Prevalence of Culturable Bacteria and Yeasts in the Nasopharynx Microbiota during the Physiological Course of Pregnancy
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Laboratory Procedures
2.3. Statistical Analyses
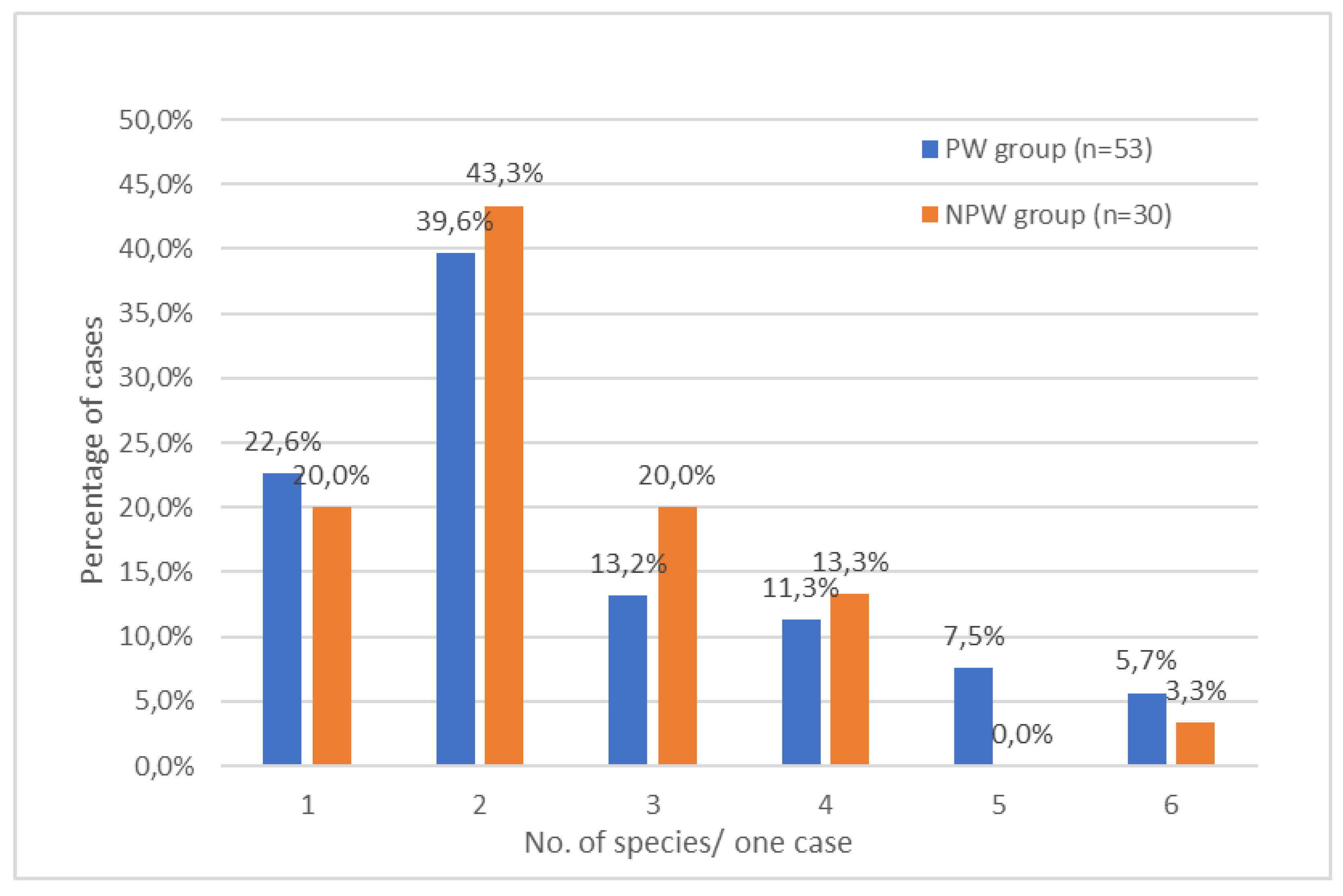
3. Results
3.1. Baseline Features of Participants
3.2. Patient Colonization with Opportunistic Microorganisms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hegewald, M.J.; Crapo, R.O. Respiratory physiology in pregnancy. Clin. Chest Med. 2011, 32, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Magon, N. Hormones in pregnancy. Niger. Med. J. 2012, 53, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Nuriel-Ohayon, M.; Neuman, H.; Koren, O. Microbial Changes during Pregnancy, Birth, and Infancy. Front. Microbiol. 2016, 7, 1031. [Google Scholar] [CrossRef] [PubMed]
- Morton, A. Physiological Changes and Cardiovascular Investigations in Pregnancy. Heart Lung Circ. 2021, 30, e6–e15. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, Z.Z.; Al-Rumaihi, S.; Al-Absi, R.S.; Farah, H.; Elamin, M.; Nader, R.; Bouabidi, S.; Suleiman, S.E.; Nasr, S.; Al-Asmakh, M. Physiological Changes and Interactions between Microbiome and the Host during Pregnancy. Front. Cell. Infect. Microbiol. 2022, 12, 824925. [Google Scholar] [CrossRef]
- Nuriel-Ohayon, M.; Neuman, H.; Ziv, O.; Belogolovski, A.; Barsheshet, Y.; Bloch, N.; Uzan, A.; Lahav, R.; Peretz, A.; Frishman, S.; et al. Progesterone Increases Bifidobacterium Relative Abundance during Late Pregnancy. Cell Rep. 2019, 27, 730–736.e3. [Google Scholar] [CrossRef] [PubMed]
- Amir, M.; Brown, J.A.; Rager, S.L.; Sanidad, K.Z.; Ananthanarayanan, A.; Zeng, M.Y. Maternal Microbiome and Infections in Pregnancy. Microorganisms 2020, 8, 1996. [Google Scholar] [CrossRef]
- Saadaoui, M.; Singh, P.; Al Khodor, S. Oral microbiome and pregnancy: A bidirectional relationship. J. Reprod. Immunol. 2021, 145, 103293. [Google Scholar] [CrossRef]
- Fujiwara, N.; Tsuruda, K.; Iwamoto, Y.; Kato, F.; Odaki, T.; Yamane, N.; Hori, Y.; Harashima, Y.; Sakoda, A.; Tagaya, A.; et al. Significant increase of oral bacteria in the early pregnancy period in Japanese women. J. Investig. Clin. Dent. 2017, 8, e12189. [Google Scholar] [CrossRef]
- Fuhler, G.M. The immune system and microbiome in pregnancy. Best Pract. Res. Clin. Gastroenterol. 2020, 44–45, 101671. [Google Scholar] [CrossRef]
- Edwards, S.M.; Cunningham, S.A.; Dunlop, A.L.; Corwin, E.J. The Maternal Gut Microbiome During Pregnancy. MCN. Am. J. Matern. Child Nurs. 2017, 42, 310–317. [Google Scholar] [CrossRef]
- Hezel, M.P.; Weitzberg, E. The oral microbiome and nitric oxide homoeostasis. Oral Dis. 2015, 21, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Kilian, M.; Chapple, I.L.C.; Hannig, M.; Marsh, P.D.; Meuric, V.; Pedersen, A.M.L.; Tonetti, M.S.; Wade, W.G.; Zaura, E. The oral microbiome—An update for oral healthcare professionals. Br. Dent. J. 2016, 221, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.B.; Rockwell, L.C.; Prioleau, M.D.; Goetzl, L. The role of the bacterial microbiota on reproductive and pregnancy health. Anaerobe 2016, 42, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Neuman, H.; Koren, O. The Pregnancy Microbiome. In Nestle Nutrition Institute Workshop Series; Karger Publishers: Basel, Switzerland, 2017; Volume 88, pp. 1–9. [Google Scholar] [CrossRef]
- Gomes, C.F.; Sousa, M.; Lourenço, I.; Martins, D.; Torres, J. Gastrointestinal diseases during pregnancy: What does the gastroenterologist need to know? Ann. Gastroenterol. 2018, 31, 385–394. [Google Scholar] [CrossRef]
