Anatomical and Functional Connectivity of Critical Deep Brain Structures and Their Potential Clinical Application in Brain Stimulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. MRI Data Acquisition
2.3. MRI Data Pre-Processing
2.4. Seed-Based Functional Connectivity Analysis for Target Generation
2.5. ROI-Based Probabilistic Tractography Analysis
2.6. Overlap of rsFC/PTG Analysis and Associations between Functional and Structural Connectivity within the Overlaps
3. Results
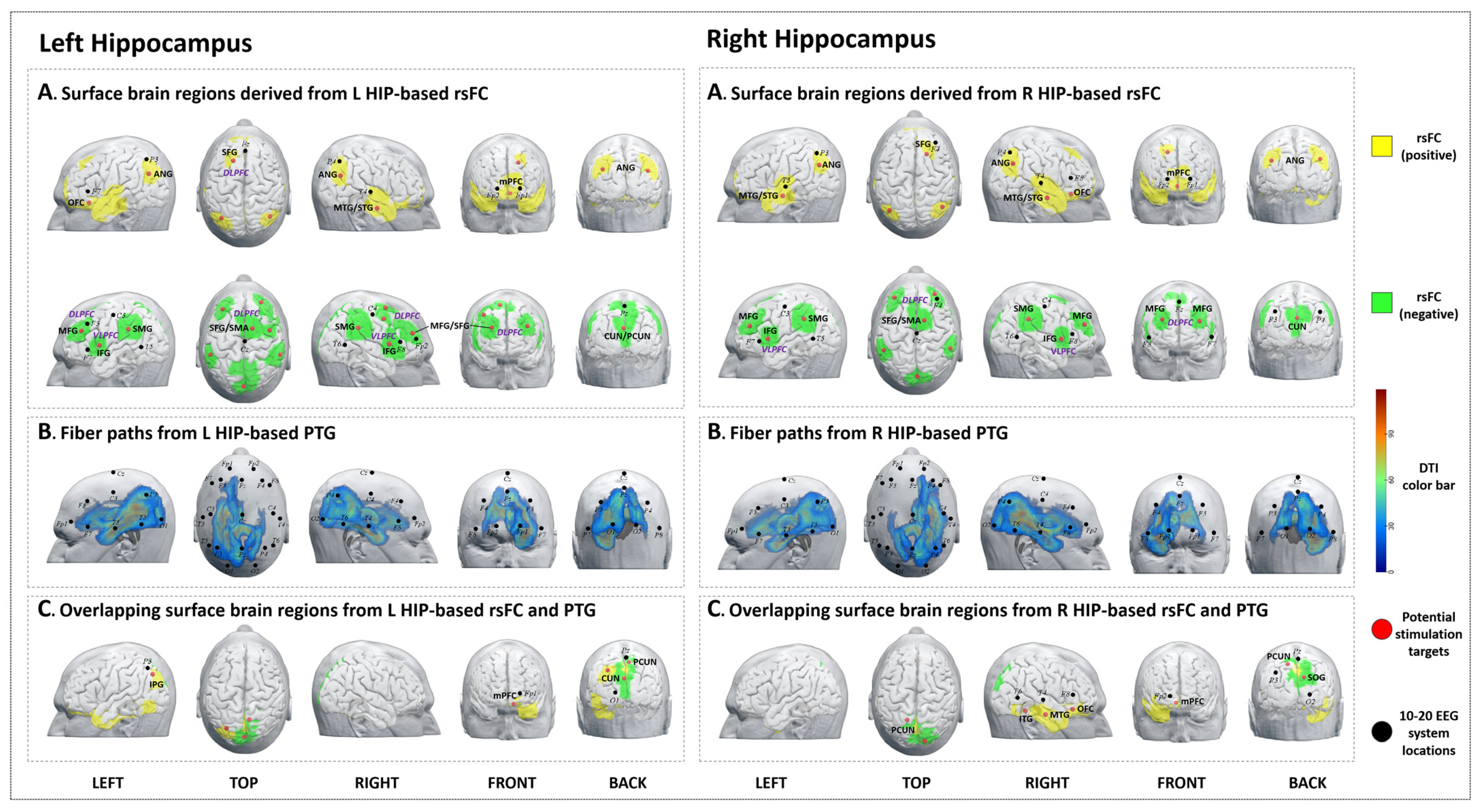
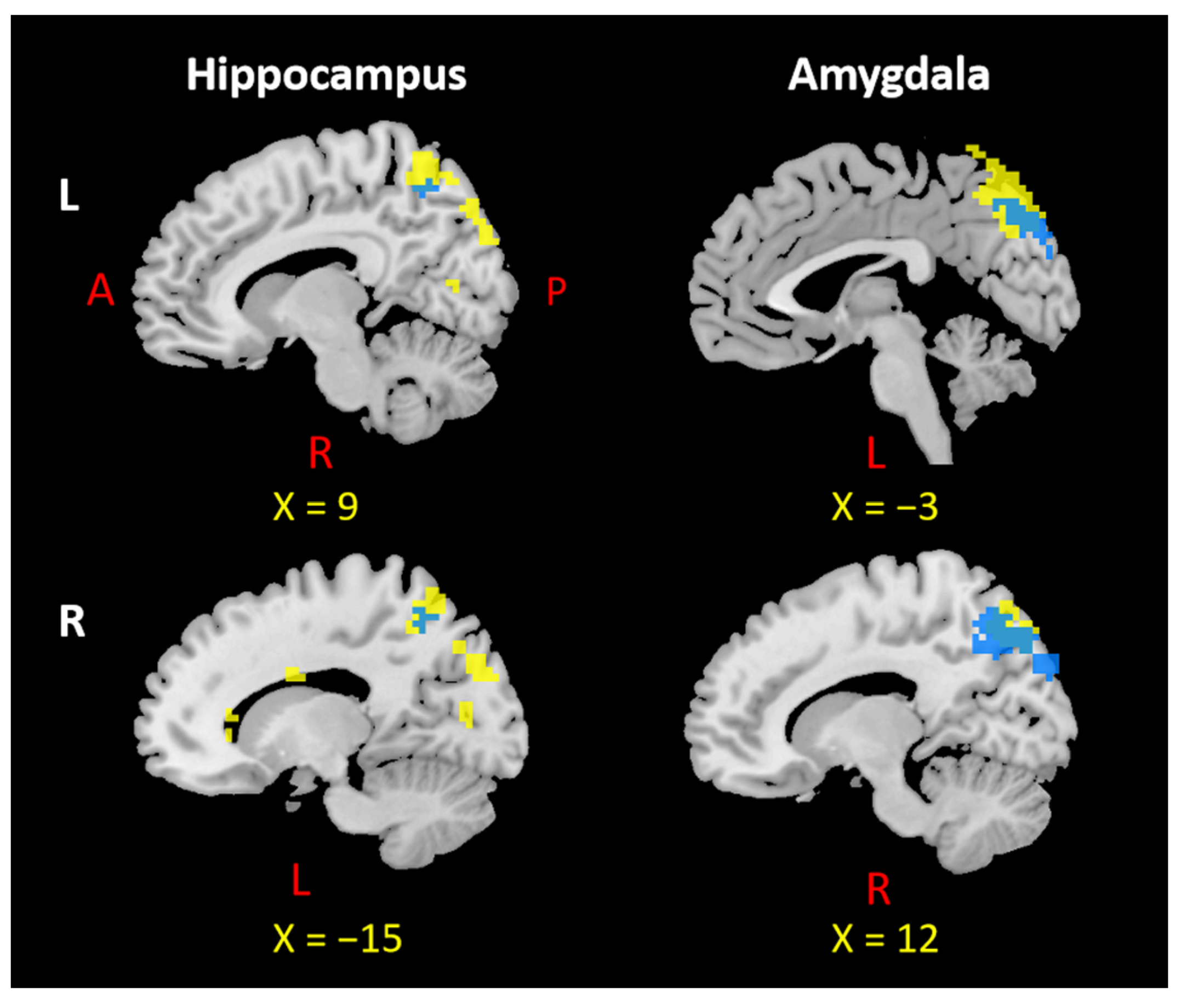
3.1. The Left Hippocampus
3.1.1. rsFC
3.1.2. PTG
3.1.3. Overlap Regions between rsFC and PTG
3.2. The Right Hippocampus
3.2.1. rsFC
3.2.2. PTG
3.2.3. Overlap Regions between rsFC and PTG
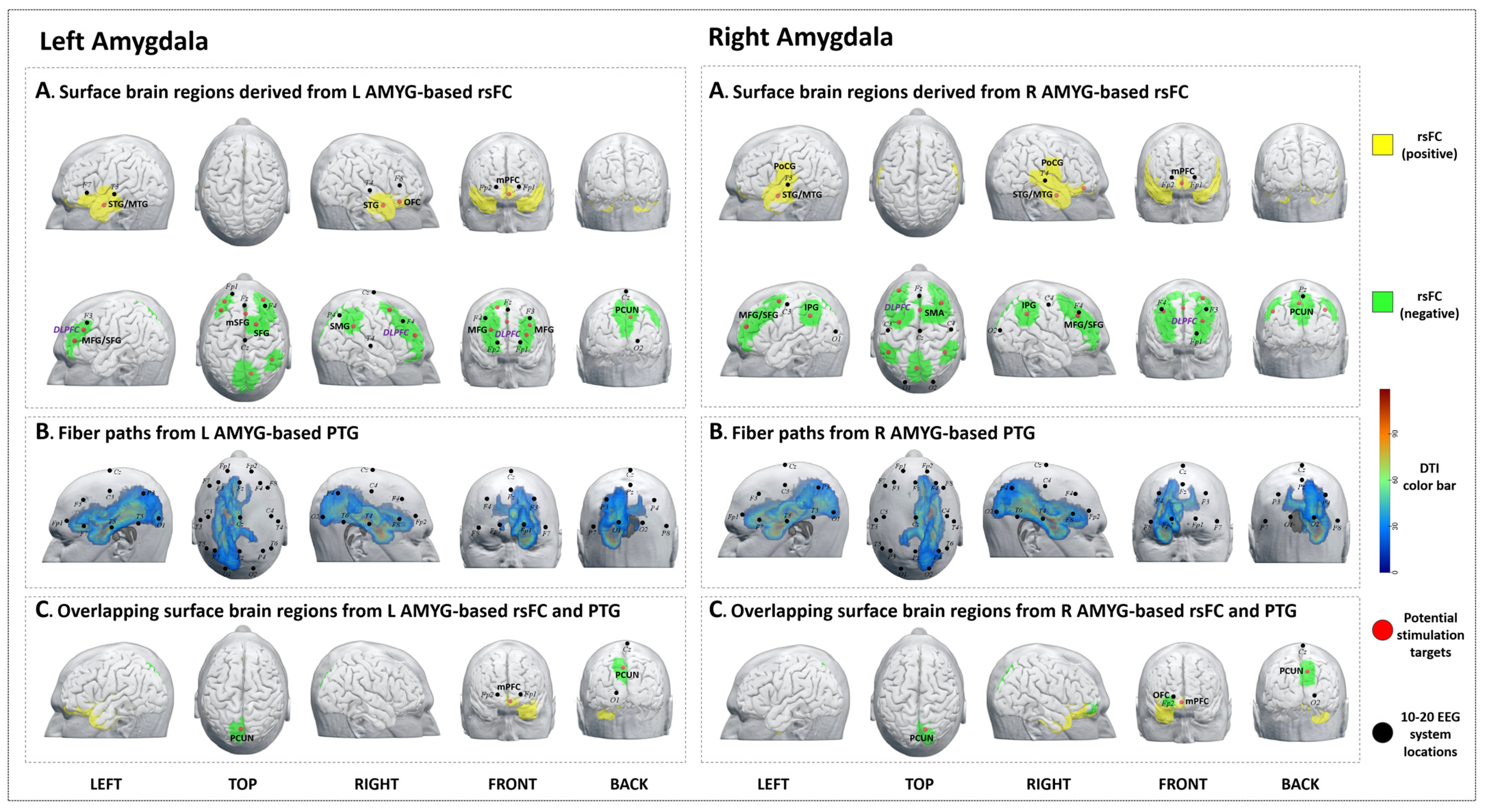
3.3. The Left Amygdala
3.3.1. rsFC
3.3.2. PTG
3.3.3. Overlap Regions between rsFC and PTG
3.4. The Right Amygdala
3.4.1. rsFC
3.4.2. PTG
3.4.3. Overlap Regions between rsFC and PTG
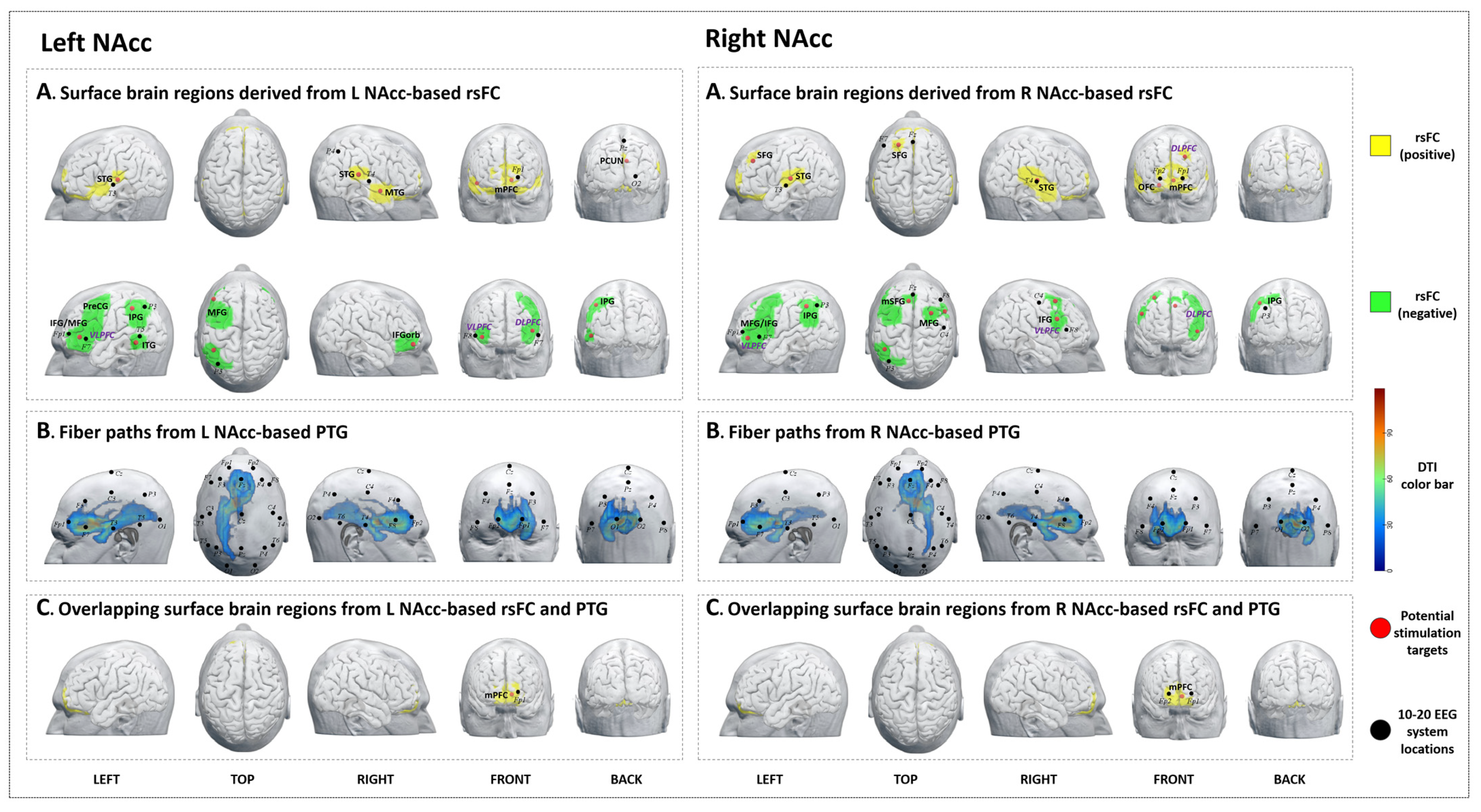
3.5. Left NAcc
3.5.1. rsFC
3.5.2. PTG
3.5.3. Overlap Regions between rsFC and PTG
3.6. Right NAcc
3.6.1. rsFC
3.6.2. PTG
3.6.3. Overlap Regions between rsFC and PTG
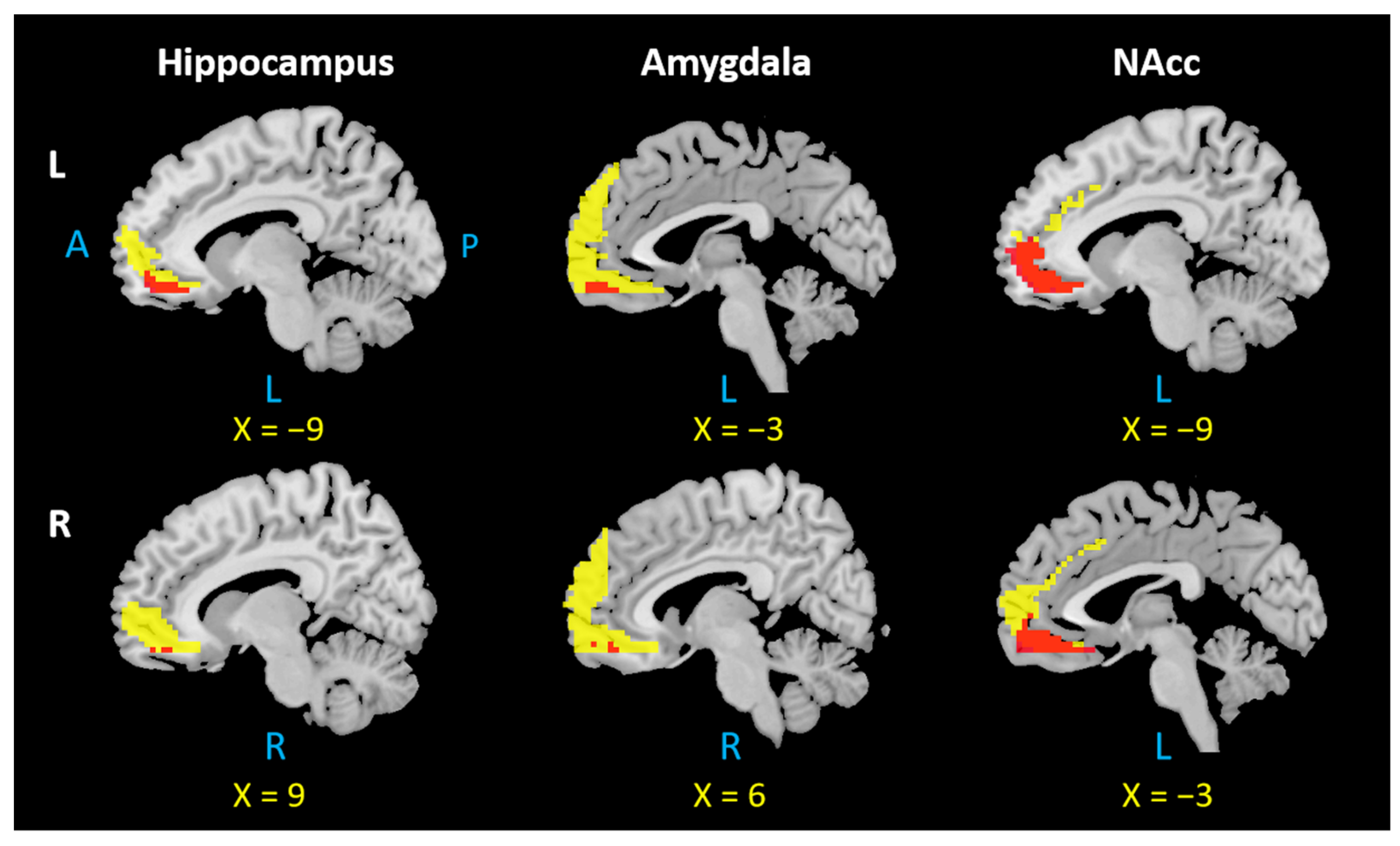
4. Overlapping Surface Regions among Three Subcortical Structures Based on rsFC/PTG
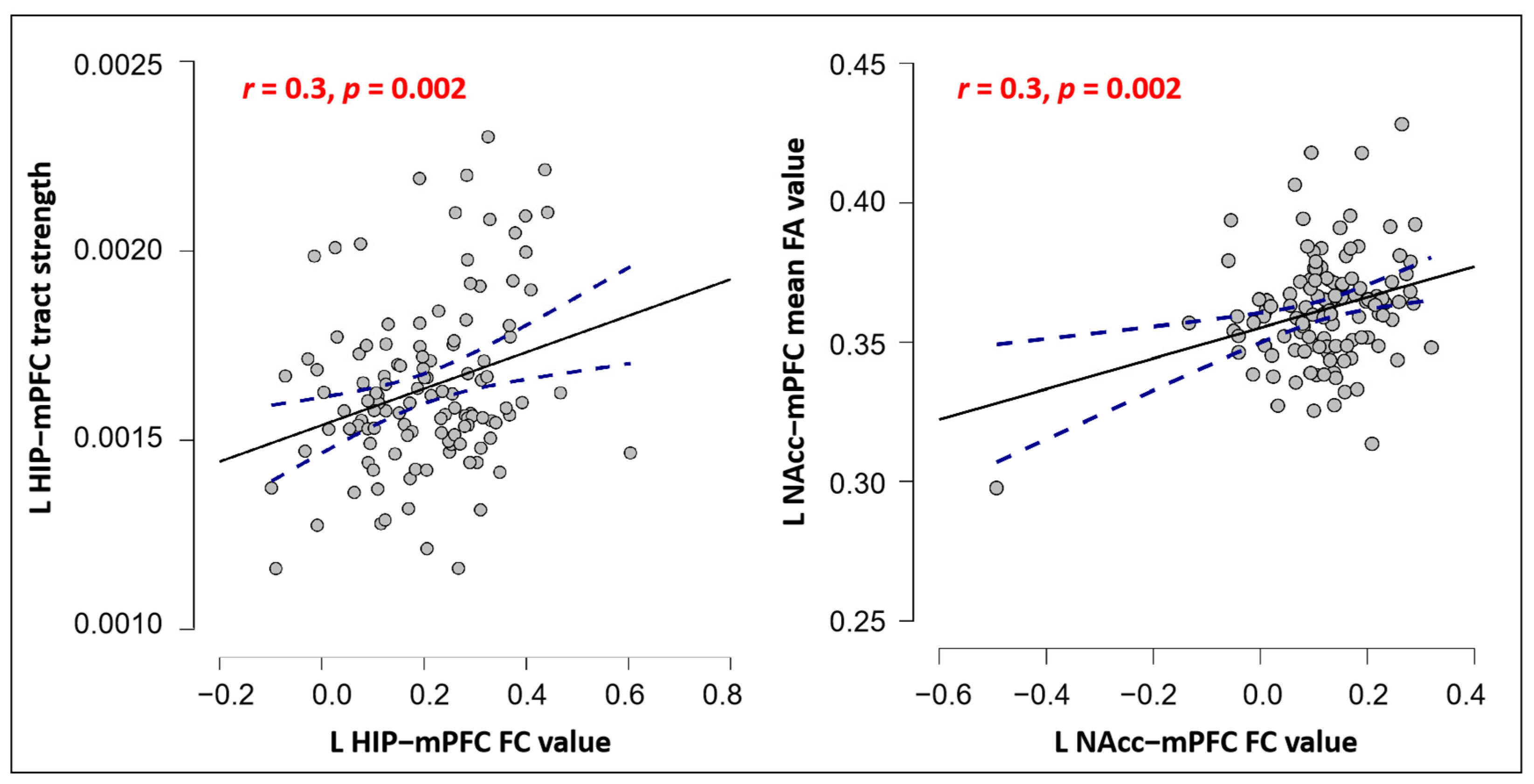
Correlations between FC and SC in mPFC among Three Subcortical Structures
5. Discussion
5.1. The mPFC Is Functionally and Anatomically Connected with the Hippocampus, Amygdala, and NAcc
5.2. The Precuneus Is Functionally and Anatomically Connected with the Hippocampus and Amygdala
5.3. Other Hippocampus Functional Connectivity Analysis Results
5.4. Other Amygdala Functional Connectivity Analysis Results
5.5. Other NAcc Functional Connectivity Analysis Results
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Knierim, J.J. The Hippocampus. Curr. Biol. 2015, 25, R1116–R1121. [Google Scholar] [CrossRef] [Green Version]
- Toda, T.; Parylak, S.L.; Linker, S.B.; Gage, F.H. The Role of Adult Hippocampal Neurogenesis in Brain Health and Disease. Mol. Psychiatry 2019, 24, 67. [Google Scholar] [CrossRef]
- Zeidman, P.; Maguire, E.A. Anterior Hippocampus: The Anatomy of Perception, Imagination and Episodic Memory. Nat. Rev. Neurosci. 2016, 17, 173–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janak, P.H.; Tye, K.M. From Circuits to Behaviour in the Amygdala. Nature 2015, 517, 284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, N.F.; Chong, P.L.H.; Lee, D.R.; Chew, Q.H.; Chen, G.; Sim, K. The Amygdala in Schizophrenia and Bipolar Disorder: A Synthesis of Structural MRI, Diffusion Tensor Imaging, and Resting-State Functional Connectivity Findings. Harv. Rev. Psychiatry 2019, 27, 150–164. [Google Scholar] [CrossRef]
- Zheng, Z.H.; Tu, J.L.; Li, X.H.; Hua, Q.; Liu, W.Z.; Liu, Y.; Pan, B.X.; Hu, P.; Zhang, W.H. Neuroinflammation Induces Anxiety-and Depressive-like Behavior by Modulating Neuronal Plasticity in the Basolateral Amygdala. Brain Behav. Immun. 2021, 91, 505–518. [Google Scholar] [CrossRef]
- Salgado, S.; Kaplitt, M.G. The Nucleus Accumbens: A Comprehensive Review. Stereotact. Funct. Neurosurg. 2015, 93, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Shlobin, N.A.; Jung, Y.; Zhang, K.K.; Warsi, N.; Kulkarni, A.V.; Ibrahim, G.M. Nucleus Accumbens: A Systematic Review of Neural Circuitry and Clinical Studies in Healthy and Pathological States. J. Neurosurg. 2022, 138, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E.E. Involvement of the Nucleus Accumbens and Dopamine System in Chronic Pain. Neurology 2016, 87, 1720–1726. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.Z. From Structure to Behavior in Basolateral Amygdala-Hippocampus Circuits. Front. Neural Circuits 2017, 11, 86. [Google Scholar] [CrossRef] [Green Version]
- Pennartz, C.M.A.; Ito, R.; Verschure, P.F.M.J.; Battaglia, F.P.; Robbins, T.W. The Hippocampal-Striatal Axis in Learning, Prediction and Goal-Directed Behavior. Trends Neurosci. 2011, 34, 548–559. [Google Scholar] [CrossRef] [PubMed]
- Bagot, R.C.; Parise, E.M.; Peña, C.J.; Zhang, H.X.; Maze, I.; Chaudhury, D.; Persaud, B.; Cachope, R.; Bolaños-Guzmán, C.A.; Cheer, J.; et al. Ventral Hippocampal Afferents to the Nucleus Accumbens Regulate Susceptibility to Depression. Nat. Commun. 2015, 6, 7062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etkin, A.; Egner, T.; Kalisch, R. Emotional Processing in Anterior Cingulate and Medial Prefrontal Cortex. Trends Cogn. Sci. 2011, 15, 85. [Google Scholar] [CrossRef] [Green Version]
- Mansouri, F.A.; Tanaka, K.; Buckley, M.J. Conflict-Induced Behavioural Adjustment: A Clue to the Executive Functions of the Prefrontal Cortex. Nat. Rev. Neurosci. 2009, 10, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Park, A.J.; Harris, A.Z.; Martyniuk, K.M.; Chang, C.Y.; Abbas, A.I.; Lowes, D.C.; Kellendonk, C.; Gogos, J.A.; Gordon, J.A. Reset of Hippocampal-Prefrontal Circuitry Facilitates Learning. Nature 2021, 591, 615–619. [Google Scholar] [CrossRef]
- Euston, D.R.; Gruber, A.J.; McNaughton, B.L. The Role of Medial Prefrontal Cortex in Memory and Decision Making. Neuron 2012, 76, 1057. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.C.; Zucca, A.; Levy, J.; Page, D.T. Social Behavior Is Modulated by Valence-Encoding MPFC-Amygdala Sub-Circuitry. Cell Rep. 2020, 32, 107899. [Google Scholar] [CrossRef]
- Goto, Y.; Grace, A.A. Limbic and Cortical Information Processing in the Nucleus Accumbens. Trends Neurosci. 2008, 31, 552–558. [Google Scholar] [CrossRef] [Green Version]
