Low and High Ankle-Brachial Index Are Both Associated with Mortality in German Nursing Home Residents—The Five-Year Follow-Up of the “Allo-Study” Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Inclusion and Exclusion Criteria
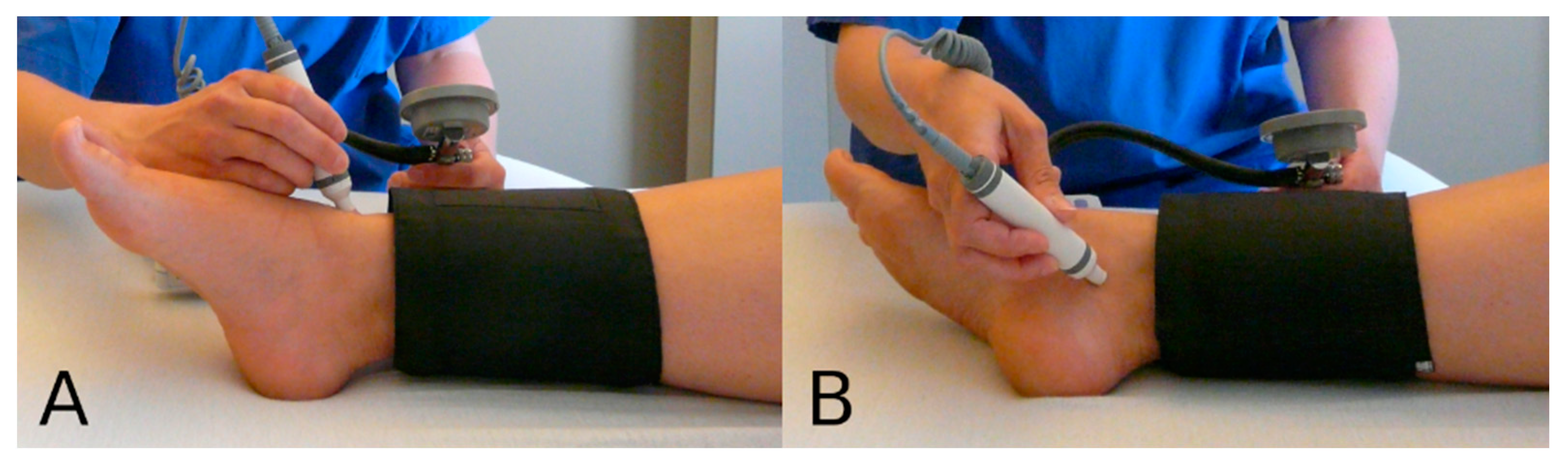
2.3. Variables of Interest
2.4. End Points
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
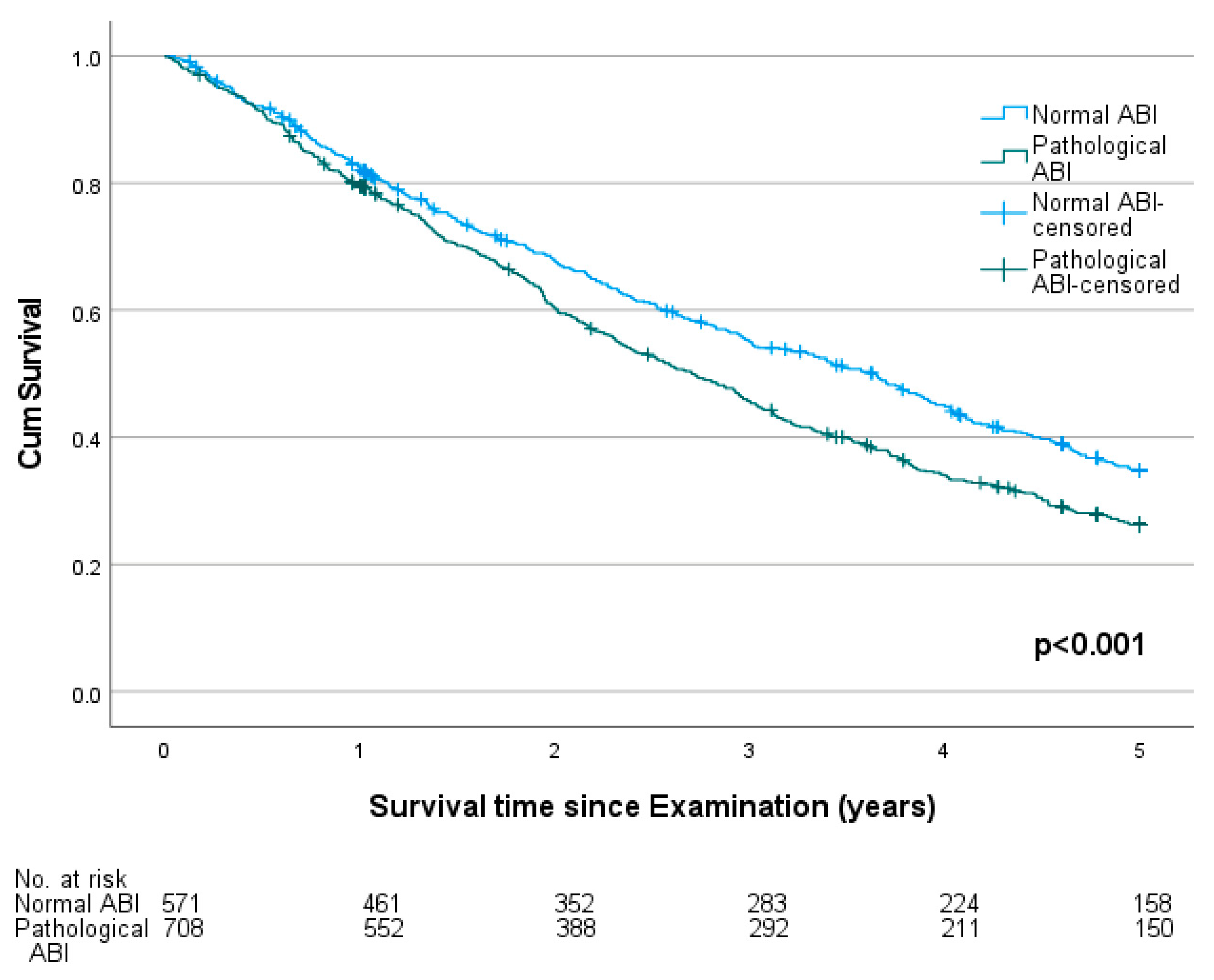
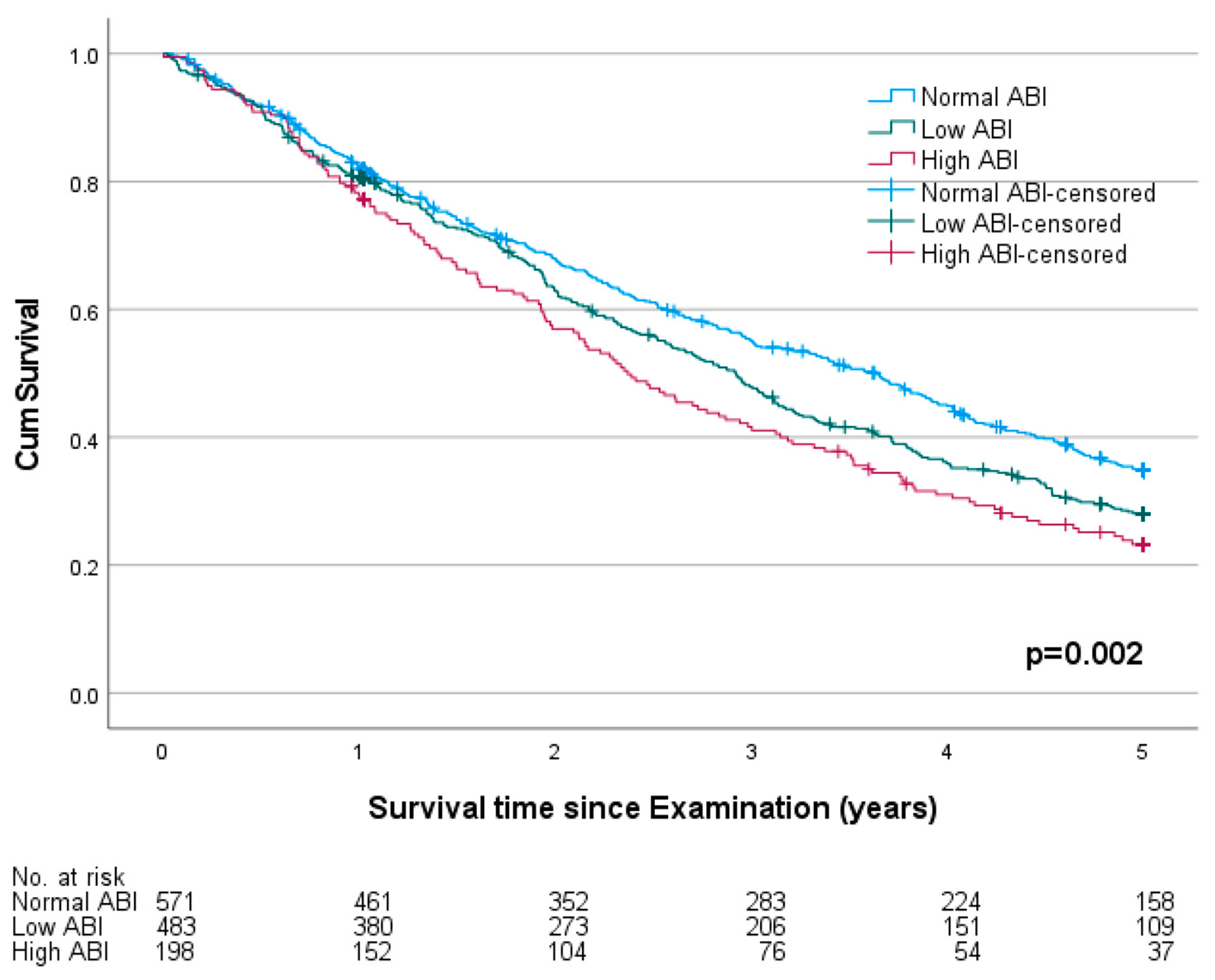
3.2. Ankle-Brachial Index and Mortality
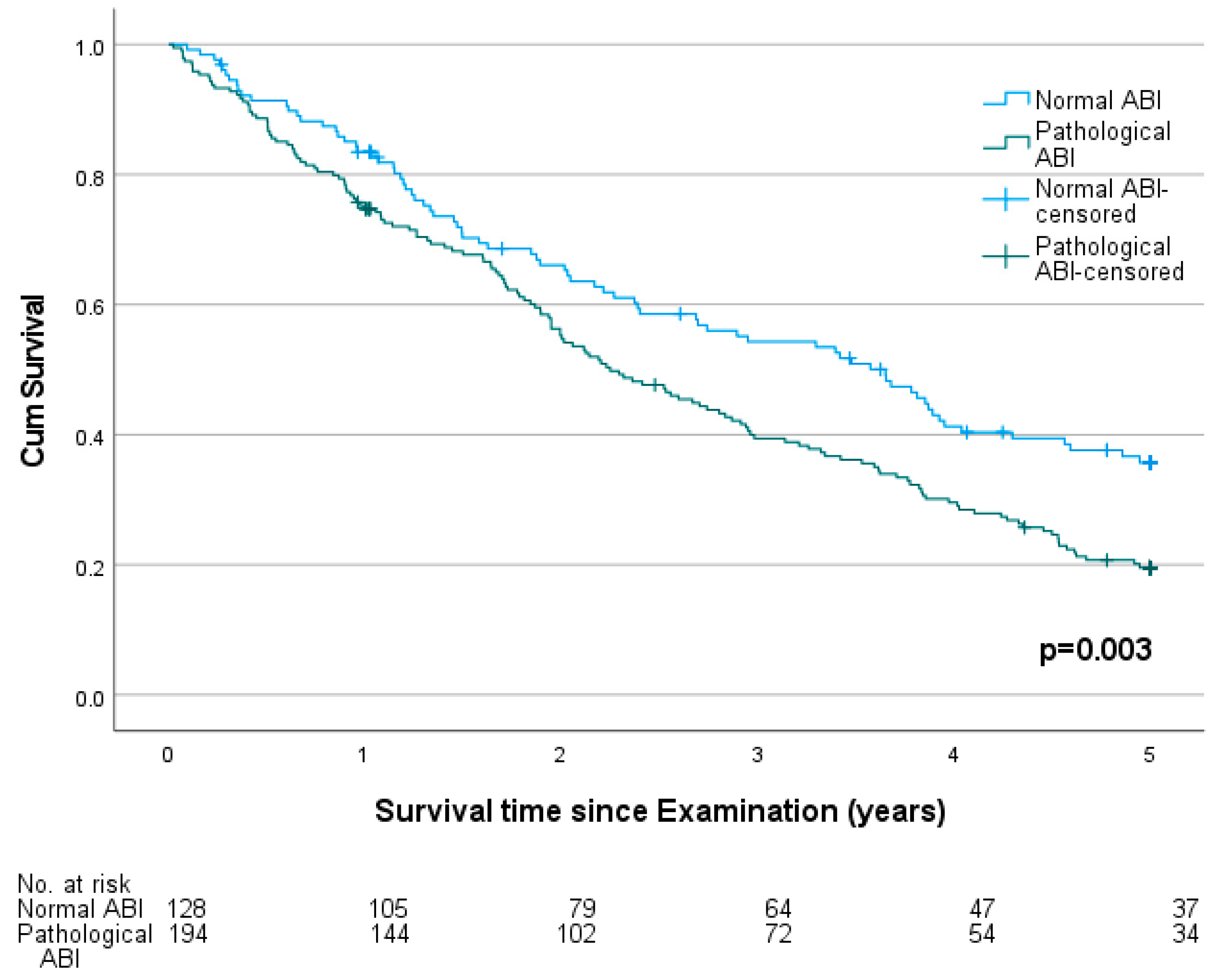
3.3. Ankle-Brachial Index, Patient History and Mortality
3.4. Medication and Mortality
4. Discussion
4.1. Prevalence of Peripheral Arterial Disease
4.2. Underdiagnosis of Peripheral Arterial Disease
4.3. Ankle-Brachial Index and Mortality
4.4. Medication and Mortality
4.5. ABI Screening
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sampson, U.K.A.; Fowkes, F.G.R.; McDermott, M.M.; Criqui, M.H.; Aboyans, V.; Norman, P.E.; Forouzanfar, M.H.; Naghavi, M.; Song, Y.; Harrell, F.E.; et al. Global and Regional Burden of Death and Disability from Peripheral Artery Disease: 21 World Regions, 1990 to 2010. Glob. Heart 2014, 9, 145–158.e21. [Google Scholar] [CrossRef] [PubMed]
- Rammos, C.; Steinmetz, M.; Lortz, J.; Mahabadi, A.A.; Petrikhovich, O.; Kirsch, K.; Hering, R.; Schulz, M.; Rassaf, T. Peripheral Artery Disease in Germany (2009–2018): Prevalence, Frequency of Specialized Ambulatory Care and Use of Guideline-Recommended Therapy—A Population-Based Study. Lancet Reg. Health Eur. 2021, 5, 100113. [Google Scholar] [CrossRef] [PubMed]
- Diehm, C.; Schuster, A.; Allenberg, J.R.; Darius, H.; Haberl, R.; Lange, S.; Pittrow, D.; von Stritzky, B.; Tepohl, G.; Trampisch, H.-J. High Prevalence of Peripheral Arterial Disease and Co-Morbidity in 6880 Primary Care Patients: Cross-Sectional Study. Atherosclerosis 2004, 172, 95–105. [Google Scholar] [CrossRef] [PubMed]
- McKenna, M.; Wolfson, S.; Kuller, L. The Ratio of Ankle and Arm Arterial Pressure as an Independent Predictor of Mortality. Atherosclerosis 1991, 87, 119–128. [Google Scholar] [CrossRef]
