebe, E.S.; Johnson, P.; Labrum, J.; Fu, F.H.; Irrgang, J.J.; Samuelsson, K.; Musahl, V. Increased lateral tibial slope predicts high-grade rotatory knee laxity pre-operatively in ACL reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 1170–1176. [Google Scholar] [CrossRef]
- Ruiz, N.; Filippi, G.J.; Gagnière, B.; Bowen, M.; Robert, H.E. The Comparative Role of the Anterior Cruciate Ligament and Anterolateral Structures in Controlling Passive Internal Rotation of the Knee: A Biomechanical Study. Arthroscopy 2016, 32, 1053–1062. [Google Scholar] [CrossRef]
- Parsons, E.M.; Gee, A.O.; Spiekerman, C.; Cavanagh, P.R. The biomechanical function of the anterolateral ligament of the knee. Am. J. Sports Med. 2015, 43, 669–674. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, M.T.; Nitri, M.; Williams, B.T.; Moulton, S.G.; Cruz, R.S.; Dornan, G.J.; Goldsmith, M.T.; LaPrade, R.F. An In Vitro Robotic Assessment of the Anterolateral Ligament, Part 1: Secondary Role of the Anterolateral Ligament in the Setting of an Anterior Cruciate Ligament Injury. Am. J. Sports Med. 2016, 44, 585–592. [Google Scholar] [CrossRef]
- Thein, R.; Boorman-Padgett, J.; Stone, K.; Wickiewicz, T.L.; Imhauser, C.W.; Pearle, A.D. Biomechanical Assessment of the Anterolateral Ligament of the Knee: A Secondary Restraint in Simulated Tests of the Pivot Shift and of Anterior Stability. J. Bone Jt. Surg. Am. 2016, 98, 937–943. [Google Scholar] [CrossRef] [Green Version]
- Noyes, F.R.; Huser, L.E.; Jurgensmeier, D.; Walsh, J.; Levy, M.S. Is an Anterolateral Ligament Reconstruction Required in ACL-Reconstructed Knees with Associated Injury to the Anterolateral Structures? A Robotic Analysis of Rotational Knee Stability. Am. J. Sports Med. 2017, 45, 1018–1027. [Google Scholar] [CrossRef]
- Ferretti, A.; Monaco, E.; Gaj, E.; Andreozzi, V.; Annibaldi, A.; Carrozzo, A.; Vieira, T.D.; Sonnery-Cottet, B.; Saithna, A. Risk Factors for Grade 3 Pivot Shift in Knees with Acute Anterior Cruciate Ligament Injuries: A Comprehensive Evaluation of the Importance of Osseous and Soft Tissue Parameters from the SANTI Study Group. Am. J. Sports Med. 2020, 48, 2408–2417. [Google Scholar] [CrossRef]
- Song, G.-Y.; Hong, L.; Zhang, H.; Zhang, J.; Li, Y.; Feng, H. Clinical Outcomes of Combined Lateral Extra-articular Tenodesis and Intra-articular Anterior Cruciate Ligament Reconstruction in Addressing High-Grade Pivot-Shift Phenomenon. Arthroscopy 2016, 32, 898–905. [Google Scholar] [CrossRef]