- Sesack, S.R.; Grace, A.A. Cortico-Basal Ganglia Reward Network: Microcircuitry. Neuropsychopharmacology 2010, 35, 27–47. [Google Scholar] [CrossRef] [Green Version]
- Sydnor, V.J.; Cieslak, M.; Duprat, R.; Deluisi, J.; Flounders, M.W.; Long, H.; Scully, M.; Balderston, N.L.; Sheline, Y.I.; Bassett, D.S.; et al. Cortical-Subcortical Structural Connections Support Transcranial Magnetic Stimulation Engagement of the Amygdala. Sci. Adv. 2022, 8, eabn5803. [Google Scholar] [CrossRef]
- Tambini, A.; Nee, D.E.; D’Esposito, M. Hippocampal-Targeted Theta-Burst Stimulation Enhances Associative Memory Formation. J. Cogn. Neurosci. 2018, 30, 1452–1472. [Google Scholar] [CrossRef] [PubMed]
- Vetkas, A.; Fomenko, A.; Germann, J.; Sarica, C.; Iorio-Morin, C.; Samuel, N.; Yamamoto, K.; Milano, V.; Cheyuo, C.; Zemmar, A.; et al. Deep Brain Stimulation Targets in Epilepsy: Systematic Review and Meta-Analysis of Anterior and Centromedian Thalamic Nuclei and Hippocampus. Epilepsia 2022, 63, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Hariz, M.; Visser-Vandewalle, V.; Zrinzo, L.; Coenen, V.A.; Sheth, S.A.; Bervoets, C.; Naesström, M.; Blomstedt, P.; Coyne, T.; et al. Deep Brain Stimulation for Refractory Obsessive-Compulsive Disorder (OCD): Emerging or Established Therapy? Mol. Psychiatry 2021, 26, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Corripio, I.; Roldán, A.; Sarró, S.; McKenna, P.J.; Alonso-Solís, A.; Rabella, M.; Díaz, A.; Puigdemont, D.; Pérez-Solà, V.; Álvarez, E.; et al. Deep Brain Stimulation in Treatment Resistant Schizophrenia: A Pilot Randomized Cross-over Clinical Trial. EBioMedicine 2020, 51, 102568. [Google Scholar] [CrossRef] [Green Version]
- Dandekar, M.P.; Fenoy, A.J.; Carvalho, A.F.; Soares, J.C.; Quevedo, J. Deep Brain Stimulation for Treatment-Resistant Depression: An Integrative Review of Preclinical and Clinical Findings and Translational Implications. Mol. Psychiatry 2018, 23, 1094–1112. [Google Scholar] [CrossRef]
- Meeres, J.; Hariz, M. Deep Brain Stimulation for Post-Traumatic Stress Disorder: A Review of the Experimental and Clinical Literature. Stereotact. Funct. Neurosurg. 2022, 100, 143–155. [Google Scholar] [CrossRef]
- Lee, D.J.; Lozano, C.S.; Dallapiazza, R.F.; Lozano, A.M. Current and Future Directions of Deep Brain Stimulation for Neurological and Psychiatric Disorders. J. Neurosurg. 2019, 131, 333–342. [Google Scholar] [CrossRef] [Green Version]
- Fox, M.D.; Buckner, R.L.; Liu, H.; Mallar Chakravarty, M.; Lozano, A.M.; Pascual-Leone, A. Resting-State Networks Link Invasive and Noninvasive Brain Stimulation across Diverse Psychiatric and Neurological Diseases. Proc. Natl. Acad. Sci. USA 2014, 111, E4367–E4375. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Parra, L.C. Can Transcranial Electric Stimulation with Multiple Electrodes Reach Deep Targets? Brain Stimul. 2019, 12, 30–40. [Google Scholar] [CrossRef]
- Louviot, S.; Tyvaert, L.; Maillard, L.G.; Colnat-Coulbois, S.; Dmochowski, J.; Koessler, L. Transcranial Electrical Stimulation Generates Electric Fields in Deep Human Brain Structures. Brain Stimul. 2022, 15, 1–12. [Google Scholar] [CrossRef]
- Warren, K.N.; Hermiller, M.S.; Nilakantan, A.S.; Voss, J.L. Stimulating the Hippocampal Posterior-Medial Network Enhances Task-Dependent Connectivity and Memory. Elife 2019, 8, e49458. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Rogers, L.M.; Gross, E.Z.; Ryals, A.J.; Dokucu, M.E.; Brandstatt, K.L.; Hermiller, M.S.; Voss, J.L. Targeted Enhancement of Cortical-Hippocampal Brain Networks and Associative Memory. Science 2014, 345, 1054–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, M.D.; Buckner, R.L.; White, M.P.; Greicius, M.D.; Pascual-Leone, A. Efficacy of Transcranial Magnetic Stimulation Targets for Depression Is Related to Intrinsic Functional Connectivity with the Subgenual Cingulate. Biol. Psychiatry 2012, 72, 595–603. [Google Scholar] [CrossRef] [Green Version]
- Luber, B.; Davis, S.W.; de Deng, Z.; Murphy, D.; Martella, A.; Peterchev, A.V.; Lisanby, S.H. Using Diffusion Tensor Imaging to Effectively Target TMS to Deep Brain Structures. Neuroimage 2022, 249, 118863. [Google Scholar] [CrossRef] [PubMed]
- Weigand, A.; Horn, A.; Caballero, R.; Cooke, D.; Stern, A.P.; Taylor, S.F.; Press, D.; Pascual-Leone, A.; Fox, M.D. Prospective Validation That Subgenual Connectivity Predicts Antidepressant Efficacy of Transcranial Magnetic Stimulation Sites. Biol. Psychiatry 2018, 84, 28–37. [Google Scholar] [CrossRef]
- Ning, L.; Makris, N.; Camprodon, J.A.; Rathi, Y. Limits and Reproducibility of Resting-State Functional MRI Definition of DLPFC Targets for Neuromodulation. Brain Stimul. 2019, 12, 129–138. [Google Scholar] [CrossRef]
- Zhu, Z.; Hubbard, E.; Guo, X.; Barbosa, D.A.N.; Popal, A.M.; Cai, C.; Jiang, H.; Zheng, Z.; Lin, J.; Gao, W.; et al. A Connectomic Analysis of Deep Brain Stimulation for Treatment-Resistant Depression. Brain Stimul. 2021, 14, 1226–1233. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, J.; Bao, T.; Wilson, G.; Park, J.; Zhao, B.; Kong, J. Locations for Noninvasive Brain Stimulation in Treating Depressive Disorders: A Combination of Meta-Analysis and Resting-State Functional Connectivity Analysis. Aust. N. Z. J. Psychiatry 2020, 54, 582–590. [Google Scholar] [CrossRef]