- Newman, A.B.; Siscovick, D.S.; Manolio, T.A.; Polka, J.; Fried, L.P.; Borhani, N.O.; Wolfson, S.K. Ankle-Arm Index as a Marker of Atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) Collaborative Research Group. Circulation 1993, 88, 837–845. [Google Scholar] [CrossRef]
- Fowkes Ankle Brachial Index Combined with Framingham Risk Score to Predict Cardiovascular Events and Mortality: A Meta-Analysis. JAMA 2008, 300, 197–208. [CrossRef]
- Xu, D.; Zou, L.; Xing, Y.; Hou, L.; Wei, Y.; Zhang, J.; Qiao, Y.; Hu, D.; Xu, Y.; Li, J.; et al. Diagnostic Value of Ankle-Brachial Index in Peripheral Arterial Disease: A Meta-Analysis. Can. J. Cardiol. 2013, 29, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Galas, N.; Becker, I.; Ficon, T.; Sakrauski, M.; Reichert, R.; Ahmad, W.; Mylonas, S.; Brunkwall, J.; Majd, P. Prescription Rate of Anti-Atherosclerotic Drugs in German Nursing Homes and Its Impact on Outcome. Vasa 2018, 48, 158–166. [Google Scholar] [CrossRef]
- Aboyans, V.; Criqui, M.H.; Abraham, P.; Allison, M.A.; Creager, M.A.; Diehm, C.; Fowkes, F.G.R.; Hiatt, W.R.; Jönsson, B.; Lacroix, P.; et al. Measurement and Interpretation of the Ankle-Brachial Index: A Scientific Statement from the American Heart Association. Circulation 2012, 126, 2890–2909. [Google Scholar] [CrossRef]
- Bo, M.; Zanocchi, M.; Poli, L.; Molaschi, M. The Ankle-Brachial Index Is Not Related to Mortality in Elderly Subjects Living in Nursing Homes. Angiology 2005, 56, 693–697. [Google Scholar] [CrossRef]
- Hirsch, A.T. Peripheral Arterial Disease Detection, Awareness, and Treatment in Primary Care. JAMA 2001, 286, 1317. [Google Scholar] [CrossRef] [PubMed]
- Goodney, P.P.; Travis, L.L.; Nallamothu, B.K.; Holman, K.; Suckow, B.; Henke, P.K.; Lucas, F.L.; Goodman, D.C.; Birkmeyer, J.D.; Fisher, E.S. Variation in the Use of Lower Extremity Vascular Procedures for Critical Limb Ischemia. Circ. Cardiovasc. Qual. Outcomes 2012, 5, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Caro, J.; Migliaccio-Walle, K.; Ishak, K.J.; Proskorovsky, I. The Morbidity and Mortality Following a Diagnosis of Peripheral Arterial Disease: Long-Term Follow-up of a Large Database. BMC Cardiovasc. Disord. 