- Rapone, B.; Ferrara, E.; Montemurro, N.; Converti, I.; Loverro, M.; Loverro, M.T.; Gnoni, A.; Scacco, S.; Siculella, L.; Corsalini, M.; et al. Oral Microbiome and Preterm Birth: Correlation or Coincidence? A Narrative Review. Open Access Maced. J. Med. Sci. 2020, 8, 123–132. [Google Scholar] [CrossRef]
- Chandra, H.; Sharma, K.K.; Tuovinen, O.H.; Sun, X.; Shukla, P. Pathobionts: Mechanisms of survival, expansion, and interaction with host with a focus on Clostridioides difficile. Gut Microbes 2021, 13, 1979882. [Google Scholar] [CrossRef]
- Bucka-Kolendo, J.; Sokołowska, B.; Winiarczyk, S. Influence of High Hydrostatic Pressure on the Identification of Lactobacillus by MALDI-TOF MS-Preliminary Study. Microorganisms 2020, 8, 813. [Google Scholar] [CrossRef]
- Dhiman, N.; Hall, L.; Wohlfiel, S.L.; Buckwalter, S.P.; Wengenack, N.L. Performance and cost analysis of matrix-assisted laser desorption ionization-time of flight mass spectrometry for routine identification of yeast. J. Clin. Microbiol. 2011, 49, 1614–1616. [Google Scholar] [CrossRef]
- Lain, K.Y.; Catalano, P.M. Metabolic changes in pregnancy. Clin. Obstet. Gynecol. 2007, 50, 938–948. [Google Scholar] [CrossRef]
- Wang, Q.; Würtz, P.; Auro, K.; Mäkinen, V.-P.; Kangas, A.J.; Soininen, P.; Tiainen, M.; Tynkkynen, T.; Jokelainen, J.; Santalahti, K.; et al. Metabolic profiling of pregnancy: Cross-sectional and longitudinal evidence. BMC Med. 2016, 14, 205. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.I.; Littman, D.R. Modulation of immune homeostasis by commensal bacteria. Curr. Opin. Microbiol. 2011, 14, 106–114. [Google Scholar] [CrossRef]
- Hui, A.W.-H.; Lau, H.-W.; Chan, T.H.-T.; Tsui, S.K.-W. The human microbiota: A new direction in the investigation of thoracic diseases. J. Thorac. Dis. 2013, 5 (Suppl. S2), S127–S131. [Google Scholar] [CrossRef] [PubMed]
- Purcell, P.; Jary, H.; Perry, A.; Perry, J.D.; Stewart, C.J.; Nelson, A.; Lanyon, C.; Smith, D.L.; Cummings, S.P.; De Soyza, A. Polymicrobial airway bacterial communities in adult bronchiectasis patients. BMC Microbiol. 2014, 14, 130. [Google Scholar] [CrossRef]
- Costello, E.K.; Lauber, C.L.; Hamady, M.; Fierer, N.; Gordon, J.I.; Knight, R. Bacterial community variation in human body habitats across space and time. Science 2009, 326, 1694–1697. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The Human Microbiome Project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef] [PubMed]
- The Human Microbiome Consortium Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [CrossRef]
- Arora, N.; Sadovsky, Y.; Dermody, T.S.; Coyne, C.B. Microbial Vertical Transmission during Human Pregnancy. Cell Host Microbe 2017, 21, 561–567. [Google Scholar] [CrossRef]
- Manti, S.; Leonardi, S.; Rezaee, F.; Harford, T.J.; Perez, M.K.; Piedimonte, G. Effects of Vertical Transmission of Respiratory Viruses to the Offspring. Front. Immunol. 2022, 13, 853009. [Google Scholar] [CrossRef]
- García-Gómez, E.; González-Pedrajo, B.; Camacho-Arroyo, I. Role of sex steroid hormones in bacterial-host interactions. Biomed Res. Int. 2013, 2013, 928290. [Google Scholar] [CrossRef]
- Kim, J.; Amar, S. Periodontal disease and systemic conditions: A bidirectional relationship. Odontology 2006, 94, 10–21. [Google Scholar] [CrossRef]
- Hornef, M. Pathogens, Commensal Symbionts, and Pathobionts: Discovery and Functional Effects on the Host. ILAR J. 2015, 56, 159–162. [Google Scholar] [CrossRef]
- Becker, K.; Heilmann, C.; Peters, G. Coagulase-negative staphylococci. Clin. Microbiol. Rev. 2014, 27, 870–926. [Google Scholar] [CrossRef]
- Michalik, M.; Samet, A.; Podbielska-Kubera, A.; Savini, V.; Międzobrodzki, J.; Kosecka-Strojek, M. Coagulase-negative staphylococci (CoNS) as a significant etiological factor of laryngological infections: A review. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 26. [Google Scholar] [CrossRef] [PubMed]