- Onggo, J.R.; Rasaratnam, H.K.; Nambiar, M.; Onggo, J.D.; Pai, V.; Damasena, I.; Riazi, A.; Babazadeh, S. Anterior Cruciate Ligament Reconstruction Alone vs. with Lateral Extra-articular Tenodesis with Minimum 2-Year Follow-up: A Meta-analysis and Systematic Review of Randomized Controlled Trials. Am. J. Sports Med. 2022, 50, 1137–1145. [Google Scholar] [CrossRef]
- Lee, D.W.; Kim, J.G.; Kim, H.T.; Cho, S.I. Evaluation of Anterolateral Ligament Healing after Anatomic Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 2020, 48, 1078–1087. [Google Scholar] [CrossRef]
- Ahn, J.H.; Lee, S.K.; Mun, J.W.; Kim, S.W. Degree of Anterolateral Ligament Injury Impacts Outcomes after Double-Bundle Anterior Cruciate Ligament Reconstruction. Arthroscopy 2021, 37, 222–230. [Google Scholar] [CrossRef]
- Sobrado, M.F.; Giglio, P.N.; Bonadio, M.B.; Helito, P.V.P.; Guimarães, T.M.; Pécora, J.R.; Gobbi, R.G.; Helito, C.P. Outcomes after Isolated Acute Anterior Cruciate Ligament Reconstruction Are Inferior in Patients with an Associated Anterolateral Ligament Injury. Am. J. Sports Med. 2020, 48, 3177–3182. [Google Scholar] [CrossRef]
- Chiba, D.; Gale, T.; Nishida, K.; Suntaxi, F.; Lesniak, B.P.; Fu, F.H.; Anderst, W.; Musahl, V. Lateral Extra-articular Tenodesis Contributes Little to Change In Vivo Kinematics after Anterior Cruciate Ligament Reconstruction: A Randomized Controlled Trial. Am. J. Sports Med. 2021, 49, 1803–1812. [Google Scholar] [CrossRef]
- Nagai, K.; Kamada, K.; Kay, J.; Hoshino, Y.; Matsushita, T.; Kuroda, R.; de Sa, D. Clinical Outcomes after Anterior Cruciate Ligament Reconstruction in Patients with a Concomitant Segond Fracture: A Systematic Review. Am. J. Sports Med. 2023, 51, 525–533. [Google Scholar] [CrossRef]
- Irrgang, J.J.; Tashman, S.; Moore, C.; Fu, F.H. Challenge accepted: Description of an ongoing NIH-funded randomized clinical trial to compare anatomic single-bundle vs. anatomic double-bundle ACL reconstruction. Arthroscopy 2012, 28, 745–747, author reply 747-8. [Google Scholar] [CrossRef]
- Fu, F.H.; Van Eck, C.F.; Tashman, S.; Irrgang, J.J.; Moreland, M.S. Anatomic anterior cruciate ligament reconstruction: A changing paradigm. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 640–648. [Google Scholar] [CrossRef]
- Tashman, S.; Collon, D.; Anderson, K.; Kolowich, P.; Anderst, W. Abnormal rotational knee motion during running after anterior cruciate ligament reconstruction. Am. J. Sports Med. 2004, 32, 975–983. [Google Scholar] [CrossRef]