2005, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.W.; Kim, B.G.; Kim, D.H.; Kim, B.O.; Byun, Y.S.; Rhee, K.J.; Lee, B.K.; Goh, C.W. Prediction of Coronary Artery Disease in Patients with Lower Extremity Peripheral Artery Disease. Int. Heart J. 2015, 56, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Man, C.; Zhang, H.; Fan, Y. High Ankle-Brachial Index and Risk of Cardiovascular or All-Cause Mortality: A Meta-Analysis. Atherosclerosis 2019, 282, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, E.J.E.; Westerink, J.; de Jong, P.A.; de Borst, G.J.; Nathoe, H.M.; Mali, W.P.T.M.; van der Graaf, Y.; van der Schouw, Y.T.; Beulens, J.W. SMART Study Group Association of High Ankle Brachial Index with Incident Cardiovascular Disease and Mortality in a High-Risk Population. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Hoek, A.G.; Zwakenberg, S.R.; Elders, P.J.M.; de Jong, P.A.; Spiering, W.; Bartstra, J.W.; Doesburg, T.; van der Heijden, A.A.; van der Schouw, Y.T.; Beulens, J.W.J.; et al. An Elevated Ankle-Brachial Index Is Not a Valid Proxy for Peripheral Medial Arterial Calcification. Atherosclerosis 2021, 323, 13–19. [Google Scholar] [CrossRef]
- Aboyans, V.; Ho, E.; Denenberg, J.O.; Ho, L.A.; Natarajan, L.; Criqui, M.H. The Association between Elevated Ankle Systolic Pressures and Peripheral Occlusive Arterial Disease in Diabetic and Nondiabetic Subjects. J. Vasc. Surg. 2008, 48, 1197–1203. [Google Scholar] [CrossRef]
- Peláez, V.C.; Ausín, L.; Ruiz Mambrilla, M.; Gonzalez-Sagrado, M.; Pérez Castrillón, J.L. Ankle-Brachial Index, Risk of Clinical Fractures, Mortality and Low Bone Mass in Nursing Home Residents. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1577–1582. [Google Scholar]
- Hajibandeh, S.; Hajibandeh, S.; Shah, S.; Child, E.; Antoniou, G.A.; Torella, F. Prognostic Significance of Ankle Brachial Pressure Index: A Systematic Review and Meta-Analysis. Vascular 2017, 25, 208–224. [Google Scholar] [CrossRef]