- Sigudu, T.T.; Oguttu, J.W.; Qekwana, D.N. Prevalence of Staphylococcus spp. from human specimens submitted to diagnostic laboratories in South Africa, 2012–2017. S. Afr. J. Infect. Dis. 2023, 38, 477. [Google Scholar] [CrossRef] [PubMed]
- Aubry, B.; Lemarié, C.; Chenouard, R.; Kempf, M.; Eveillard, M.; Pailhoriès, H. Performance of penicillinase detection tests in Staphylococcus epidermidis: Comparison of different phenotypic methods. BMC Microbiol. 2020, 20, 240. [Google Scholar] [CrossRef]
- Stratmann, J.A.; Lacko, R.; Ballo, O.; Shaid, S.; Gleiber, W.; Vehreschild, M.J.G.T.; Wichelhaus, T.; Reinheimer, C.; Göttig, S.; Kempf, V.A.J.; et al. Colonization with multi-drug-resistant organisms negatively impacts survival in patients with non-small cell lung cancer. PLoS ONE 2020, 15, e0242544. [Google Scholar] [CrossRef]
- França, A.; Gaio, V.; Lopes, N.; Melo, L.D.R. Virulence Factors in Coagulase-Negative Staphylococci. Pathogens 2021, 10, 170. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, J.; He, Y.; Lv, Z.; Liang, Z.; Chen, J.; Li, P.; Liu, J.; Yang, H.; Tao, A.; et al. Exploring the Role of Staphylococcus aureus in Inflammatory Diseases. Toxins 2022, 14, 464. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, T.; Lu, W.; Duan, X.; Luo, X.; Liu, S.; Chen, Y.; Li, Y.; Chen, J.; Liao, J.; et al. Altered Airway Microbiota Composition in Patients With Pulmonary Hypertension. Hypertension 2020, 76, 1589–1599. [Google Scholar] [CrossRef] [PubMed]
- Soares, L.C.; Pereira, I.A.; Pribul, B.R.; Oliva, M.S.; Coelho, S.M.O.; Souza, M.M.S. Antimicrobial resistance and detection of mecA and blaZ genes in coagulase-negative Staphylococcus isolated from bovine mastitis. Pesqui. Vet. Bras. 2012, 32, 692–696. [Google Scholar] [CrossRef]
- Biesbroek, G.; Tsivtsivadze, E.; Sanders, E.A.M.; Montijn, R.; Veenhoven, R.H.; Keijser, B.J.F.; Bogaert, D. Early respiratory microbiota composition determines bacterial succession patterns and respiratory health in children. Am. J. Respir. Crit. Care Med. 2014, 190, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, S.; Rivera-Hernandez, T.; Curren, B.F.; Harbison-Price, N.; De Oliveira, D.M.P.; Jespersen, M.G.; Davies, M.R.; Walker, M.J. Pathogenesis, epidemiology and control of Group A Streptococcus infection. Nat. Rev. Microbiol. 2023, 21, 431–447. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xu, Y.; Zhao, H.; Wang, X.; Rao, L.; Guo, Y.; Yi, X.; Hu, L.; Chen, S.; Han, L.; et al. Methicillin-resistant Staphylococcus aureus in China: A multicentre longitudinal study and whole-genome sequencing. Emerg. Microbes Infect. 2022, 11, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Truque, N.; Tedeschi, S.; Saye, E.J.; McKenna, B.D.; Langdon, W.; Wright, J.P.; Alsentzer, A.; Arnold, S.; Saville, B.R.; Wang, W.; et al. Relationship between maternal and neonatal Staphylococcus aureus colonization. Pediatrics 2012, 129, e1252-9. [Google Scholar] [CrossRef]
- Mishra, A.K.; Yadav, P.; Mishra, A. A Systemic Review on Staphylococcal Scalded Skin Syndrome (SSSS): A Rare and Critical Disease of Neonates. Open Microbiol. J. 2016, 10, 150–159. [Google Scholar] [CrossRef]
- Basavaraju, A.; Durga, S.V.; Vanitha, B. Variations in the oral anaerobic microbial flora in relation to pregnancy. J. Clin. Diagn. Res. 2012, 6, 1489–1491. [Google Scholar] [CrossRef]
- Borgo, P.V.; Rodrigues, V.A.A.; Feitosa, A.C.R.; Xavier, K.C.B.; Avila-Campos, M.J. Association between periodontal condition and subgingival microbiota in women during pregnancy: A longitudinal study. J. Appl. Oral Sci. 2014, 22, 528–533. [Google Scholar] [CrossRef]