- Cao, J.; Huang, Y.; Meshberg, N.; Hodges, S.A.; Kong, J. Neuroimaging-Based Scalp Acupuncture Locations for Dementia. J. Clin. Med. 2020, 9, 2477. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, B.; Wilson, G.; Kong, J. New Perspective for Non-Invasive Brain Stimulation Site Selection in Mild Cognitive Impairment: Based on Meta- and Functional Connectivity Analyses. Front. Aging Neurosci. 2019, 11, 228. [Google Scholar] [CrossRef] [Green Version]
- Cutini, S.; Scatturin, P.; Zorzi, M. A New Method Based on ICBM152 Head Surface for Probe Placement in Multichannel FNIRS. Neuroimage 2011, 54, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Woolrich, M.W.; Jbabdi, S.; Patenaude, B.; Chappell, M.; Makni, S.; Behrens, T.; Beckmann, C.; Jenkinson, M.; Smith, S.M. Bayesian Analysis of Neuroimaging Data in FSL. Neuroimage 2009, 45, S173–S186. [Google Scholar] [CrossRef] [PubMed]
- Lambert, C.; Zrinzo, L.; Nagy, Z.; Lutti, A.; Hariz, M.; Foltynie, T.; Draganski, B.; Ashburner, J.; Frackowiak, R. Confirmation of Functional Zones within the Human Subthalamic Nucleus: Patterns of Connectivity and Sub-Parcellation Using Diffusion Weighted Imaging. Neuroimage 2012, 60, 83–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasques, X.; Richardet, R.; Hill, S.L.; Slater, D.; Chappelier, J.C.; Pralong, E.; Bloch, J.; Draganski, B.; Cif, L. Automatic Target Validation Based on Neuroscientific Literature Mining for Tractography. Front. Neuroanat. 2015, 9, 66. [Google Scholar] [CrossRef] [Green Version]
- Robinson, J.L.; Barron, D.S.; Kirby, L.A.J.; Bottenhorn, K.L.; Hill, A.C.; Murphy, J.E.; Katz, J.S.; Salibi, N.; Eickhoff, S.B.; Fox, P.T. Neurofunctional Topography of the Human Hippocampus. Hum. Brain Mapp. 2015, 36, 5018–5037. [Google Scholar] [CrossRef] [Green Version]
- Jung, W.H.; Lee, S.; Lerman, C.; Kable, J.W. Amygdala Functional and Structural Connectivity Predicts Individual Risk Tolerance. Neuron 2018, 98, 394–404.e4. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Vangel, M.; Chen, H.; Eshel, M.; Cheng, M.; Lu, T.; Kong, J. The Impaired Subcortical Pathway From Superior Colliculus to the Amygdala in Boys With Autism Spectrum Disorder. Front. Integr. Neurosci. 2022, 16, 66439. [Google Scholar] [CrossRef]
- Liu, W.; Chen, C.; Wang, F.; Guo, S.; Hao, Y.; Li, S. Development Trend and Current Situation of Acupuncture-Moxibustion Indications. World J. Acupunct. Moxibustion 2020, 30, 245–250. [Google Scholar] [CrossRef]
- Raichle, M.E.; MacLeod, A.M.; Snyder, A.Z.; Powers, W.J.; Gusnard, D.A.; Shulman, G.L. INAUGURAL ARTICLE by a Recently Elected Academy Member:A Default Mode of Brain Function. Proc. Natl. Acad. Sci. USA 2001, 98, 676. [Google Scholar] [CrossRef] [Green Version]
- Andrews-Hanna, J.R.; Smallwood, J.; Spreng, R.N. The Default Network and Self-Generated Thought: Component Processes, Dynamic Control, and Clinical Relevance. Ann. N. Y. Acad. Sci. 2014, 1316, 29–52. [Google Scholar] [CrossRef] [Green Version]
- Mohan, A.; Roberto, A.J.; Mohan, A.; Lorenzo, A.; Jones, K.; Carney, M.J.; Liogier-Weyback, L.; Hwang, S.; Lapidus, K.A.B. Focus: The Aging Brain: The Significance of the Default Mode Network (DMN) in Neurological and Neuropsychiatric Disorders: A Review. Yale J. Biol. Med. 2016, 89, 49. [Google Scholar]
- Davis, M.-C.; Hill, A.T.; Fitzgerald, P.B.; Bailey, N.W.; Sullivan, C.; Stout, J.C.; Hoy, K.E. Medial Prefrontal Transcranial Alternating Current Stimulation for Apathy in Huntington’s Disease. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2023, 126, 110776. [Google Scholar] [CrossRef] [PubMed]
- Csifcsák, G.; Boayue, N.M.; Puonti, O.; Thielscher, A.; Mittner, M. Effects of Transcranial Direct Current Stimulation for Treating Depression: A Modeling Study. J. Affect. Disord. 2018, 234, 164–173. [Google Scholar] [CrossRef] [Green Version]
- Adams, T.G.; Cisler, J.M.; Kelmendi, B.; George, J.R.; Kichuk, S.A.; Averill, C.L.; Anticevic, A.; Abdallah, C.G.; Pittenger, C. Transcranial Direct Current Stimulation Targeting the Medial Prefrontal Cortex Modulates Functional Connectivity and Enhances Safety Learning in Obsessive-Compulsive Disorder: Results from Two Pilot Studies. Depress. Anxiety 2022, 39, 37–48. [Google Scholar] [CrossRef]
- Abend, R.; Sar-el, R.; Gonen, T.; Jalon, I.; Vaisvaser, S.; Bar-Haim, Y.; Hendler, T. Modulating Emotional Experience Using Electrical Stimulation of the Medial-Prefrontal Cortex: A Preliminary TDCS-FMRI Study. Neuromodulation 2019, 22, 884–893. [Google Scholar] [CrossRef]
- Gu, S.; Pasqualetti, F.; Cieslak, M.; Telesford, Q.K.; Yu, A.B.; Kahn, A.E.; Medaglia, J.D.; Vettel, J.M.; Miller, M.B.; Grafton, S.T.; et al. Controllability of Structural Brain Networks. Nat. Commun. 2015, 6, 8414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rolls, E.T. The Hippocampus, Ventromedial Prefrontal Cortex, and Episodic and Semantic Memory. Prog. Neurobiol. 2022, 217, 102334. [Google Scholar] [CrossRef] [PubMed]
- Bonnici, H.M.; Chadwick, M.J.; Lutti, A.; Hassabis, D.; Weiskopf, N.; Maguire, E.A. Detecting Representations of Recent and Remote Autobiographical Memories in VmPFC and Hippocampus. J. Neurosci. 2012, 32, 16982–16991. [Google Scholar] [CrossRef] [Green Version]
- Tao, J.; Liu, J.; Egorova, N.; Chen, X.; Sun, S.; Xue, X.; Huang, J.; Zheng, G.; Wang, Q.; Chen, L.; et al. Increased Hippocampus-Medial Prefrontal Cortex Resting-State Functional Connectivity and Memory Function after Tai Chi Chuan Practice in Elder Adults. Front. Aging Neurosci. 2016, 8, 25. [Google Scholar] [CrossRef] [Green Version]
