Long-Term Outcomes after Multimodal Treatment for Clival Chordoma: Efficacy of the Endonasal Transclival Approach with Early Adjuvant Radiation Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population and Treatment Modalities
2.2. Surgical Strategy
2.3. Endonasal Transclival Approach
2.4. Statistical Analysis
3. Results
3.1. Tumor Locations and Invasion of Critical Structures
3.2. Overall Outcomes
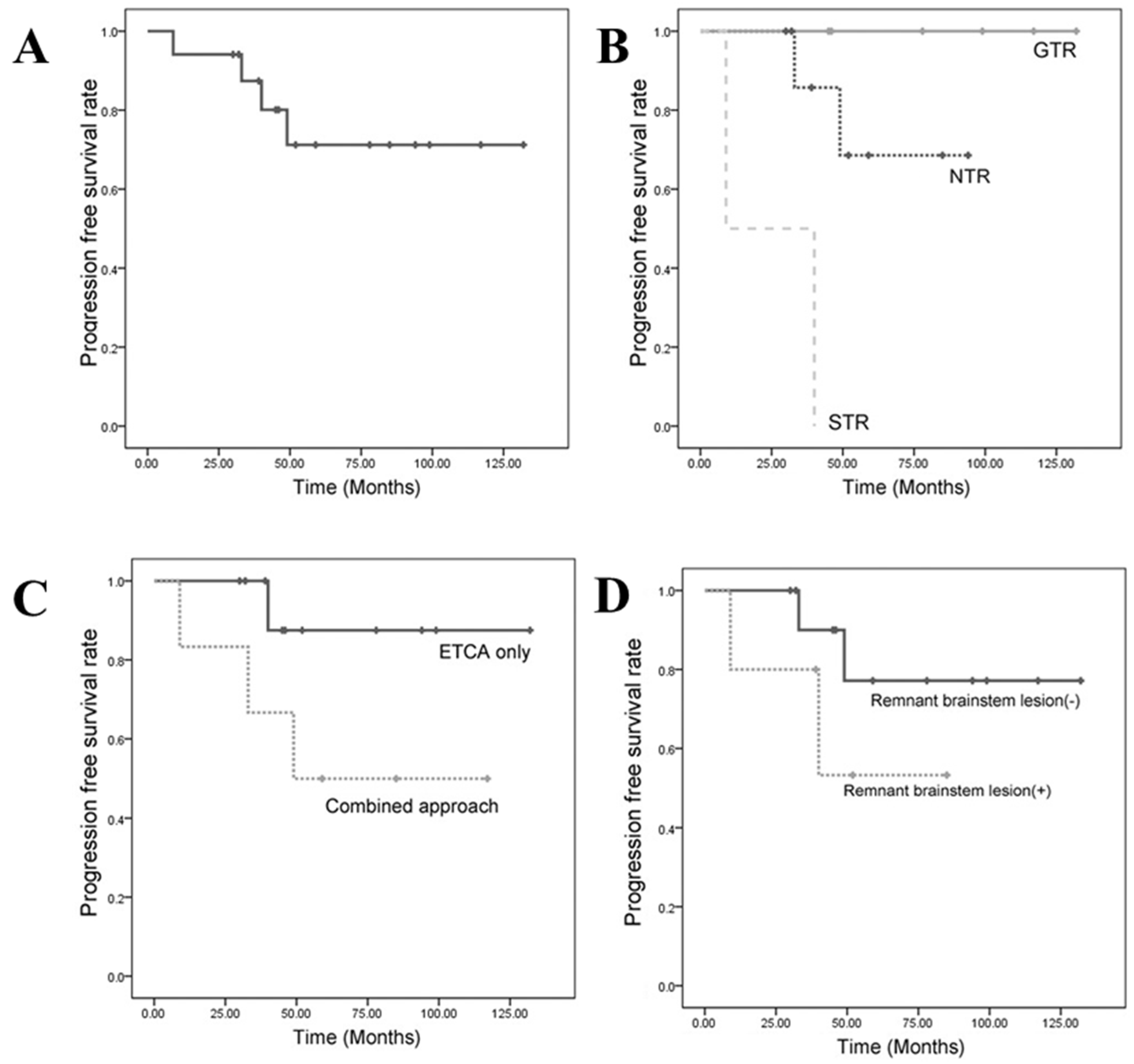
3.3. Progression-Free Survival (PFS)
3.3.1. PFS Based on the Extent of Resection
3.3.2. Progression-Free Survival Based on the Surgical Approach
3.3.3. Progression-Free Survival Based on a Remnant Brainstem Lesion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erdem, E.; Angtuaco, E.C.; Van Hemert, R.; Park, J.S.; Al-Mefty, O. Comprehensive review of intracranial chordoma. Radiographics 2003, 23, 995–1009. [Google Scholar] [CrossRef] [Green Version]
- Pallini, R.; Maira, G.; Pierconti, F.; Falchetti, M.L.; Alvino, E.; Cimino-Reale, G.; Fernandez, E.; D’Ambrosio, E.; Larocca, L.M. Chordoma of the skull base: Predictors of tumor recurrence. J. Neurosurg. 2003, 98, 812–822. [Google Scholar] [CrossRef] [Green Version]
- Yoneoka, Y.; Tsumanuma, I.; Fukuda, M.; Tamura, T.; Morii, K.; Tanaka, R.; Fujii, Y. Cranial base chordoma—Long term outcome and review of the literature. Acta Neurochir. 2008, 150, 773–778. [Google Scholar] [CrossRef]
- Ito, E.; Saito, K.; Okada, T.; Nagatani, T.; Nagasaka, T. Long-term control of clival chordoma with initial aggressive surgical resection and gamma knife radiosurgery for recurrence. Acta Neurochir. 2010, 152, 57–67. [Google Scholar] [CrossRef]
- Di Maio, S.; Temkin, N.; Ramanathan, D.; Sekhar, L.N. Current comprehensive management of cranial base chordomas: 10-year meta-analysis of observational studies. J. Neurosurg. 2011, 115, 1094–1105. [Google Scholar] [CrossRef]
- Walcott, B.P.; Nahed, B.V.; Mohyeldin, A.; Coumans, J.V.; Kahle, K.T.; Ferreira, M.J. Chordoma: Current concepts, management, and future directions. Lancet Oncol. 2012, 13, e69–e76. [Google Scholar] [CrossRef]
- Fraser, J.F.; Nyquist, G.G.; Moore, N.; Anand, V.K.; Schwartz, T.H. Endoscopic endonasal transclival resection of chordomas: Operative technique, clinical outcome, and review of the literature. J. Neurosurg. 2010, 112, 1061–1069. [Google Scholar] [CrossRef]
- Crockard, H.A.; Steel, T.; Plowman, N.; Singh, A.; Crossman, J.; Revesz, T.; Holton, J.L.; Cheeseman, A. A multidisciplinary team approach to skull base chordomas. J. Neurosurg. 2001, 95, 175–183. [Google Scholar] [CrossRef] [Green Version]