- Kocur, M.; Kloos, W.E.; Schleifer, K.-H. The Genus Micrococcus BT—The Prokaryotes: Volume 3: Archaea. Bacteria: Firmicutes, Actinomycetes; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.-H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; pp. 961–971. ISBN 978-0-387-30743-5. [Google Scholar]
- Živković Zarić, R.S.; Pejčić, A.V.; Janković, S.M.; Kostić, M.J.; Milosavljević, M.N.; Milosavljević, M.J.; Opančina, V.D. Antimicrobial treatment of Kocuria kristinae invasive infections: Systematic review. J. Chemother. 2019, 31, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Rutsch, F.; Uekötter, A.; Kipp, F.; König, J.; Marquardt, T.; Peters, G.; von Eiff, C. Kocuria rhizophila adds to the emerging spectrum of micrococcal species involved in human infections. J. Clin. Microbiol. 2008, 46, 3537–3539. [Google Scholar] [CrossRef] [PubMed]
- Moissenet, D.; Becker, K.; Mérens, A.; Ferroni, A.; Dubern, B.; Vu-Thien, H. Persistent bloodstream infection with Kocuria rhizophila related to a damaged central catheter. J. Clin. Microbiol. 2012, 50, 1495–1498. [Google Scholar] [CrossRef] [PubMed]
- Kandi, V.; Palange, P.; Vaish, R.; Bhatti, A.B.; Kale, V.; Kandi, M.R.; Bhoomagiri, M.R. Emerging Bacterial Infection: Identification and Clinical Significance of Kocuria Species. Cureus 2016, 8, e731. [Google Scholar] [CrossRef] [PubMed]
- Corvec, S. Clinical and Biological Features of Cutibacterium (Formerly Propionibacterium) avidum, an Underrecognized Microorganism. Clin. Microbiol. Rev. 2018, 31, e00064-17. [Google Scholar] [CrossRef] [PubMed]
- Wanderer, J.P.; Leffert, L.R.; Mhyre, J.M.; Kuklina, E.V.; Callaghan, W.M.; Bateman, B.T. Epidemiology of obstetric-related ICU admissions in Maryland: 1999–2008. Crit. Care Med. 2013, 41, 1844–1852. [Google Scholar] [CrossRef]
- Karumanchi, S.A. Angiogenic factors in pre-eclampsia: Implications for clinical practice. BJOG 2018, 125, 1396. [Google Scholar] [CrossRef]
- Sagkrioti, M.; Glass, S.; Arealis, G. Evaluation of the effectiveness of skin preparation methods for the reduction of Cutibacterium acnes (formerly Propionibacterium acnes) in shoulder surgery: A systematic review. Shoulder Elb. 2022, 14, 583–597. [Google Scholar] [CrossRef]
- Olaniyi, K.S.; Moodley, J.; Mahabeer, Y.; Mackraj, I. Placental Microbial Colonization and Its Association With Pre-eclampsia. Front. Cell. Infect. Microbiol. 2020, 10, 413. [Google Scholar] [CrossRef]
- Michael, G.B.; Kadlec, K.; Sweeney, M.T.; Brzuszkiewicz, E.; Liesegang, H.; Daniel, R.; Murray, R.W.; Watts, J.L.; Schwarz, S. ICEPmu1, an integrative conjugative element (ICE) of Pasteurella multocida: Structure and transfer. J. Antimicrob. Chemother. 2012, 67, 91–100. [Google Scholar] [CrossRef]
- Adeolu, M.; Alnajar, S.; Naushad, S.; Gupta, R.S. Genome-based phylogeny and taxonomy of the “Enterobacteriales”: Proposal for Enterobacterales ord. nov. divided into the families Enterobacteriaceae, Erwiniaceae fam. nov., Pectobacteriaceae fam. nov., Yersiniaceae fam. nov., Hafniaceae fam. nov., Morgan. Int. J. Syst. Evol. Microbiol. 2016, 66, 5575–5599. [Google Scholar] [CrossRef]
- Rodríguez-Medina, N.; Barrios-Camacho, H.; Duran-Bedolla, J.; Garza-Ramos, U. Klebsiella variicola: An emerging pathogen in humans. Emerg. Microbes Infect. 2019, 8, 973–988. [Google Scholar] [CrossRef]
- Gomez-Arango, L.F.; Barrett, H.L.; McIntyre, H.D.; Callaway, L.K.; Morrison, M.; Nitert, M.D. Contributions of the maternal oral and gut microbiome to placental microbial colonization in overweight and obese pregnant women. Sci. Rep. 2017, 7, 2860. [Google Scholar] [CrossRef]