- Arya, S.; Khakharia, A.; Binney, Z.O.; DeMartino, R.R.; Brewster, L.P.; Goodney, P.P.; Wilson, P.W.F. Association of Statin Dose with Amputation and Survival in Patients With Peripheral Artery Disease. Circulation 2018, 137, 1435–1446. [Google Scholar] [CrossRef]
- Aboyans, V.; Ricco, J.-B.; Bartelink, M.-L.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.-P.; Czerny, M.; Carlo, M.D.; Debus, S.; et al. Editor’s Choice—2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in Collaboration with the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2018, 55, 305–368. [Google Scholar] [CrossRef] [PubMed]
- Frank, U.; Nikol, S.; Belch, J.; Boc, V.; Brodmann, M.; Carpentier, P.H.; Chraim, A.; Canning, C.; Dimakakos, E.; Gottsäter, A.; et al. ESVM Guideline on Peripheral Arterial Disease. Vasa 2019, 48, 1–79. [Google Scholar] [CrossRef] [PubMed]
- Belch, J.L.F.; Brodmann, M.; Baumgartner, I.; Binder, C.J.; Casula, M.; Heiss, C. Lipid-Lowering and Anti-Thrombotic Therapy in Patients with Peripheral Arterial Disease: European Atherosclerosis Society/European Society of Vascular Medicine Joint Statement. J. Vasc. Surg. 2022, 75, 1116. [Google Scholar] [CrossRef]
- Gerhard-Herman, M.D.; Gornik, H.L.; Barrett, C.; Barshes, N.R.; Corriere, M.A.; Drachman, D.E.; Fleisher, L.A.; Fowkes, F.G.R.; Hamburg, N.M.; Kinlay, S.; et al. 2016 AHA/ACC Guideline on the Management of Patients with Lower Extremity Peripheral Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017, 135, e726–e779. [Google Scholar] [CrossRef]
- Berger, J.S.; Krantz, M.J.; Kittelson, J.M.; Hiatt, W.R. Aspirin for the Prevention of Cardiovascular Events in Patients with Peripheral Artery Disease: A Meta-Analysis of Randomized Trials. JAMA 2009, 301, 1909–1919. [Google Scholar] [CrossRef] [PubMed]
- Fowkes, F.G.R.; Price, J.F.; Stewart, M.C.W.; Butcher, I.; Leng, G.C.; Pell, A.C.H.; Sandercock, P.A.G.; Fox, K.A.A.; Lowe, G.D.O.; Murray, G.D.; et al. Aspirin for Prevention of Cardiovascular Events in a General Population Screened for a Low Ankle Brachial Index: A Randomized Controlled Trial. JAMA 2010, 303, 841–848. [Google Scholar] [CrossRef]