- Jia, L.; Sun, Z.; Shi, D.; Wang, M.; Jia, J.; He, Y.; Xue, F.; Ren, Y.; Yang, J.; Ma, X. Effects of Different Patterns of Electric Stimulation of the Ventromedial Prefrontal Cortex on Hippocampal-Prefrontal Coherence in a Rat Model of Depression. Behav. Brain Res. 2019, 356, 179–188. [Google Scholar] [CrossRef]
- Motzkin, J.C.; Philippi, C.L.; Wolf, R.C.; Baskaya, M.K.; Koenigs, M. Ventromedial Prefrontal Cortex Is Critical for the Regulation of Amygdala Activity in Humans. Biol. Psychiatry 2015, 77, 276–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jafari, E.; Alizadehgoradel, J.; Pourmohseni Koluri, F.; Nikoozadehkordmirza, E.; Refahi, M.; Taherifard, M.; Nejati, V.; Hallajian, A.H.; Ghanavati, E.; Vicario, C.M.; et al. Intensified Electrical Stimulation Targeting Lateral and Medial Prefrontal Cortices for the Treatment of Social Anxiety Disorder: A Randomized, Double-Blind, Parallel-Group, Dose-Comparison Study. Brain Stimul. 2021, 14, 974–986. [Google Scholar] [CrossRef]
- Yeh, N.; Payne, J.D.; Kim, S.Y.; Kensinger, E.A.; Koen, J.D.; Rose, N.S. Medial Prefrontal Cortex Has a Causal Role in Selectively Enhanced Consolidation of Emotional Memories after a 24-Hour Delay: A TBS Study. J. Neurosci. 2021, 41, 6273–6280. [Google Scholar] [CrossRef]
- Haber, S.N.; Knutson, B. The Reward Circuit: Linking Primate Anatomy and Human Imaging. Neuropsychopharmacology 2010, 35, 4–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.; Li, W.; Shen, W.; Edwards, R.R.; Gollub, R.L.; Wilson, G.; Park, J.; Ortiz, A.; Cao, J.; Gerber, J.; et al. Impaired Mesocorticolimbic Connectivity Underlies Increased Pain Sensitivity in Chronic Low Back Pain. Neuroimage 2020, 218, 116969. [Google Scholar] [CrossRef] [PubMed]
- Navratilova, E.; Porreca, F. Reward and Motivation in Pain and Pain Relief. Nat. Neurosci. 2014, 17, 1304–1312. [Google Scholar] [CrossRef]
- Lim, L.W.; Janssen, M.L.F.; Kocabicak, E.; Temel, Y. The Antidepressant Effects of Ventromedial Prefrontal Cortex Stimulation Is Associated with Neural Activation in the Medial Part of the Subthalamic Nucleus. Behav. Brain Res. 2015, 279, 17–21. [Google Scholar] [CrossRef]
- Shirvalkar, P.; Prosky, J.; Chin, G.; Ahmadipour, P.; Sani, O.G.; Desai, M.; Schmitgen, A.; Dawes, H.; Shanechi, M.M.; Starr, P.A.; et al. First-in-Human Prediction of Chronic Pain State Using Intracranial Neural Biomarkers. Nat. Neurosci. 2023, 26, 1090–1099. [Google Scholar] [CrossRef]
- Honey, C.J.; Sporns, O.; Cammoun, L.; Gigandet, X.; Thiran, J.P.; Meuli, R.; Hagmann, P. Predicting Human Resting-State Functional Connectivity from Structural Connectivity. Proc. Natl. Acad. Sci. USA 2009, 106, 2035–2040. [Google Scholar] [CrossRef] [Green Version]
- Khalsa, S.; Mayhew, S.D.; Chechlacz, M.; Bagary, M.; Bagshaw, A.P. The Structural and Functional Connectivity of the Posterior Cingulate Cortex: Comparison between Deterministic and Probabilistic Tractography for the Investigation of Structure–Function Relationships. Neuroimage 2014, 102, 118–127. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Dai, Z.; Gong, G.; Zhou, C.; He, Y. Understanding Structural-Functional Relationships in the Human Brain. Neuroscientist 2014, 21, 290–305. [Google Scholar] [CrossRef] [PubMed]
- Metz, A.E.; Yau, H.J.; Centeno, M.V.; Apkarian, A.V.; Martina, M. Morphological and Functional Reorganization of Rat Medial Prefrontal Cortex in Neuropathic Pain. Proc. Natl. Acad. Sci. USA 2009, 106, 2423–2428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, S.A.; Keaser, M.L.; Meiller, T.F.; Seminowicz, D.A. Altered Structure and Function in the Hippocampus and Medial Prefrontal Cortex in Patients with Burning Mouth Syndrome. Pain 2014, 155, 1472–1480. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ma, N.; Hu, G.; Nousayhah, A.; Xue, C.; Qi, W.; Xu, W.; Chen, S.; Rao, J.; Liu, W.; et al. RTMS Modulates Precuneus-Hippocampal Subregion Circuit in Patients with Subjective Cognitive Decline. Aging 2020, 13, 1314–1331. [Google Scholar] [CrossRef]
- Koch, G.; Bonnì, S.; Pellicciari, M.C.; Casula, E.P.; Mancini, M.; Esposito, R.; Ponzo, V.; Picazio, S.; di Lorenzo, F.; Serra, L.; et al. Transcranial Magnetic Stimulation of the Precuneus Enhances Memory and Neural Activity in Prodromal Alzheimer’s Disease. Neuroimage 2018, 169, 302–311. [Google Scholar] [CrossRef] [Green Version]
- Ferri, J.; Schmidt, J.; Hajcak, G.; Canli, T. Emotion Regulation and Amygdala-Precuneus Connectivity: Focusing on Attentional Deployment. Cogn. Affect. Behav. Neurosci. 2016, 16, 991–1002. [Google Scholar] [CrossRef] [Green Version]
- Cullen, K.R.; Westlund, M.K.; Klimes-Dougan, B.; Mueller, B.A.; Houri, A.; Eberly, L.E.; Lim, K.O. Abnormal Amygdala Resting-State Functional Connectivity in Adolescent Depression. JAMA Psychiatry 2014, 71, 1138–1147. [Google Scholar] [CrossRef] [Green Version]
- Stoddard, J.; Hsu, D.; Reynolds, R.C.; Brotman, M.A.; Ernst, M.; Pine, D.S.; Leibenluft, E.; Dickstein, D.P. Aberrant Amygdala Intrinsic Functional Connectivity Distinguishes Youths with Bipolar Disorder from Those with Severe Mood Dysregulation. Psychiatry Res. 2015, 231, 120–125. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, A.A.; Densmore, M.; Frewen, P.A.; Théberge, J.; Neufeld, R.W.J.; McKinnon, M.C.; Lanius, R.A. The Dissociative Subtype of Posttraumatic Stress Disorder: Unique Resting-State Functional Connectivity of Basolateral and Centromedial Amygdala Complexes. Neuropsychopharmacology 2015, 40, 2317–2326. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, P.; Vilgis, V.; Rhoads, S.; Chahal, R.; Fassbender, C.; Leibenluft, E.; Dixon, J.F.; Pakyurek, M.; van den Bos, W.; Hinshaw, S.P.; et al. Associations of Irritability With Functional Connectivity of Amygdala and Nucleus Accumbens in Adolescents and Young Adults With ADHD. J. Atten. Disord. 2022, 26, 1040–1050. [Google Scholar] [CrossRef]