- Anderst, W.; Zauel, R.; Bishop, J.; Demps, E.; Tashman, S. Validation of three-dimensional model-based tibio-femoral tracking during running. Med. Eng. Phys. 2009, 31, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Grood, E.S.; Suntay, W.J. A joint coordinate system for the clinical description of three-dimensional motions: Application to the knee. J. Biomech. Eng. 1983, 105, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Higgins, L.D.; Taylor, M.K.; Park, D.; Ghodadra, N.; Marchant, M.; Pietrobon, R.; Cook, C. Reliability and validity of the International Knee Documentation Committee (IKDC) Subjective Knee Form. Jt. Bone Spine 2007, 74, 594–599. [Google Scholar] [CrossRef]
- Roos, E.M.; Roos, H.P.; Lohmander, S.; Ekdahl, C.; Beynnon, B.D. Knee Injury and Osteoarthritis Outcome Score (KOOS)—Development of a self-administered outcome measure. J. Orthop. Sports Phys. Ther. 1998, 28, 88–96. [Google Scholar] [CrossRef]
- Hartigan, D.E.; Carroll, K.W.; Kosarek, F.J.; Piasecki, D.P.; Fleischli, J.F.; D’Alessandro, D.F. Visibility of Anterolateral Ligament Tears in Anterior Cruciate Ligament-Deficient Knees with Standard 1.5-Tesla Magnetic Resonance Imaging. Arthroscopy 2016, 32, 2061–2065. [Google Scholar] [CrossRef]
- Terry, G.C.; Norwood, L.A.; Hughston, J.C.; Caldwell, K.M. How iliotibial tract injuries of the knee combine with acute anterior cruciate ligament tears to influence abnormal anterior tibial displacement. Am. J. Sports Med. 1993, 21, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, A.; Monaco, E.; Fabbri, M.; Maestri, B.; DE Carli, A. Prevalence and Classification of Injuries of Anterolateral Complex in Acute Anterior Cruciate Ligament Tears. Arthroscopy 2017, 33, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Monaco, E.; Ferretti, A.; Labianca, L.; Maestri, B.; Speranza, A.; Kelly, M.J.; D’arrigo, C. Navigated knee kinematics after cutting of the ACL and its secondary restraint. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 870–877. [Google Scholar] [CrossRef]
- Deneweth, J.M.; Bey, M.J.; McLean, S.G.; Lock, T.R.; Kolowich, P.A.; Tashman, S. Tibiofemoral joint kinematics of the anterior cruciate ligament-reconstructed knee during a single-legged hop landing. Am. J. Sports Med. 2010, 38, 1820–1828. [Google Scholar] [CrossRef]