- Debus, E.S.; Nehler, M.R.; Govsyeyev, N.; Bauersachs, R.M.; Anand, S.S.; Patel, M.R.; Fanelli, F.; Capell, W.H.; Brackin, T.; Hinterreiter, F.; et al. Effect of Rivaroxaban and Aspirin in Patients with Peripheral Artery Disease Undergoing Surgical Revascularization: Insights from the VOYAGER PAD Trial. Circulation 2021, 144, 1104–1116. [Google Scholar] [CrossRef]
- Anand, S.S.; Hiatt, W.; Dyal, L.; Bauersachs, R.; Berkowitz, S.D.; Branch, K.R.H.; Debus, S.; Fox, K.A.A.; Liang, Y.; Muehlhofer, E.; et al. Low-Dose Rivaroxaban and Aspirin among Patients with Peripheral Artery Disease: A Meta-Analysis of the COMPASS and VOYAGER Trials. Eur. J. Prev. Cardiol. 2022, 29, e181–e189. [Google Scholar] [CrossRef]
- Li, L.; Geraghty, O.C.; Mehta, Z.; Rothwell, P.M. Age-Specific Risks, Severity, Time Course, and Outcome of Bleeding on Long-Term Antiplatelet Treatment after Vascular Events: A Population-Based Cohort Study. Lancet 2017, 390, 490–499. [Google Scholar] [CrossRef]
- Bauersachs, R.M.; Herold, J. Oral Anticoagulation in the Elderly and Frail. Hamostaseologie 2020, 40, 74–83. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on Cardiovascular Disease Prevention in Clinical Practice: Developed by the Task Force for Cardiovascular Disease Prevention in Clinical Practice with Representatives of the European Society of Cardiology and 12 Medical Societies With the Special Contribution of the European Association of Preventive Cardiology (EAPC). Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- US Preventive Services Task Force; Curry, S.J.; Krist, A.H.; Owens, D.K.; Barry, M.J.; Caughey, A.B.; Davidson, K.W.; Doubeni, C.A.; Epling, J.W.; Kemper, A.R.; et al. Screening for Peripheral Artery Disease and Cardiovascular Disease Risk Assessment with the Ankle-Brachial Index: US Preventive Services Task Force Recommendation Statement. JAMA 2018, 320, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Lindholt, J.S.; Søgaard, R. Population Screening and Intervention for Vascular Disease in Danish Men (VIVA): A Randomised Controlled Trial. Lancet 2017, 390, 2256–2265. [Google Scholar] [CrossRef]