- Chen, C.Y.; Yen, J.Y.; Wang, P.W.; Liu, G.C.; Yen, C.F.; Ko, C.H. Altered Functional Connectivity of the Insula and Nucleus Accumbens in Internet Gaming Disorder: A Resting State FMRI Study. Eur. Addict. Res. 2016, 22, 192–200. [Google Scholar] [CrossRef]
- Liu, R.; Wang, Y.; Chen, X.; Zhang, Z.; Xiao, L.; Zhou, Y. Anhedonia Correlates with Functional Connectivity of the Nucleus Accumbens Subregions in Patients with Major Depressive Disorder. Neuroimage Clin. 2021, 30, 102599. [Google Scholar] [CrossRef]
- Hebscher, M.; Voss, J.L. Testing Network Properties of Episodic Memory Using Non-Invasive Brain Stimulation. Curr. Opin. Behav. Sci. 2020, 32, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Poh, J.S.; Wen, D.J.; Broekman, B.F.P.; Chong, Y.S.; Yap, F.; Shek, L.P.; Gluckman, P.D.; Fortier, M.v.; Qiu, A. Functional and Structural Networks of Lateral and Medial Orbitofrontal Cortex as Potential Neural Pathways for Depression in Childhood. Depress. Anxiety 2019, 36, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Nakamura, M.; Nishikawa, Y.; Komori, Y.; Nishiyama, S.; Takayanagi, Y.; Furuichi, A.; Kido, M.; Sasabayashi, D.; Higuchi, Y.; et al. Potential Role of Orbitofrontal Surface Morphology on Social and Cognitive Functions in High-Risk Subjects for Psychosis and Schizophrenia Patients. Psychiatry Res. Neuroimaging 2019, 283, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Colasanti, A.; Guo, Q.; Giannetti, P.; Wall, M.B.; Newbould, R.D.; Bishop, C.; Onega, M.; Nicholas, R.; Ciccarelli, O.; Muraro, P.A.; et al. Hippocampal Neuroinflammation, Functional Connectivity, and Depressive Symptoms in Multiple Sclerosis. Biol. Psychiatry 2016, 80, 62–72. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Li, J.; Wang, J.; Fan, X.; Hu, M.; Shen, Y.; Chen, H.; Zhao, J. Hippocampal and Orbital Inferior Frontal Gray Matter Volume Abnormalities and Cognitive Deficit in Treatment-Naive, First-Episode Patients with Schizophrenia. Schizophr. Res. 2014, 152, 339–343. [Google Scholar] [CrossRef]
- Yan, R.; Tao, S.W.; Liu, H.Y.; Chen, Y.; Shi, J.B.; Yang, Y.Y.; Zhu, R.X.; Yao, Z.J.; Lu, Q. Abnormal Alterations of Regional Spontaneous Neuronal Activity in Inferior Frontal Orbital Gyrus and Corresponding Brain Circuit Alterations: A Resting-State FMRI Study in Somatic Depression. Front. Psychiatry 2019, 10, 267. [Google Scholar] [CrossRef]
- Li, Q.; Fu, Y.; Liu, C.; Meng, Z. Transcranial Direct Current Stimulation of the Dorsolateral Prefrontal Cortex for Treatment of Neuropsychiatric Disorders. Front. Behav. Neurosci. 2022, 16, 893955. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Chen, H.; Hu, G.; Yu, S.; Ruan, X.; Luo, Z.; Wei, X.; Xie, Y. Altered Global Synchronizations in Patients With Parkinson’s Disease: A Resting-State FMRI Study. Front. Aging Neurosci. 2019, 11, 139. [Google Scholar] [CrossRef] [Green Version]
- Sigurdsson, T.; Duvarci, S. Hippocampal-Prefrontal Interactions in Cognition, Behavior and Psychiatric Disease. Front. Syst. Neurosci. 2016, 9, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermiller, M.S.; Chen, Y.F.; Parrish, T.B.; Voss, J.L. Evidence for Immediate Enhancement of Hippocampal Memory Encoding by Network-Targeted Theta-Burst Stimulation during Concurrent FMRI. J. Neurosci. 2020, 40, 7155–7168. [Google Scholar] [CrossRef] [PubMed]
- Schwab, S.; Afyouni, S.; Chen, Y.; Han, Z.; Guo, Q.; Dierks, T.; Wahlund, L.O.; Grieder, M.; Babiloni, C. Functional Connectivity Alterations of the Temporal Lobe and Hippocampus in Semantic Dementia and Alzheimer’s Disease. J. Alzheimers Dis. 2020, 76, 1461–1475. [Google Scholar] [CrossRef] [PubMed]
- Burgess, N.; Maguire, E.A.; O’Keefe, J. The Human Hippocampus and Spatial and Episodic Memory. Neuron 2002, 35, 625–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakaguchi, Y.; Sakurai, Y. Left-Right Functional Difference of the Rat Dorsal Hippocampus for Short-Term Memory and Long-Term Memory. Behav. Brain Res. 2020, 382, 112478. [Google Scholar] [CrossRef]
- Roy, A.K.; Shehzad, Z.; Margulies, D.S.; Kelly, A.M.C.; Uddin, L.Q.; Gotimer, K.; Biswal, B.B.; Castellanos, F.X.; Milham, M.P. Functional Connectivity of the Human Amygdala Using Resting State FMRI. Neuroimage 2009, 45, 614–626. [Google Scholar] [CrossRef] [Green Version]
- Mao, Y.; Zuo, X.; Ding, C.; Qiu, J. OFC and Its Connectivity with Amygdala as Predictors for Future Social Anxiety in Adolescents. Dev. Cogn. Neurosci. 2020, 44, 100804. [Google Scholar] [CrossRef]
- Kim, M.J.; Loucks, R.A.; Palmer, A.L.; Brown, A.C.; Solomon, K.M.; Marchante, A.N.; Whalen, P.J. The Structural and Functional Connectivity of the Amygdala: From Normal Emotion to Pathological Anxiety. Behav. Brain Res. 2011, 223, 403–410. [Google Scholar] [CrossRef] [Green Version]
- Qi, R.; Liu, C.; Ke, J.; Xu, Q.; Ye, Y.; Jia, L.; Wang, F.; Zhang, L.J.; Lu, G.M. Abnormal Amygdala Resting-State Functional Connectivity in Irritable Bowel Syndrome. AJNR Am. J. Neuroradiol. 2016, 37, 1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, L.; Li, H.; Liu, J.; Jiang, J.; Li, B.; Li, X.; Zhang, S.; Gao, Y.; Liang, K.; Hu, X.; et al. Disorganized Functional Architecture of Amygdala Subregional Networks in Obsessive-Compulsive Disorder. Commun. Biol. 2022, 5, 1184. [Google Scholar] [CrossRef]