- Sen, C.; Triana, A. Cranial chordomas: Results of radical excision. Neurosurg. Focus 2001, 10, E3. [Google Scholar] [CrossRef]
- Tzortzidis, F.; Elahi, F.; Wright, D.; Natarajan, S.K.; Sekhar, L.N. Patient outcome at long-term follow-up after aggressive microsurgical resection of cranial base chordomas. Neurosurgery 2006, 59, 230–237. [Google Scholar] [CrossRef]
- Carrabba, G.; Dehdashti, A.R.; Gentili, F. Surgery for clival lesions: Open resection versus the expanded endoscopic endonasal approach. Neurosurg. Focus 2008, 25, E7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatemi, N.; Dusick, J.R.; Gorgulho, A.A.; Mattozo, C.A.; Moftakhar, P.; De Salles, A.A.; Kelly, D.F. Endonasal microscopic removal of clival chordomas. Surg. Neurol. 2008, 69, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Stippler, M.; Gardner, P.A.; Snyderman, C.H.; Carrau, R.L.; Prevedello, D.M.; Kassam, A.B. Endoscopic endonasal approach for clival chordomas. Neurosurgery 2009, 64, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Koutourousiou, M.; Gardner, P.A.; Tormenti, M.J.; Henry, S.L.; Stefko, S.T.; Kassam, A.B.; Fernandez-Miranda, J.C.; Snyderman, C.H. Endoscopic endonasal approach for resection of cranial base chordomas: Outcomes and learning curve. Neurosurgery 2012, 71, 614–624. [Google Scholar] [CrossRef]
- Fraser, J.F.; Nyquist, G.G.; Moore, N.; Anand, V.K.; Schwartz, T.H. Endoscopic endonasal minimal access approach to the clivus: Case series and technical nuances. Neurosurgery 2010, 67, ons150–ons158. [Google Scholar] [CrossRef]
- Dehdashti, A.R.; Karabatsou, K.; Ganna, A.; Witterick, I.; Gentili, F. Expanded endoscopic endonasal approach for treatment of clival chordomas: Early results in 12 patients. Neurosurgery 2008, 63, 299–307. [Google Scholar] [CrossRef]
- Hsu, W.; Kosztowski, T.A.; Zaidi, H.A.; Gokaslan, Z.L.; Wolinsky, J.P. Image-guided, endoscopic, transcervical resection of cervical chordoma. J. Neurosurg. Spine 2010, 12, 431–435. [Google Scholar] [CrossRef]
- Kano, H.; Iqbal, F.O.; Sheehan, J.; Mathieu, D.; Seymour, Z.A.; Niranjan, A.; Flickinger, J.C.; Kondziolka, D.; Pollock, B.E.; Rosseau, G.; et al. Stereotactic radiosurgery for chordoma: A report from the North American Gamma Knife Consortium. Neurosurgery 2011, 68, 379–389. [Google Scholar] [CrossRef]
- Takahashi, S.; Kawase, T.; Yoshida, K.; Hasegawa, A.; Mizoe, J.E. Skull base chordomas: Efficacy of surgery followed by carbon ion radiotherapy. Acta Neurochir. 2009, 151, 759–769. [Google Scholar] [CrossRef]