- Souto, R.; Silva-Boghossian, C.M.; Colombo, A.P.V. Prevalence of Pseudomonas aeruginosa and Acinetobacter spp. in subgingival biofilm and saliva of subjects with chronic periodontal infection. Braz. J. Microbiol. 2014, 45, 495–501. [Google Scholar] [CrossRef]
- Fechney, J.M.; Browne, G.V.; Prabhu, N.; Irinyi, L.; Meyer, W.; Hughes, T.; Bockmann, M.; Townsend, G.; Salehi, H.; Adler, C.J. Preliminary study of the oral mycobiome of children with and without dental caries. J. Oral Microbiol. 2019, 11, 1536182. [Google Scholar] [CrossRef] [PubMed]
- Rizzetto, L.; De Filippo, C.; Cavalieri, D. Richness and diversity of mammalian fungal communities shape innate and adaptive immunity in health and disease. Eur. J. Immunol. 2014, 44, 3166–3181. [Google Scholar] [CrossRef] [PubMed]
- Crovetto, F.; Selma-Royo, M.; Crispi, F.; Carbonetto, B.; Pascal, R.; Larroya, M.; Casas, I.; Tortajada, M.; Escudero, N.; Muñoz-Almagro, C.; et al. Nasopharyngeal Microbiota Profiling of Pregnant Women with SARS-CoV-2 Infection. Sci. Rep. 2022, 12, 13404. [Google Scholar] [CrossRef] [PubMed]
- Dutkiewicz, J.; Mackiewicz, B.; Kinga Lemieszek, M.; Golec, M.; Milanowski, J. Pantoea agglomerans: A mysterious bacterium of evil and good. Part III. Deleterious effects: Infections of humans, animals and plants. Ann. Agric. Environ. Med. 2016, 23, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Y.; Ascher-Landsberg, J.; Cohen, A.; Lessing, J.B.; Grisaru, D. The Role of C-Reactive Protein Measurement as a Diagnostic Aid in Early Pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 176, 64–67. [Google Scholar] [CrossRef]
- Pelzer, E.S.; Allan, J.A.; Theodoropoulos, C.; Ross, T.; Beagley, K.W.; Knox, C.L. Hormone-dependent bacterial growth, persistence and biofilm formation—A pilot study investigating human follicular fluid collected during IVF cycles. PLoS ONE 2012, 7, e49965. [Google Scholar] [CrossRef]
- Balan, P.; Brandt, B.W.; Chong, Y.S.; Crielaard, W.; Wong, M.L.; Lopez, V.; He, H.G.; Seneviratne, C.J. Subgingival Microbiota during Healthy Pregnancy and Pregnancy Gingivitis. JDR Clin. Transl. Res. 2021, 6, 343–351. [Google Scholar] [CrossRef]
- Kelley, S.T.; Liu, W.; Quintana, P.J.E.; Hoh, E.; Dodder, N.G.; Mahabee-Gittens, E.M.; Padilla, S.; Ogden, S.; Frenzel, S.; Sisk-Hackworth, L.; et al. Altered microbiomes in thirdhand smoke-exposed children and their home environments. Pediatr. Res. 2021, 90, 1153–1160. [Google Scholar] [CrossRef]
- Paropkari, A.D.; Leblebicioglu, B.; Christian, L.M.; Kumar, P.S. Smoking, pregnancy and the subgingival microbiome. Sci. Rep. 2016, 6, 30388. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, Y.; Romero, R.; Mele, L.; Wapner, R.J.; Iams, J.D.; Dudley, D.J.; Spong, C.Y.; Peaceman, A.M.; Leveno, K.J.; Harper, M.; et al. Maternal serum interleukin-6, C-reactive protein, and matrix metalloproteinase-9 concentrations as risk factors for preterm birth < 32 weeks and adverse neonatal outcomes. Am. J. Perinatol. 2010, 27, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Dodds, W.G.; Iams, J.D. Maternal C-reactive protein and preterm labor. J. Reprod. Med. 1987, 32, 527–530. [Google Scholar] [PubMed]
- Hirsch, W.; Koppitz, D.; Morack, G.; Gerhardt, C. C-reactive protein in the maternal serum and risk of fetal infection in premature rupture of the fetal membranes and threatened premature labor. Zentralbl. Gynakol. 1989, 111, 1411–1416. [Google Scholar] [PubMed]
- Bek, K.M.; Nielsen, F.R.; Qvist, I.; Rasmussen, P.E.; Tobiassen, M. C-reactive protein (CRP) and pregnancy. An early indicator of chorioamnionitis. A review. Eur. J. Obstet. Gynecol. Reprod. Biol. 1990, 35, 29–33. [Google Scholar] [CrossRef]