- Yu, R.; Liu, B.; Wang, L.; Chen, J.; Liu, X. Enhanced Functional Connectivity between Putamen and Supplementary Motor Area in Parkinson’s Disease Patients. PLoS ONE 2013, 8, e59717. [Google Scholar] [CrossRef] [Green Version]
- Cole, E.J.; Stimpson, K.H.; Bentzley, B.S.; Gulser, M.; Cherian, K.; Tischler, C.; Nejad, R.; Pankow, H.; Choi, E.; Aaron, H.; et al. Stanford Accelerated Intelligent Neuromodulation Therapy for Treatment-Resistant Depression. Am. J. Psychiatry 2020, 177, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Cole, E.J.; Phillips, A.L.; Bentzley, B.S.; Stimpson, K.H.; Nejad, R.; Barmak, F.; Veerapal, C.; Khan, N.; Cherian, K.; Felber, E.; et al. Stanford Neuromodulation Therapy (SNT): A Double-Blind Randomized Controlled Trial. Am. J. Psychiatry 2022, 179, 132–141. [Google Scholar] [CrossRef]
- Mitchell, D.G.V.; Nakic, M.; Fridberg, D.; Kamel, N.; Pine, D.S.; Blair, R.J.R. The Impact of Processing Load on Emotion. Neuroimage 2007, 34, 1299–1309. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, G.; Milardi, D.; Bertino, S.; Basile, G.A.; Di Mauro, D.; Calamuneri, A.; Chillemi, G.; Silvestri, G.; Anastasi, G.; Bramanti, A.; et al. The Limbic and Sensorimotor Pathways of the Human Amygdala: A Structural Connectivity Study. Neuroscience 2018, 385, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Radua, J.; Phillips, M.L.; Russell, T.; Lawrence, N.; Marshall, N.; Kalidindi, S.; El-Hage, W.; McDonald, C.; Giampietro, V.; Brammer, M.J.; et al. Neural Response to Specific Components of Fearful Faces in Healthy and Schizophrenic Adults. Neuroimage 2010, 49, 939–946. [Google Scholar] [CrossRef] [Green Version]
- Dyck, M.; Loughead, J.; Kellermann, T.; Boers, F.; Gur, R.C.; Mathiak, K. Cognitive versus Automatic Mechanisms of Mood Induction Differentially Activate Left and Right Amygdala. Neuroimage 2011, 54, 2503–2513. [Google Scholar] [CrossRef] [PubMed]
- Cauda, F.; Cavanna, A.E.; D’agata, F.; Sacco, K.; Duca, S.; Geminiani, G.C. Functional Connectivity and Coactivation of the Nucleus Accumbens: A Combined Functional Connectivity and Structure-Based Meta-Analysis. J. Cogn. Neurosci. 2011, 23, 2864–2877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bracht, T.; Soravia, L.; Moggi, F.; Stein, M.; Grieder, M.; Federspiel, A.; Tschümperlin, R.; Batschelet, H.M.; Wiest, R.; Denier, N. The Role of the Orbitofrontal Cortex and the Nucleus Accumbens for Craving in Alcohol Use Disorder. Transl. Psychiatry 2021, 11, 267. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T.; Cheng, W.; Feng, J. The Orbitofrontal Cortex: Reward, Emotion and Depression. Brain Commun. 2020, 2, fcaa196. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Liu, H.; Du, W.; Chao, F.; Zhang, L.; Wang, K.; Huang, C.; Gao, Y.; Tang, Y. Stimulated Left DLPFC-Nucleus Accumbens Functional Connectivity Predicts the Anti-Depression and Anti-Anxiety Effects of RTMS for Depression. Transl. Psychiatry 2018, 7, 3. [Google Scholar] [CrossRef]
- Zhao, J.; Mo, L.; Bi, R.; He, Z.; Chen, Y.; Xu, F.; Xie, H.; Zhang, D. The VLPFC versus the DLPFC in Downregulating Social Pain Using Reappraisal and Distraction Strategies. J. Neurosci. 2021, 41, 1331–1339. [Google Scholar] [CrossRef]
- Yang, R.; Zhao, X.; Liu, J.; Yao, X.; Hou, F.; Xu, Y.; Feng, Q. Functional Connectivity Changes of Nucleus Accumbens Shell Portion in Left Mesial Temporal Lobe Epilepsy Patients. Brain Imaging Behav. 2020, 14, 2659–2667. [Google Scholar] [CrossRef]
- Prasad, S.; Reddam, V.; Stezin, A.; Yadav, R.; Saini, J.; Pal, P. Abnormal Subcortical Volumes and Cortical Thickness in Parkinson’s Disease with Impulse Control Disorders. Ann. Ind. Acad. Neurol. 2019, 22, 426–431. [Google Scholar] [CrossRef]
- Biundo, R.; Weis, L.; Facchini, S.; Formento-Dojot, P.; Vallelunga, A.; Pilleri, M.; Weintraub, D.; Antonini, A. Patterns of Cortical Thickness Associated with Impulse Control Disorders in Parkinson’s Disease. Mov. Disord. 2015, 30, 688–695. [Google Scholar] [CrossRef]
- Johnson, M.D.; Lim, H.H.; Netoff, T.I.; Connolly, A.T.; Johnson, N.; Roy, A.; Holt, A.; Lim, K.O.; Carey, J.R.; Vitek, J.L.; et al. Neuromodulation for Brain Disorders: Challenges and Opportunities. IEEE Trans. Biomed. Eng. 2013, 60, 610. [Google Scholar] [CrossRef] [PubMed]
- Antal, A.; Luber, B.; Brem, A.K.; Bikson, M.; Brunoni, A.R.; Cohen Kadosh, R.; Dubljević, V.; Fecteau, S.; Ferreri, F.; Flöel, A.; et al. Non-Invasive Brain Stimulation and Neuroenhancement. Clin. Neurophysiol. Pract. 2022, 7, 146–165. [Google Scholar] [CrossRef]
- Terranova, C.; Rizzo, V.; Cacciola, A.; Chillemi, G.; Calamuneri, A.; Milardi, D.; Quartarone, A. Is There a Future for Non-Invasive Brain Stimulation as a Therapeutic Tool? Front. Neurol. 2019, 10, 1146. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Hallett, M.; Rossini, P.M.; Pascual-Leone, A.; Avanzini, G.; Bestmann, S.; Berardelli, A.; Brewer, C.; Canli, T.; Cantello, R.; et al. Safety, Ethical Considerations, and Application Guidelines for the Use of Transcranial Magnetic Stimulation in Clinical Practice and Research. Clin. Neurophysiol. 2009, 120, 2008–2039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| rsFC | Cluster Size | Peak T | Peak Coordinate | Identified Brain Regions | 10–20 EEG System Locations | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Brain regions from resting-state functional connectivity analysis * | |||||||