- Amichetti, M.; Amelio, D.; Minniti, G. Radiosurgery with photons or protons for benign and malignant tumours of the skull base: A review. Radiat. Oncol. 2012, 7, 210. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, S.; Foote, R.L.; Brown, P.D.; Pollock, B.E.; Link, M.J.; Garces, Y.I. Radiosurgery for cranial base chordomas and chondrosarcomas. Neurosurgery 2005, 56, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Bugoci, D.M.; Girvigian, M.R.; Chen, J.C.; Miller, M.M.; Rahimian, J. Photon-based fractionated stereotactic radiotherapy for postoperative treatment of skull base chordomas. Am. J. Clin. Oncol. 2013, 36, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Colli, B.; Al-Mefty, O. Chordomas of the craniocervical junction: Follow-up review and prognostic factors. J. Neurosurg. 2001, 95, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Crockard, H.A.; Cheeseman, A.; Steel, T.; Revesz, T.; Holton, J.L.; Plowman, N.; Singh, A.; Crossman, J. A multidisciplinary team approach to skull base chondrosarcomas. J. Neurosurg. 2001, 95, 184–189. [Google Scholar] [CrossRef] [Green Version]
- Sen, C.; Triana, A.I.; Berglind, N.; Godbold, J.; Shrivastava, R.K. Clival chordomas: Clinical management, results, and complications in 71 patients. J. Neurosurg. 2010, 113, 1059–1071. [Google Scholar] [CrossRef]
- Gagliardi, F.; Boari, N.; Riva, P.; Mortini, P. Current therapeutic options and novel molecular markers in skull base chordomas. Neurosurg. Rev. 2012, 35, 1–14. [Google Scholar] [CrossRef]
- Koutourousiou, M.; Snyderman, C.H.; Fernandez-Miranda, J.; Gardner, P.A. Skull base chordomas. Otolaryngol. Clin. N. Am. 2011, 44, 1155–1171. [Google Scholar] [CrossRef]
- Varga, P.P.; Szoverfi, Z.; Fisher, C.G.; Boriani, S.; Gokaslan, Z.L.; Dekutoski, M.B.; Chou, D.; Quraishi, N.A.; Reynolds, J.J.; Luzzati, A.; et al. Surgical treatment of sacral chordoma: Prognostic variables for local recurrence and overall survival. Eur. Spine J. 2015, 24, 1092–1101. [Google Scholar] [CrossRef]
- Asano, S.; Kawahara, N.; Kirino, T. Intradural spinal seeding of a clival chordoma. Acta Neurochir. 2003, 145, 599–603. [Google Scholar] [CrossRef]
- Nishigaya, K.; Kaneko, M.; Ohashi, Y.; Nukui, H. Intradural retroclival chordoma without bone involvement: No tumor regrowth 5 years after operation: Case report. J. Neurosurg. 1998, 88, 764–768. [Google Scholar] [CrossRef]