- Aguirre, A.; Sharma, K.; Arora, A.; Humphries, M.D. Early ABI Testing May Decrease Risk of Amputation for Patients with Lower Extremity Ulcers. Ann. Vasc. Surg. 2022, 79, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Rodway, A.D.; Cheal, D.; Allan, C.; Pazos-Casal, F.; Hanna, L.; Field, B.C.T.; Pankhania, A.; Aston, P.J.; Skene, S.S.; Maytham, G.D.; et al. Ankle Doppler for Cuffless Ankle Brachial Index Estimation and Peripheral Artery Disease Diagnosis Independent of Diabetes. J. Clin. Med. 2022, 12, 97. [Google Scholar] [CrossRef] [PubMed]
- Collingridge Moore, D.; Payne, S.; Keegan, T.; Van den Block, L.; Deliens, L.; Gambassi, G.; Heikkila, R.; Kijowska, V.; Pasman, H.R.; Pivodic, L.; et al. Length of Stay in Long-Term Care Facilities: A Comparison of Residents in Six European Countries. Results of the PACE Cross-Sectional Study. BMJ Open 2020, 10, e033881. [Google Scholar] [CrossRef]

| Total (n = 1333) | Normal ABI (n = 592) | Low ABI (n = 503) | High ABI (n = 208) | p | ||
|---|---|---|---|---|---|---|
| Age—y | 84 (78–89) | 84 (78–89) | 84 (77–89) | 85.5 (79–89.75) | 0.137 # | |
| sex | male | 393 (29.5) | 173 (29.2) | 137 (27.2) | 72 (34.6) | 0.14 |
| female | 940 (70.5) | 419 (70.8) | 366 (72.8) | 136 (65.4) | ||
| Tobacco use | 573 (43.9) | 241 (41.3) | 240 (48.8) | 83 (41.3) | 0.033 * | |
| Coronary artery disease | 338 (25.4) | 136 (23.0) | 135 (26.8) | 58 (27.9) | 0.22 | |
| Peripheral vascular disease (previously known) | 138 (10.4) | 33 (5.6) | 78 (15.5) | 22 (10.6) | <0.001 * | |
| Carotid disease | 79 (6.0) | 31 (5.2) | 34 (6.9) | 13 (6.3) | 0.52 | |
| Hypertension | 1044 (78.4) | 428 (72.4) | 417 (82.9) | 173 (83.2) | <0.001 * | |
| Dyslipidemia | 382 (28.7) | 176 (29.7) | 143 (28.5) | 57 (27.4) | 0.79 | |
| Diabetes mellitus | 401 (30.1) | 147 (24.8) | 160 (31.9) | 81 (38.9) | <0.001 * | |
| Renal insufficiency | 305 (22.9) | 99 (16.7) | 127 (25.3) | 68 (32.7) | <0.001 * | |
| Cardiac arrhythmia | 390 (29.3) | 165 (27.9) | 132 (26.4) | 83 (39.9) | <0.001 * | |