- Wirestam, L.; Pihl, S.; Saleh, M.; Wetterö, J.; Sjöwall, C. Plasma C-Reactive Protein and Pentraxin-3 Reference Intervals During Normal Pregnancy. Front. Immunol. 2021, 12, 722118. [Google Scholar] [CrossRef]
- Can, U.; Buyukinan, M.; Yerlikaya, F.H. Serum Levels of Soluble Urokinase Plasminogen Activator Receptor as a New Inflammatory Marker in Adolescent Obesity. Indian J. Med. Res. 2017, 145, 327–333. [Google Scholar] [CrossRef]
- Ridker, P.M. C-Reactive Protein and the Prediction of Cardiovascular Events among Those at Intermediate Risk: Moving an Inflammatory Hypothesis toward Consensus. J. Am. Coll. Cardiol. 2007, 49, 2129–2138. [Google Scholar] [CrossRef]
- Zhou, B.; Shu, B.; Yang, J.; Liu, J.; Xi, T.; Xing, Y. C-Reactive Protein, Interleukin-6 and the Risk of Colorectal Cancer: A Meta-Analysis. Cancer Causes Control 2014, 25, 1397–1405. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Heidari-Bakavoli, A.R.; Shoeibi, S.; Mirhafez, S.R.; Moohebati, M.; Esmaily, H.; Ghazavi, H.; Saberi Karimian, M.; Parizadeh, S.M.R.; Mohammadi, M.; et al. Association of Serum Hs-CRP Levels With the Presence of Obesity, Diabetes Mellitus, and Other Cardiovascular Risk Factors. J. Clin. Lab. Anal. 2016, 30, 672–676. [Google Scholar] [CrossRef]
- Fink, N.R.; Chawes, B.; Bønnelykke, K.; Thorsen, J.; Stokholm, J.; Rasmussen, M.A.; Brix, S.; Bisgaard, H. Levels of Systemic Low-Grade Inflammation in Pregnant Mothers and Their Offspring Are Correlated. Sci. Rep. 2019, 9, 3043. [Google Scholar] [CrossRef] [PubMed]
| No. (%) of Participants (n = 83) | |||||
|---|---|---|---|---|---|
| Parameters | PW Group (n = 53) | NPW Group (n = 30) | |||
| Age (years) | 19–45 | 53 (100.0) | 30 (100.0) | ||
| Age range | 19–35 | 48 (90.6) | 19 (63.3) | ||
| 36–45 | 5 (9.4) | 11 (36.7) | |||
| M ± SD | 29.68 ± 4.63 | 31.47 ± 8.64 | |||
| Min–Max (Me) | 19.0–41.0 (30.0) | 22.0–45.0 (29.0) | |||
| Place of residence | Village | 21 (39.6) | 5 (16.7) | ||
| City | 31 (58.5) | 25 (83.3) | |||
| No information (a) | 1 (1.9) | 0 (0.0) | |||
| Smoking status | |||||
| Smoking | Never | 33 (68.8) | 19 (79.2) | ||
| In the past | 13 (27.1) | 1 (4.2) | |||
| Current | 2 (4.2) | 4 (16.7) | |||
| No information (a) | 5 (9.4) | 6 (20.0) | |||
| Passive smoking | No | 44 (83.0) | 20 (66.7) | ||
| Yes | 9 (17.0) | 10 (33.3) | |||
| Pets | No | 26 (49.1) | 15 (50.0) | ||
| Yes | 27 (50.9) | 15 (50.0) | |||
| Pregnancy trimester | First | 5 (9.4) | |||
| Second | 10 (18.9) | ||||
| Third | 38 (71.7) | ||||
| Weeks of gestation | M ± SD | 29.30 ± 9.70 | |||
| Min–Max (Me) | 5.0–40.0 (33.0) | ||||
| Pregnancy | First | 20 (37.7) | |||
| Second | 14 (26.4) | ||||
| Third | 14 (26.4) | ||||
| Fourth | 5 (9.4) | ||||
| CRP (mg/L) (b) (n = 50) | CRP range | 0–5 | 30 (60.0) | ||
| 5.1–10 | 14 (28.0) | ||||
| > 10 | 6 (12.0) | ||||
| M ± SD | 4.64 ± 3.87 | ||||
| Min–Max (Me) | 0.04–13.83 (3.54) | ||||
| Microbial Colonizers | Number of Colonized Cases (n = 83) | ||
|---|---|---|---|
| PW Group (n = 53) | NPW Group (n = 30) | P | |
| Frequency of case colonized with various Gram-stain phenotypes of microorganisms | |||
| Only Gram-positive bacteria | 38 (71.7) | 19 (63.3) | 0.467 |
| Only Gram-negative bacteria | 3 (5.6) | 3 (10.0) | 0.662 |
| Gram-positive and Gram-negative bacteria | 10 (18.9) | 8 (26.7) | 0.420 |
| Gram-positive bacteria and yeasts* | 2 (3.8) | 0 (0.0) | 0.533 |
| Frequency of case colonized with selected groups of opportunistic pathogens | |||
| Gram-positive bacteria | |||
| Family: Staphylococcaceae | 48 (90.6) | 27 (90.0) | 1.000 |
| Family: Streptococcaceae | 2 (3.8) | 4 (13.3) | 0.182 |
| Other Gram-positive | 26 (49.1) | 14 (46.7) | 1.000 |