- Roberti, F.; Sekhar, L.N.; Jones, R.V.; Wright, D.C. Intradural cranial chordoma: A rare presentation of an uncommon tumor: Surgical experience and review of the literature. J. Neurosurg. 2007, 106, 270–274. [Google Scholar] [CrossRef]
- Ito, E.; Saito, K.; Nagatani, T.; Ishiyama, J.; Terada, K.; Yoshida, M.; Wakabayashi, T. Intradural cranial chordoma. World Neurosurg. 2010, 73, 194–197. [Google Scholar] [CrossRef]
- Bhat, D.I.; Yasha, M.; Rojin, A.; Sampath, S.; Shankar, S.K. Intradural clival chordoma: A rare pathological entity. J. Neuro-Oncol. 2010, 96, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Warnick, R.E.; Raisanen, J.; Kaczmar, T., Jr.; Davis, R.L.; Prados, M.D. Intradural chordoma of the tentorium cerebelli: Case report. J. Neurosurg. 1991, 74, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Frank, G.; Sciarretta, V.; Calbucci, F.; Farneti, G.; Mazzatenta, D.; Pasquini, E. The endoscopic transnasal transsphenoidal approach for the treatment of cranial base chordomas and chondrosarcomas. Neurosurgery 2006, 59, ONS50–ONS57. [Google Scholar] [CrossRef]
- Cai, R.; Barnett, G.H.; Novak, E.; Chao, S.T.; Suh, J.H. Principal risk of peritumoral edema after stereotactic radiosurgery for intracranial meningioma is tumor-brain contact interface area. Neurosurgery 2010, 66, 513–522. [Google Scholar] [CrossRef] [Green Version]
- Martin, M.P.; Olson, S. Intradural drop metastasis of a clival chordoma. J. Clin. Neurosci. 2009, 16, 1105–1107. [Google Scholar] [CrossRef] [PubMed]
- Arnautovic, K.I.; Al-Mefty, O. Surgical seeding of chordomas. J. Neurosurg. 2001, 95, 798–803. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.K.; Murata, H.; Draf, W. Clivus chordoma: Is it enough to image the primary site? Skull Base 2010, 20, 111–113. [Google Scholar] [CrossRef] [Green Version]




| Case | Age /Sex | F/U (Mos) | MTV (cm3) | Level | Preoperative Status | Strategy | EOR | Postoperative Status | Adjuvant RTx | Disease Progression |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 49/F | 46 | 1.76 | I | Diplopia | ETCA | GTR | No symptom | Stable | |
| 2 | 37/F | 45 | 0.84 | I | Diplopia | ETCA | GTR | No symptom | Stable | |
| 3 | 59/F | 132 | 1.00 | I | Diplopia | ETCA | GTR | No symptom | Stable | |
| 4 | 49/F | 30 | 5.65 | I | Headache, regrowth after TSA | ETCA | STR | No symptom Transient DI (resolved) | PBRT | Stable |
| 5 | 12/F | 78 | 5.34 | II | Headache | ETCA | GTR | No symptom | Stable | |
| 6 | 47/F | 36 | 5.67 | II | Regrowth after previous GKRS quadriparesis | ETCA | GTR | Remained quadriparesis | Stable | |
| 7 | 4/M | 94 | 5.76 | II | Diplopia | ETCA | NTR | No symptom | PBRT | Stable |