- Tashman, S.; Kolowich, P.; Collon, D.; Anderson, K.; Anderst, W. Dynamic function of the ACL-reconstructed knee during running. Clin. Orthop. Relat. Res. 2007, 454, 66–73. [Google Scholar] [CrossRef]
- Tashman, S.; Zandiyeh, P.; Irrgang, J.J.; Musahl, V.; West, R.V.; Shah, N.; Fu, F.H. Anatomic single- and double-bundle ACL reconstruction both restore dynamic knee function: A randomized clinical trial-part II: Knee kinematics. Knee Surg. Sports Traumatol. Arthrosc. 2021, 29, 2676–2683. [Google Scholar] [CrossRef]
- Miller, R.M.; Rahnemai-Azar, A.A.; Sürer, L.; Arilla, F.V.; Fu, F.H.; Debski, R.E.; Musahl, V. Tensile properties of a split quadriceps graft for ACL reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Rayes, J.; Ouanezar, H.; Haidar, I.M.; Ngbilo, C.; Fradin, T.; Vieira, T.D.; Freychet, B.; Sonnery-Cottet, B. Revision Anterior Cruciate Ligament Reconstruction Using Bone-Patellar Tendon-Bone Graft Combined with Modified Lemaire Technique Versus Hamstring Graft Combined with Anterolateral Ligament Reconstruction: A Clinical Comparative Matched Study with a Mean Follow-up of 5 Years from The SANTI Study Group. Am. J. Sports Med. 2022, 50, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Marom, N.; Jahandar, H.; Fraychineaud, T.J.; Zayyad, Z.A.; Ouanezar, H.; Hurwit, D.; Zhu, A.; Wickiewicz, T.L.; Pearle, A.D.; Imhauser, C.W.; et al. Lateral Extra-articular Tenodesis Alters Lateral Compartment Contact Mechanics under Simulated Pivoting Maneuvers: An In Vitro Study. Am. J. Sports Med. 2021, 49, 2898–2907. [Google Scholar] [CrossRef] [PubMed]
- Schon, J.M.; Moatshe, G.; Brady, A.W.; Cruz, R.S.; Chahla, J.; Dornan, G.J.; Turnbull, T.L.; Engebretsen, L.; LaPrade, R.F. Anatomic Anterolateral Ligament Reconstruction Leads to Overconstraint at Any Fixation Angle: Response. Am. J. Sports Med. 2016, 44, NP58–NP59. [Google Scholar] [CrossRef]
- Magnussen, R.A.; Verlage, M.; Flanigan, D.C.; Kaeding, C.C.; Spindler, K.P. Patient-Reported Outcomes and Their Predictors at Minimum 10 Years after Anterior Cruciate Ligament Reconstruction: A Systematic Review of Prospectively Collected Data. Orthop. J. Sports Med. 2015, 3, 2325967115573706. [Google Scholar] [CrossRef] [PubMed]
- Irrgang, J.J.; Tashman, S.; Patterson, C.G.; Musahl, V.; West, R.; Oostdyk, A.; Galvin, B.; Poploski, K.; Fu, F.H. Anatomic single vs. double-bundle ACL reconstruction: A randomized clinical trial-Part 1: Clinical outcomes. Knee Surg. Sports Traumatol. Arthrosc. 2021, 29, 2665–2675. [Google Scholar] [CrossRef]