| Gram-negative bacteria | |||
| Order: Enterobacterales | 7 (13.2) | 6 (20.0) | 0.532 |
| Enterobacteriaceae | 4 (7.5) | 5 (16.7) | 0.273 |
| Morganellaceae | 1 (1.9) | 0 (0.0) | 1.000 |
| Erwiniaceae | 2 (3.8) | 1 (3.3) | 1.000 |
| Order: Pseudomonadales | 4 (7.5) | 2 (6.7) | 1.000 |
| Other Gram-negative | 5 (9.4) | 2 (6.7) | 1.000 |
| Yeasts | |||
| Rhodotorula spp. | 2 (3.8) | 0 (0.0) | 0.146 |
| Family | Species | Number (%) of Colonized Cases | χ2 | p | |
|---|---|---|---|---|---|
| PW Group (n = 53) | NPW Group (n = 30) | ||||
| Gram-positive bacteria | |||||
| Staphylococcaceae | Staphylococcus aureus | 14 (26.4) | 7 (23.3) | 0.096 | 0.756 |
| Staphylococcus epidermidis | 42 (79.2) | 21 (70.0) | 0.895 | 0.344 | |
| Staphylococcus haemolyticus | 3 (5.7) | 4 (13.3) | 1.460 | 0.227 | |
| Staphylococcus warneri | 3 (5.7) | 4 (13.3) | 1.460 | 0.227 | |
| Staphylococcus hominis | 6 (11.3) | 1 (3.3) | 1.583 | 0.208 | |
| Staphylococcus pasteuri | 1 (1.9) | 1 (3.3) | 0.170 | 0.680 | |
| Staphylococcus lugduensis | 0 (0.0) | 1 (3.3) | 1.788 | 0.181 | |
| Staphylococcus capitis | 2 (3.8) | 0 (0.0) | 1.160 | 0.281 | |
| Staphylococcus cohnii | 1 (1.9) | 0 (0.0) | 0.573 | 0.449 | |
| Staphylococcus saprophyticus | 3 (5.7) | 0 (0.0) | 1.762 | 0.184 | |
| Staphylococcus simulans | 1 (1.9) | 0 (0.0) | 0.573 | 0.449 | |
| Gemella haemolysans | 1 (1.9) | 0 (0.0) | 0.573 | 0.449 | |
| Streptococcaceae | Streptococcus pneumoniae | 1 (1.9) | 0 (0.0) | 0.573 | 0.449 |
| Streptococcus mitis | 1 (1.9) | 0 (0.0) | 0.573 | 0.449 | |
| Streptococcus parasanguinis | 1 (1.9) | 3 (10.0) | 2.749 | 0.097 | |
| Streptococcus salivarius | 0 (0.0) | 1 (3.3) | 1.788 | 0.181 | |
| Enterococcaceae | Enterococcus faecalis | 1 (1.9) | 1 (3.3) | 0.170 | 0.680 |
| Micrococcaceae | Micrococcus luteus | 4 (7.5) | 2 (6.7) | 0.022 | 0.882 |
| Kocuria rhizophila | 1 (1.9) | 0 (0.0) | 0.573 | 0.449 | |
| Kocuria palustris | 0 (0.0) | 1 (3.3) | 1.788 | 0.181 | |
| Corynebacteriaceae | Corynebacterium pseudodiphtheriticum | 2 (3.8) | 1 (3.3) | 0.011 | 0.918 |
| Corynebacterium accolens | 8 (15.1) | 7 (23.3) | 0.878 | 0.349 | |
| Corynebacterium tuberculostearicum | 3 (5.7) | 3 (10.0) | 0.538 | 0.463 | |
| Corynebacterium propinquum | 3 (5.7) | 1 (3.3) | 0.226 | 0.634 | |
| Corynebacterium amycolatum | 2 (3.8) | 0 (0.0) | 1.160 | 0.281 | |
| Bacillaceae | Bacillus cereus | 8 (15.1) | 1 (3.3) | 2.741 | 0.098 |
| Bacillus pumilus | 2 (3.8) | 0 (0.0) | 1.16 | 0.281 | |
| Lactobacillaceae | Lactobacillus salivarius | 0 (0.0) | 1 (3.3) | 1.788 | 0.181 |
| Paenibacillaceae | Paenibacillus spp. | 1 (1.9) | 0 (0.0) | 0.573 | 0.449 |
| Propionibacteriaceae | Cutibacterium granulosum | 1 (1.9) | 0 (0.0) | 0.573 | 0.449 |
| Cutibacterium avidum | 2 (3.8) | 0 (0.0) | 1.160 | 0.281 | |
| Cutibacterium acnes | 1 (1.9) | 0 (0.0) | 0.573 | 0.449 | |
| Gram-negative bacteria | |||||
| Enterobacteriaceae | Citrobacter koseri | 1 (1.9) | 1 (3.3) | 0.170 | 0.680 |
| Escherichia coli | 1 (1.9) | 1 (3.3) | 0.170 | 0.680 | |
| Klebsiella variicola | 1 (1.9) | 0 (0.0) | 0.584 | 0.445 | |
| Klebsiella oxytoca | 1 (1.9) | 0 (0.0) | 0.573 | 0.449 | |
| Enterobacter aerogenes | 0 (0.0) | 3 (10.0) | 5.499 | 0.019 * | |
| Raoultella ornithinolytica | 0 (0.0) | 1 (3.3) | 1.788 | 0.181 | |
| Pantoea agglomerans | 2 (3.8) | 0 (0.0) | 1.160 | 0.281 | |
| Pantoea septica | 0 (0.0) | 1 (3.3) | 1.788 | 0.181 | |
| Morganellaceae | Proteus mirabilis | 1 (1.9) | 0 (0.0) | 0.573 | 0.449 |
| Xantomonadaceae | Stenotrophomonas maltophilia | 1 (1.9) | 1 (3.3) | 0.170 | 0.680 |
| Pseudomonadaceae | Pseudomonas aeruginosa | 1 (1.9) | 0 (0.0) | 0.573 | 0.449 |