| Positive | 66 | 14.60 | −27 | 30 | −15 | L Orbitofrontal cortex | ~2 cm inferior to F7 |
| 181 | 13.66 | −39 | −69 | 33 | L Angular | ~3 cm inferior to P3 | |
| 44 | 10.69 | −18 | 30 | 45 | L Superior frontal gyrus | ~3 cm left posterior to Fz | |
| 194 | 13.07 | 45 | −66 | 30 | R Angular | ~3 cm inferior to P4 | |
| 280 | 16.39 | 63 | −6 | −24 | R Middle/Superior temporal gyrus | ~3 cm anterior and inferior to T4 | |
| 571 | 20.42 | −3 | 42 | −12 | Bil Medial prefrontal cortex | ~0.5 cm inferior to the midpoint Fp2–Fp1 | |
| Negative | 213 | −7.38 | −36 | 42 | 30 | L Middle frontal gyrus | ~1 cm anterior and inferior to F3 |
| 76 | −5.98 | −54 | 12 | 6 | L Inferior frontal gyrus | ~2 cm posterior to F7 | |
| 237 | −9.63 | −57 | −36 | 33 | L Supramarginal gyrus | ~midpoint to C3–T5 | |
| 221 | −8.80 | 15 | 9 | 69 | R Superior frontal gyrus/ Supplementary motor area | ~2 cm right anterior to Cz | |
| 435 | −9.77 | 63 | −36 | 36 | R Supramarginal gyrus | ~midpoint to C4–T6 | |
| 78 | −6.85 | 45 | 6 | 51 | R Middle frontal gyrus | ~2 cm anterior and inferior to C4 | |
| 176 | −7.99 | 57 | 15 | 9 | R Inferior frontal gyrus | ~2 cm posterior to F8 | |
| 354 | −9.06 | 30 | 54 | 27 | R Middle/superior frontal gyrus | ~1 cm posterior and superior to Fp2 | |
| 431 | −6.47 | 0 | −90 | 27 | L Cuneus/R Precuneus | ~3 cm inferior to Pz | |
| Overlapping brain regions from functional and anatomical connectivity analysis † | |||||||
| Positive | 46 | 10.22 | −30 | −75 | 42 | L Inferior parietal gyrus | ~1 cm posterior and inferior to P3 |
| 30 | 16.55 | −9 | 42 | −12 | L Medial prefrontal cortex | ~2 cm left inferior to Fp1 | |
| Negative | 289 | −6.47 | 0 | −90 | 27 | Bil Cuneus | ~3 cm right superior to O1 |
| 56 | −5.70 | 9 | −60 | 57 | R Precuneus | ~0.5 cm right inferior to Pz | |
| rsFC | Cluster Size | Peak T | Peak Coordinate | Identified Brain Regions | 10–20 EEG System Locations | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Brain regions from resting state functional connectivity analysis * | |||||||
| Positive | 131 | 11.90 | −60 | −9 | −18 | L Middle/Superior temporal gyrus | ~1 cm inferior to T3 |
| 100 | 12.21 | −42 | −69 | 33 | L Angular | ~2 cm inferior to P3 | |
| 68 | 12.62 | 21 | 27 | 48 | R Superior frontal gyrus | ~2 cm left posterior to F4 | |
| 224 | 14.01 | 48 | −63 | 36 | R Angular | ~2 cm anterior and inferior to P4 | |
| 694 | 20.39 | 63 | −6 | −21 | R Middle/Superior temporal gyrus | ~3 cm anterior and inferior to T4 | |
| 56 | 13.76 | 30 | 33 | −15 | R Orbitofrontal cortex | ~3 cm inferior to F8 | |
| 512 | 20.52 | 3 | 33 | −15 | Bil Medial prefrontal cortex | ~1 cm inferior to the midpoint Fp2–Fp1 | |
| Negative | 215 | −7.88 | −36 | 48 | 24 | L Middle frontal gyrus | ~midpoint to Fz–F7 |
| 134 | −7.70 | −45 | 18 | 3 | L Inferior frontal gyrus | ~1 cm posterior to F7 | |
| 260 | −10.64 | −54 | −42 | 36 | L Supramarginal gyrus | ~0.5 cm posterior to the midpoint C3–T5 | |
| 174 | −9.34 | 15 | 9 | 69 | R Superior frontal gyrus/ Supplementary motor area | ~junction of 1/3 and 2/3 Cz–F4 | |
| 275 | −9.03 | 63 | −39 | 36 | R Supramarginal gyrus | ~midpoint to C4–T6 | |
| 90 | −7.63 | 54 | 15 | 3 | R Inferior frontal gyrus | ~2 cm posterior and inferior to F8 | |
| 212 | −8.87 | 33 | 54 | 27 | R Middle frontal gyrus | ~midpoint to Fz–F8 | |
| 262 | −6.92 | 3 | −87 | 30 | Bil Cuneus | ~1 cm inferior to the midpoint of P3–P4 | |
| Overlapping brain regions from functional and anatomical connectivity analysis † | |||||||
| Positive | 38 | 6.58 | 45 | −45 | −15 | R Inferior temporal gyrus | ~3 cm anterior and inferior to T6 |
| 482 | 19.17 | 60 | −12 | −21 | R Middle temporal gyrus | ~3 cm anterior and inferior to T4 | |
| 51 | 12.61 | 36 | 33 | −12 | R Orbitofrontal cortex | ~3 cm inferior to F8 | |
| 33 | 14.64 | 9 | 42 | −15 | R Medial prefrontal cortex | ~1 cm right inferior to Fp2 | |
| Negative | 30 | −4.90 | −15 | −57 | 57 | L Precuneus | ~midpoint to Pz–P3 |
| 407 | −5.96 | 15 | −93 | 33 | R Superior occipital gyrus | ~midpoint to Pz–O2 | |
| rsFC | Cluster Size | Peak T | Peak Coordinate | Identified Brain Regions | 10–20 System Locations | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Brain regions from resting state functional connectivity analysis * | |||||||
| Positive | 717 | 25.43 | −27 | 3 | −21 | L Superior/Middle temporal gyrus | ~3 cm inferior and anterior to T3 |
| 414 | 15.67 | 27 | 6 | −21 | R Superior temporal gyrus | ~3 cm inferior and anterior to T4 | |
| 36 | 11.49 | 33 | 33 | −15 | R Orbitofrontal cortex | ~3 cm inferior to F8 | |
| 144 | 12.05 | −3 | 42 | −15 | Bil Medial prefrontal cortex | ~0.5 cm inferior to the midpoint of Fp1–Fp2 | |
| Negative | 158 | −6.02 | −33 | 54 | 12 | L Middle/Superior frontal gyrus | ~1 cm right superior to Fp1 |
| 46 | −5.17 | −39 | 39 | 30 | L Middle frontal gyrus | ~1 cm anterior and inferior to F3 | |
| 54 | −7.19 | 3 | 33 | 36 | Bil Medial superior frontal gyrus | ~1 cm posterior to Fz | |
| 156 | −7.39 | 21 | 15 | 63 | R Superior frontal gyrus | ~midpoint to F4–Cz | |
| 249 | −8.18 | 48 | −45 | 36 | R Supramarginal gyrus | ~midpoint to P4–T4 | |
| 658 | −8.78 | 33 | 57 | 21 | R Middle frontal gyrus | ~midpoint to F4–Fp2 | |
| 732 | −7.92 | 12 | −69 | 45 | R Precuneus | ~midpoint to Cz–O2 | |
| Overlapping brain regions from functional and anatomical connectivity analysis † | |||||||
| Positive | 66 | 11.29 | −3 | 45 | −15 | Bil Medial prefrontal cortex | ~1 cm inferior to the midpoint of Fp2–Fp1 |