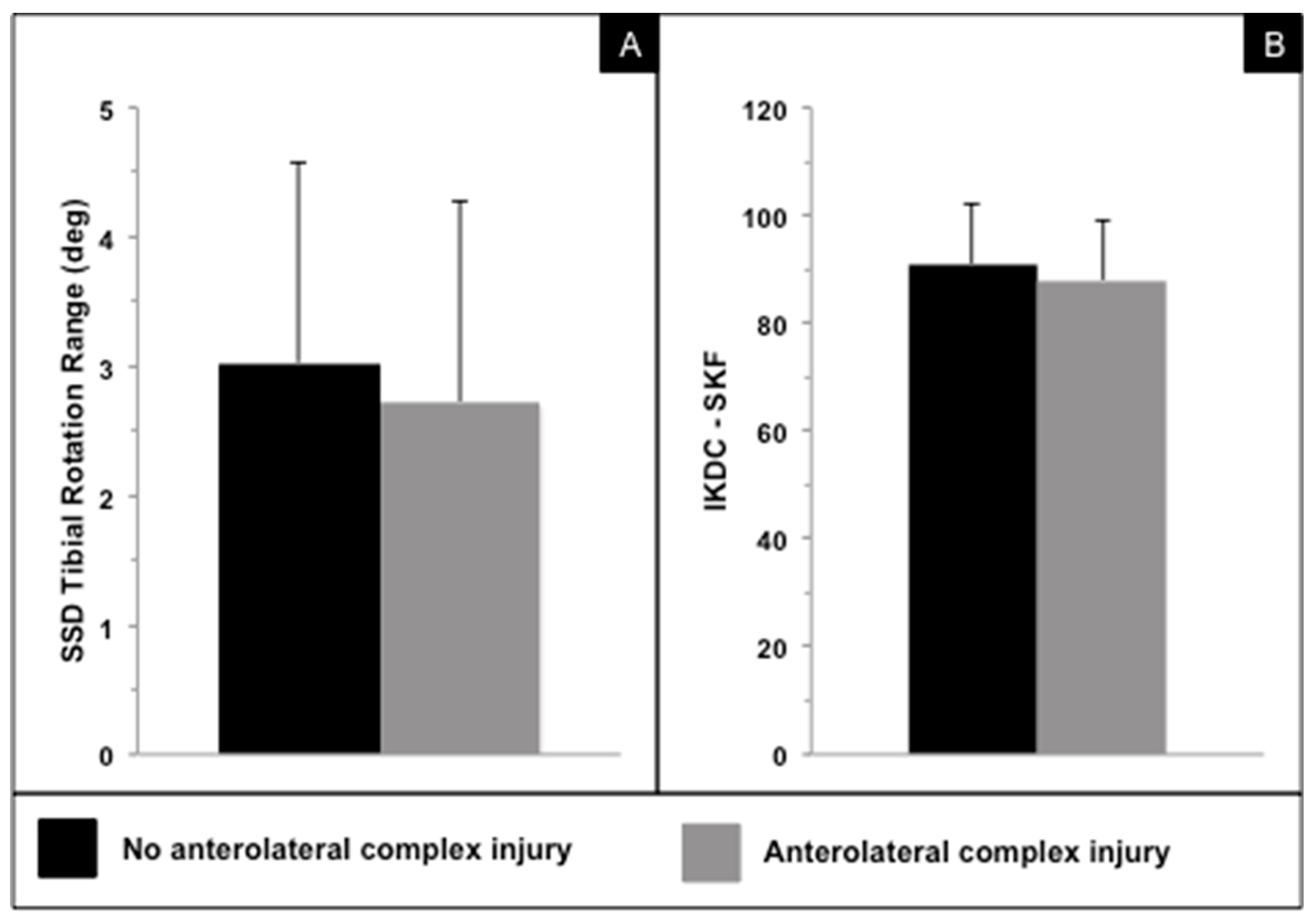
| No ALC Injury | ALC Injury | p-Value | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Side-to-side difference | |||
| Peak internal tibial rotation (deg) | 0.3 (3.3) * | 1.9 (3.2) * | 0.310 |
| Internal–external tibial rotation range (deg) | 3.0 (1.5) | 2.7 (1.6) | 0.443 |
| Peak adduction (deg) | 0.6 (1.0) ** | 0.8 (1.3) ** | 0.663 |
| Abduction–adduction range (deg) | 1.0 (0.6) | 0.9 (0.8) | 0.917 |
| Peak anterior tibial translation (mm) | 2.0 (2.4) *** | 0.6 (2.8) *** | 0.436 |
| Anterior–posterior tibial translation range (mm) | 3.7 (2.3) | 5.0 (3.3) | 0.233 |
| No ALC Injury | ALC Injury | p-Value | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| IKDC—SKF | 91.0 (10.9) | 88.0 (11.2) | 0.348 |
| KOOS—Symptoms | 89.8 (7.7) | 84.5 (11.1) | 0.181 |
| KOOS—Pain | 95.7 (6.6) | 93.1 (13.6) | 0.775 |
| KOOS—ADL | 99.1 (2.1) | 96.9 (11.3) | 0.976 |
| KOOS—Sport/Rec | 91.3 (13.4) | 90.3 (11.7) | 0.620 |
| KOOS—QOL | 87.9 (17.6) | 77.5 (24.8) | 0.169 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herbst, E.; Costello, J.; Popchak, A.J.; Tashman, S.; Irrgang, J.J.; Fu, F.H.; Musahl, V. Untreated Injuries to the Anterolateral Capsular Structures Do Not Affect Outcomes and Kinematics after Anatomic Anterior Cruciate Ligament Reconstruction. J. Clin. Med. 2023, 12, 4408. https://doi.org/10.3390/jcm12134408
Herbst E, Costello J, Popchak AJ, Tashman S, Irrgang JJ, Fu FH, Musahl V. Untreated Injuries to the Anterolateral Capsular Structures Do Not Affect Outcomes and Kinematics after Anatomic Anterior Cruciate Ligament Reconstruction. Journal of Clinical Medicine. 2023; 12(13):4408. https://doi.org/10.3390/jcm12134408
Chicago/Turabian StyleHerbst, Elmar, Joanna Costello, Adam J. Popchak, Scott Tashman, James J. Irrgang, Freddie H. Fu, and Volker Musahl. 2023. "Untreated Injuries to the Anterolateral Capsular Structures Do Not Affect Outcomes and Kinematics after Anatomic Anterior Cruciate Ligament Reconstruction" Journal of Clinical Medicine 12, no. 13: 4408. https://doi.org/10.3390/jcm12134408
APA StyleHerbst, E., Costello, J., Popchak, A. J., Tashman, S., Irrgang, J. J., Fu, F. H., & Musahl, V. (2023). Untreated Injuries to the Anterolateral Capsular Structures Do Not Affect Outcomes and Kinematics after Anatomic Anterior Cruciate Ligament Reconstruction. Journal of Clinical Medicine, 12(13), 4408. https://doi.org/10.3390/jcm12134408