| Chronic heart failure | 281 (21.1) | 109 (18.4) | 113 (22.5) | 53 (25.5) | 0.063 | |
| Ischaemic stroke | 297 (22.4) | 107 (18.1) | 127 (25.4) | 57 (27.4) | 0.003 * | |
| Cognitive dysfunction | 643 (48.2) | 295 (49.8) | 237 (47.1) | 98 (47.1) | 0.62 | |
| COPD | 150 (11.3) | 55 (9.3) | 66 (13.1) | 28 (13.5) | 0.083 | |
| Cancer | 219 (16.4) | 104 (17.6) | 67 (13.3) | 41 (19.7) | 0.058 | |
| Aspirin | 481 (37.8) | 193 (33.3) | 216 (43.8) | 72 (36.2) | 0.002 * | |
| Clopidogrel | 49 (3.9) | 16 (2.8) | 24 (4.9) | 9 (4.5) | 0.17 | |
| Oral anticoagulants | 206 (15.8) | 98 (16.6) | 61 (12.1) | 47 (22.6) | 0.002 * | |
| Beta blockers | 594 (45.6) | 245 (41.4) | 239 (47.5) | 110 (52.9) | 0.009 * | |
| ACE inhibitors | 659 (50.6) | 276 (46.6) | 277 (55.1) | 106 (51.0) | 0.020 * | |
| Calcium channel blockers | 291 (22.3) | 128 (21.6) | 122 (24.3) | 41 (19.7) | 0.36 | |
| Diuretics | 733 (56.3) | 311 (52.6) | 296 (58.8) | 126 (60.6) | 0.047 * | |
| Statins | 358 (27.5) | 174 (29.4) | 129 (25.6) | 55 (26.4) | 0.36 | |
| Oral antidiabetics | 145 (11.1) | 51 (8.6) | 72 (14.3) | 22 (10.6) | 0.011 * | |
| NASIDs | 130 (10.0) | 58 (9.8) | 45 (8.9) | 27 (13.0) | 0.26 | |
| Metamizole | 511 (39.2) | 223 (37.7) | 202 (40.2) | 86 (41.3) | 0.56 | |
| Opioid | 247 (19.0) | 104 (17.6) | 108 (21.5) | 35 (16.8) | 0.18 | |
| Psychotropic medications | 762 (58.6) | 345 (58.3) | 290 (57.8) | 127 (61.4) | 0.67 | |
| Hazard Ratio | 95.0% Confidence Interval of the Hazard Ratio | p | |
|---|---|---|---|
| Age | 1.045 | 1.04–1.06 | <0.001 |
| Female sex | 0.764 | 0.65–0.9 | 0.001 |
| ABI category | 0.031 | ||
| 1.205 | 1.03–1.41 | 0.021 |
| 1.238 | 1.01–1.52 | 0.040 |
| Renal insufficiency | 1.322 | 1.12–1.56 | <0.001 |
| Ischaemic stroke | 1.160 | 0.98–1.38 | 0.087 |
| Cognitive dysfunction | 1.170 | 1.01–1.35 | 0.032 |
| COPD | 1.537 | 1.25–1.89 | <0.001 |
| Cancer | 1.295 | 1.08–1.56 | 0.006 |
| Study Cohort (n = 1333) | Pathological ABI Subgroup (n = 729) | Low ABI Subgroup (n = 503) | Known CAD Subgroup (n = 322) | |
|---|---|---|---|---|