| Negative | 211 | −7.44 | −3 | −75 | 48 | Bil Precuneus | ~midpoint to Cz–O1 |
| rsFC | Cluster Size | Peak T | Peak Coordinate | Identified Brain Regions | 10–20 System Locations | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Brain regions from resting state functional connectivity analysis * | |||||||
| Positive | 414 | 18.78 | −24 | 3 | −21 | L Superior/Middle temporal gyrus/Postcentral gyrus | ~3 cm inferior and anterior to T3 |
| 783 | 31.06 | 27 | 3 | −24 | R Superior/Middle temporal gyrus/Postcentral gyrus | ~3 cm inferior and anterior to T4 | |
| 148 | 13.34 | 3 | 48 | −12 | Bil Medial prefrontal cortex | ~1 cm inferior to the midpoint of Fp2–Fp1 | |
| Negative | 508 | −7.72 | −33 | 57 | 21 | L Middle/Superior frontal gyrus | ~midpoint to Fp1–F3 |
| 52 | −5.18 | −33 | 3 | 63 | L Middle/Superior frontal gyrus | ~1 cm anterior and superior to C3 | |
| 187 | −6.15 | −51 | −48 | 39 | L Inferior parietal gyrus | ~midpoint to C3–O1 | |
| 142 | −7.75 | 3 | 24 | 45 | Bil Supplementary motor area | ~midpoint to Fz–Cz | |
| 237 | −8.34 | 45 | −48 | 42 | R Inferior parietal gyrus | ~midpoint to C4–O2 | |
| 787 | −8.83 | 39 | 36 | 39 | R Middle/Superior frontal gyrus | ~close to F4 | |
| 469 | −8.81 | −3 | −75 | 54 | Bil Precuneus | ~1 cm left inferior to Pz | |
| Overlapping brain regions from functional and anatomical connectivity analysis † | |||||||
| Positive | 37 | 12.53 | 6 | 48 | −12 | R Medial prefrontal cortex | ~1 cm right inferior to Fp2 |
| Negative | 36 | −4.66 | 21 | 57 | −3 | R Orbitofrontal cortex | ~close to Fp2 |
| 216 | −7.78 | 12 | −72 | 45 | R Precuneus | ~midpoint to Cz–O2 | |
| rsFC | Cluster Size | Peak T | Peak Coordinate | Identified Brain Regions | 10–20 System Locations | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Brain regions from resting state functional connectivity analysis * | |||||||
| Positive | 108 | 8.54 | −48 | −21 | 6 | L Superior temporal gyrus | ~1 cm posterior and superior to T3 |
| 60 | 7.28 | 60 | −33 | 15 | R Superior temporal gyrus | ~2 cm posterior and superior to T4 | |
| 79 | 7.48 | 54 | 3 | −12 | R Middle temporal gyrus | ~3 cm anterior and inferior to T4 | |
| 771 | 16.50 | −9 | 42 | −6 | L Medial prefrontal cortex | ~1 cm left inferior to Fp1 | |
| 297 | 8.16 | 9 | −60 | 30 | R Precuneus | ~midpoint to Pz–O2 | |
| Negative | 768 | −7.21 | −48 | 42 | 3 | L Inferior/Middle frontal gyrus/Precentral gyrus | ~1 cm anterior to F7, on the line F7–Fp1 |
| 273 | −6.07 | −48 | −45 | 51 | L Inferior parietal gyrus | ~2 cm anterior to P3 | |
| 31 | −4.43 | −57 | −51 | −6 | L Inferior temporal gyrus | ~1 cm anterior and inferior to T5 | |
| 273 | −5.83 | 45 | 57 | −9 | R Inferior frontal gyrus | ~1 cm anterior to F8 | |
| Overlapping brain regions from functional and anatomical connectivity analysis † | |||||||
| Positive | 524 | 16.50 | −9 | 42 | −6 | Bil Medial prefrontal cortex | ~1 cm left anterior to Fp1 |
| Negative | − | − | − | − | − | − | − |
| rsFC | Cluster Size | Peak T | Peak Coordinate | Identified Brain Regions | 10–20 System Locations | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Brain regions from resting state functional connectivity analysis * | |||||||
| Positive | 34 | 7.10 | −24 | 42 | 39 | L Superior frontal gyrus | ~midpoint to F7–Fz |
| 147 | 8.77 | −60 | −21 | 9 | L Superior temporal gyrus | ~1 cm posterior and superior to T3 | |
| 383 | 7.59 | 57 | −15 | 6 | R Superior temporal gyrus | ~close to T4 | |
| 56 | 8.18 | 24 | 33 | −15 | R Orbitofrontal cortex | ~1 cm inferior to Fp2 | |
| 713 | 18.17 | −3 | 45 | −6 | Bil Medial prefrontal cortex | ~midpoint to Fp1–Fp2 | |
| Negative | 409 | −6.20 | −48 | 51 | −3 | L Middle/Inferior frontal gyrus | ~0.5 cm inferior to the midpoint of Fp1–F7 |
| 147 | −4.69 | −48 | −48 | 51 | L Inferior parietal gyrus | ~2 cm anterior to P3 | |
| 40 | −5.41 | −6 | 36 | 45 | L Medial superior frontal gyrus | ~1 cm left posterior to Fz | |
| 31 | −4.66 | 33 | 15 | 60 | R Middle frontal gyrus | ~midpoint to Fz–C4 | |
| 38 | −4.63 | 57 | 18 | 30 | R Inferior frontal gyrus | ~midpoint to C4–F8 | |
| Overlapping brain regions from functional and anatomical connectivity analysis † | |||||||
| Positive | 586 | 18.17 | −3 | 45 | −6 | Bil Medial prefrontal cortex | ~0.5 cm inferior to the midpoint of Fp2–Fp1 |
| Negative | − | − | − | − | − | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, Q.; Sacca, V.; Zhu, M.; Ursitti, A.K.; Kong, J. Anatomical and Functional Connectivity of Critical Deep Brain Structures and Their Potential Clinical Application in Brain Stimulation. J. Clin. Med. 2023, 12, 4426. https://doi.org/10.3390/jcm12134426
Kong Q, Sacca V, Zhu M, Ursitti AK, Kong J. Anatomical and Functional Connectivity of Critical Deep Brain Structures and Their Potential Clinical Application in Brain Stimulation. Journal of Clinical Medicine. 2023; 12(13):4426. https://doi.org/10.3390/jcm12134426
Chicago/Turabian StyleKong, Qiao, Valeria Sacca, Meixuan Zhu, Amy Katherine Ursitti, and Jian Kong. 2023. "Anatomical and Functional Connectivity of Critical Deep Brain Structures and Their Potential Clinical Application in Brain Stimulation" Journal of Clinical Medicine 12, no. 13: 4426. https://doi.org/10.3390/jcm12134426
APA StyleKong, Q., Sacca, V., Zhu, M., Ursitti, A. K., & Kong, J. (2023). Anatomical and Functional Connectivity of Critical Deep Brain Structures and Their Potential Clinical Application in Brain Stimulation. Journal of Clinical Medicine, 12(13), 4426. https://doi.org/10.3390/jcm12134426