| 8 | 43/M | 32 | 25.1 | II | Diplopia | ETCA | NTR | No symptom | GKRS | Stable |
| 9 | 32/M | 52 | 22.95 | II | Dysequilibrium | ETCA | NTR | No symptom CSF leak (resolved) | PBRT | Stable |
| 10 | 42/F | 39 | 66.8 | III | Regrowth after previous surgery | ETCA | NTR | No symptom Transient facial palsy (resolved) | PBRT | Stable |
| 11 | 57/F | 117 | 26.4 | III | Trigeminal neuralgia | ETCA + TCSA | GTR | No symptom | Stable | |
| 12 | 48/F | 59 | 10.1 | III | Diplopia | ETCA + TCSA | NTR | No symptom | PBRT | Stable |
| 13 | 51/F | 85 | 48.7 | III | Diplopia | ETCA + APTA | NTR | No symptom | PBRT | Stable |
| 14 | 48/M | 61 | 9.37 | III | Regrowth after previous GKRS ophthalmoplegia | ETCA + TCSA | NTR | Remained ophthalmoplegia | GKRS | Regrowth at 49 months → Reoperation |
| 15 | 34/F | 117 | 10.2 | III | Diplopia, trigeminal neuralgia | ATPA | NTR | No symptom | GKRS | Regrowth at 33 months → GKRS |
| 16 | 35/F | 9 | 10.3 | III | Altered mental status | ATPA | STR | Unchanged | Death at 9 months | |
| 17 | 13/F | 62 | 98.75 | IV | Dysphagia, dysarthria | ETCA | STR | No symptom | GKRS | Regrowth at 40 months → Reoperation + GKRS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, H.D.; Chung, J.C.; Park, K.S.; Chung, S.Y.; Park, M.S.; Ryu, S.; Kim, S.M. Long-Term Outcomes after Multimodal Treatment for Clival Chordoma: Efficacy of the Endonasal Transclival Approach with Early Adjuvant Radiation Therapy. J. Clin. Med. 2023, 12, 4460. https://doi.org/10.3390/jcm12134460
Yoo HD, Chung JC, Park KS, Chung SY, Park MS, Ryu S, Kim SM. Long-Term Outcomes after Multimodal Treatment for Clival Chordoma: Efficacy of the Endonasal Transclival Approach with Early Adjuvant Radiation Therapy. Journal of Clinical Medicine. 2023; 12(13):4460. https://doi.org/10.3390/jcm12134460
Chicago/Turabian StyleYoo, Hyun Dong, Jong Chul Chung, Ki Seok Park, Seung Young Chung, Moon Sun Park, Seungjun Ryu, and Seong Min Kim. 2023. "Long-Term Outcomes after Multimodal Treatment for Clival Chordoma: Efficacy of the Endonasal Transclival Approach with Early Adjuvant Radiation Therapy" Journal of Clinical Medicine 12, no. 13: 4460. https://doi.org/10.3390/jcm12134460
APA StyleYoo, H. D., Chung, J. C., Park, K. S., Chung, S. Y., Park, M. S., Ryu, S., & Kim, S. M. (2023). Long-Term Outcomes after Multimodal Treatment for Clival Chordoma: Efficacy of the Endonasal Transclival Approach with Early Adjuvant Radiation Therapy. Journal of Clinical Medicine, 12(13), 4460. https://doi.org/10.3390/jcm12134460