| Pseudomonas congelans | 1 (1.9) | 0 (0.0) | 0.573 | 0.449 | |
| Pseudomonas spp. | 1 (1.9) | 0 (0.0) | 0.584 | 0.445 | |
| Pasteurellaceae | Haemophilus influenzae | 1 (1.9) | 0 (0.0) | 0.573 | 0.449 |
| Haemophilus parainfluenzae | 3 (5.7) | 1 (3.3) | 0.226 | 0.634 | |
| Moraxellaceae | Acinetobacter tandoii | 0 (0.0) | 1 (3.3) | 1.788 | 0.181 |
| Moraxella_sg_Branhamella catarrhalis | 1 (1.9) | 0 (0.0) | 0.573 | 0.449 | |
| Neisseriaceae | Neisseria mucosa | 0 (0.0) | 1 (3.3) | 1.788 | 0.181 |
| Yeasts | |||||
| Sporidiobolaceae | Rhodotorula mucilaginosa | 1 (1.9) | 0 (0.0) | 0.573 | 0.449 |
| Rhodotorula minuta | 1 (1.9) | 0 (0.0) | 0.573 | 0.449 | |
| Group | Number of Staphylococcus Species per Sample | Staphylococcus Species | Number (%) of Colonized Cases | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CoPS | CoNS | ||||||||||||
| S. aureus | S. epidermidis | S. hominis | S. warneri | S. capitis | S. saprophyticus | S. haemolyticus | S. pasteuri | S. simulans | S. cohnii | S. lugduensis | |||
| PW (n = 53) | 1 | - | - | - | - | - | - | - | - | - | - | 5 (9.4) | |
| - | - | - | - | - | - | - | - | - | - | 22 (41.5) | |||
| 2 | - | - | - | - | - | - | - | - | - | 6 (11.3) | |||
| - | - | - | - | - | - | - | - | - | 4 (7.5) | ||||
| - | - | - | - | - | - | - | - | - | 1 (1.9) | ||||
| - | - | - | - | - | - | - | - | - | 2 (3.8) | ||||
| - | - | - | - | - | - | - | - | - | 2 (3.8) | ||||
| - | - | - | - | - | - | - | - | - | 1 (1.9) | ||||
| 3 | - | - | - | - | - | - | - | - | 1 (1.9) | ||||
| - | - | - | - | - | - | - | - | 1 (1.9) | |||||
| - | - | - | - | - | - | - | - | 1 (1.9) | |||||
| - | - | - | - | - | - | - | - | 1 (1.9) | |||||
| 5 | - | - | - | - | - | - | 1 (1.9) | ||||||
| NPW (n = 30) | 1 | - | - | - | - | - | - | - | - | - | - | 2 (6.7) | |
| - | - | - | - | - | - | - | - | - | - | - | 9 (30.0) | ||
| - | - | - | - | - | - | - | - | - | - | 2 (6.7) | |||
| - | - | - | - | - | - | - | - | - | - | 1 (3.3) | |||
| - | - | - | - | - | - | - | - | - | - | 1 (3.3) | |||
| 2 | - | - | - | - | - | - | - | - | - | 2 (6.7) | |||
| - | - | - | - | - | - | - | - | - | 1 (3.3) | ||||
| - | - | - | - | - | - | - | - | - | 2 (6.7) | ||||
| - | - | - | - | - | - | - | - | - | 3 (10.0) | ||||
| - | - | - | - | - | - | - | - | - | 1 (3.3) | ||||
| - | - | - | - | - | - | - | - | - | 1 (3.3) | ||||
| 3 | - | - | - | - | - | - | - | - | 2 (6.7) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosikowska, U.; Dłuski, D.F.; Pietras-Ożga, D.; Leszczyńska-Gorzelak, B.; Andrzejczuk, S. Prevalence of Culturable Bacteria and Yeasts in the Nasopharynx Microbiota during the Physiological Course of Pregnancy. J. Clin. Med. 2023, 12, 4447. https://doi.org/10.3390/jcm12134447
Kosikowska U, Dłuski DF, Pietras-Ożga D, Leszczyńska-Gorzelak B, Andrzejczuk S. Prevalence of Culturable Bacteria and Yeasts in the Nasopharynx Microbiota during the Physiological Course of Pregnancy. Journal of Clinical Medicine. 2023; 12(13):4447. https://doi.org/10.3390/jcm12134447
Chicago/Turabian StyleKosikowska, Urszula, Dominik Franciszek Dłuski, Dorota Pietras-Ożga, Bożena Leszczyńska-Gorzelak, and Sylwia Andrzejczuk. 2023. "Prevalence of Culturable Bacteria and Yeasts in the Nasopharynx Microbiota during the Physiological Course of Pregnancy" Journal of Clinical Medicine 12, no. 13: 4447. https://doi.org/10.3390/jcm12134447
APA StyleKosikowska, U., Dłuski, D. F., Pietras-Ożga, D., Leszczyńska-Gorzelak, B., & Andrzejczuk, S. (2023). Prevalence of Culturable Bacteria and Yeasts in the Nasopharynx Microbiota during the Physiological Course of Pregnancy. Journal of Clinical Medicine, 12(13), 4447. https://doi.org/10.3390/jcm12134447