| Statins | Longer survival, p = 0.001 | Longer survival, p = 0.002 | Longer survival, p = 0.006 | Longer survival, p = 0.007 |
| Diuretics | Shorter survival, p = 0.002 | Shorter survival, p = 0.027 | (p = 0.075) | Shorter survival, p = 0.045 |
| ACE inhibitors | Longer survival, p = 0.024 | (p = 0.05) | (p = 0.55) | (p = 0.10) |
| Metamizol | (p = 0.075) | Shorter survival, p = 0.036 | (p = 0.10) | (p = 0.78) |
| Nonsteroidal anti-inflammatory drugs | (p = 0.19) | (p = 0.277) | (p = 0.42) | Shorter survival, p = 0.026 |
| Psychotropic medications | (p = 0.066) | (p = 0.57) | (p = 0.068) | Shorter survival, p = 0.018 |
| No statistically significant difference in survival time in all three groups: Aspirin, Clopidogrel, Oral anticoagulation, Beta blockers, Calcium channel blockers, Opioids, Oral antidiabetics | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorn, A.; Dorweiler, B.; Ahmad, W.; Mylonas, S.; Becker, I.; Majd, P. Low and High Ankle-Brachial Index Are Both Associated with Mortality in German Nursing Home Residents—The Five-Year Follow-Up of the “Allo-Study” Cohort. J. Clin. Med. 2023, 12, 4411. https://doi.org/10.3390/jcm12134411
Dorn A, Dorweiler B, Ahmad W, Mylonas S, Becker I, Majd P. Low and High Ankle-Brachial Index Are Both Associated with Mortality in German Nursing Home Residents—The Five-Year Follow-Up of the “Allo-Study” Cohort. Journal of Clinical Medicine. 2023; 12(13):4411. https://doi.org/10.3390/jcm12134411
Chicago/Turabian StyleDorn, Anna, Bernhard Dorweiler, Wael Ahmad, Spyridon Mylonas, Ingrid Becker, and Payman Majd. 2023. "Low and High Ankle-Brachial Index Are Both Associated with Mortality in German Nursing Home Residents—The Five-Year Follow-Up of the “Allo-Study” Cohort" Journal of Clinical Medicine 12, no. 13: 4411. https://doi.org/10.3390/jcm12134411
APA StyleDorn, A., Dorweiler, B., Ahmad, W., Mylonas, S., Becker, I., & Majd, P. (2023). Low and High Ankle-Brachial Index Are Both Associated with Mortality in German Nursing Home Residents—The Five-Year Follow-Up of the “Allo-Study” Cohort. Journal of Clinical Medicine, 12(13), 4411. https://doi.org/10.3390/jcm12134411






