Abstract
The therapy of neuroblastoma relies, amongst other things, on administering chemotherapeutics and radioactive compounds, e.g., the (meta-iodobenzyl)guanidine [131I]mIBG. For special applications (conditioning before stem cell transplantation), busulfan and melphalan (M) proved to be effective. However, both drugs are not used for normal chemotherapy in neuroblastoma because of their side effects. The alkylating drug melphalan contains a (Cl-CH2-CH2-)2N- group in the para-position of the phenyl moiety of the essential amino acid phenylalanine (Phe) and can, therefore, be taken up by virtually all kinds of cells by amino acid transporters. In contrast, mIBG isotopologs are taken up more selectively by neuroblastoma cells via the noradrenaline transporter (NAT). The present study aimed at synthesising and studying hybrid molecules of benzylguanidine (BG) and the alkylating motif of M. Such hybrids should combine the preferential uptake of BGs into neuroblastoma cells with the cytotoxicity of M. Besides the hybrid of BG with the dialkylating group (Cl-CH2-CH2-)2N- bound in the para-position as in M (pMBG), we also synthesised mMBG, which is BG meta-substituted by a (Cl-CH2-CH2-)2N- group. Furthermore, two monoalkylating hybrid molecules were synthesised: the BG para-substituted by a (Cl-CH2-CH2-)NH- group (pM*BG) and the BG meta-substituted by a (Cl-CH2-CH2-)NH- group (mM*BG). The effects of the four new compounds were studied with human neuroblastoma cell lines (SK-N-SH, Kelly, and LS) with regard to uptake, viability, and proliferation by standard test systems. The dialkylating hybrid molecules pMBG and mMBG were at least as effective as M, whereas the monoalkylating hybrid molecules pM*BG and mM*BG were more effective than M. Considering the preferred uptake via the noradrenaline transporter by neuroblastoma cells, we conclude that they might be well suited for therapy.
1. Introduction
Neuroblastoma is a malignant tumour of the sympathetic nervous system in childhood [] with a poor prognosis in stage IV patients []. Characteristically, most neuroblastoma cells are able to synthesise catecholamines (dopamine, noradrenaline) [] and they can express the noradrenaline transporter (NAT) for the reuptake of released dopamine and noradrenaline []. Besides catecholamines, a few other compounds can be taken up by the NAT, among them (meta-iodobenzyl)guanidine (mIBG) []. From the 1980s until now [], radio-iodine-labelled mIBG {first [131I]mIBG [,], (Figure 1, line 1), soon replaced by [123I]mIBG}, has been used for scintigraphic diagnosis [], and [131I]mIBG for therapy [] of neuroblastoma. It is noteworthy that the benzylguanidine (BG) with 131iodine bound in the meta-position {[131I]mIBG, Figure 1, line 1)} is taken up by NAT-expressing cells equally well as its para-isomer [131I]pIBG. However, in clinical praxis, the meta-substituted forms {[131I]mIBG)/[123I]mIBG} are used exclusively because of the presence of deiodinases in humans and animals; they remove iodine preferentially from the para- and not as readily from the meta-position (lower thyroid radioactivity concentration due to lower liberated iodine using the meta-substituted forms) [,,].
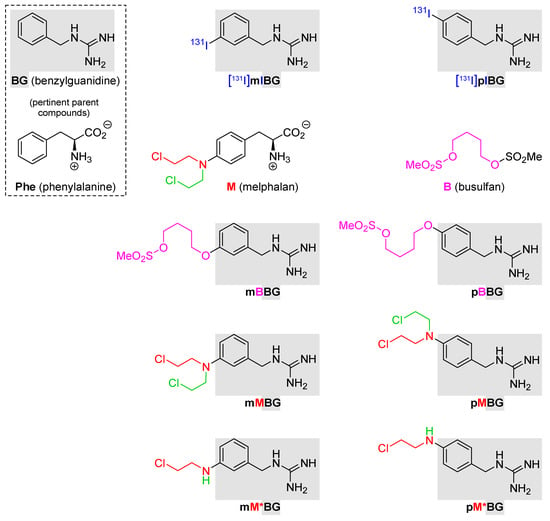
Figure 1.
Structural formulas of the compounds described in this study. Line 1: benzylguanidine (BG) and the radiotherapeuticals [131I]mIBG and [131I]pIBG. Line 2: phenylalanine (Phe; essential amino acid); melphalan (M), and busulfan (B). Line 3: mBBG and pBBG. Line 4: hybrids with dialkylating entities copied from M (mMBG and pMBG). Line 5: hybrids with monoalkylating entities inspired by M (mM*BG and pM*BG). M: 4-[N,N-Bis(2-chloroethyl)amino]phenylalanine; mMBG: {3-[N,N-Bis(2-chloroethyl)amino]-benzyl}guanidine; pMBG: {4-[N,N-Bis(2-chloroethyl)amino]-benzyl}guanidine; mM*BG: {3-[N-(2-Chloroethyl)amino]benzyl}-guanidine; pM*BG: {4-[N-(2-Chloroethyl)amino]benzyl}-guanidine; B: 5-[(Methanesulfonyl)oxy]pentyl methanesulfonate; mBBG: 5-[3-(Guanidinomethyl)phenoxy]-pentyl methanesulfonate; pBBG: 5-[4-(Guanidinomethyl)phenoxy]pentyl methanesulfonate.
For the treatment of high-risk neuroblastoma before a hematopoietic stem cell transplantation, the alkylating drugs melphalan (M) [] and busulfan (B; traded as “myleran” (GlaxoSmithKline, Stevenage, UK) or “busulfex IV” (Otsuka America Pharmaceutical, Inc, Rockville, MD, USA) [] are used for conditioning; (Figure 1, line 2). Investigations by the Society for Pediatric Oncology (SIOP; HR-NBL1/SIOPEN) showed that, in such cases, the use of a combination of M and B is superior to the formerly used protocol using a combination of M, carboplatin, and etoposide []. Despite the high effectivity of M and B, they are not included in chemotherapy protocols for neuroblastoma without subsequent stem cell rescue because of their severe toxicities. The unspecific toxicity of M is not surprising since the carrier molecule of the dialkylating group is phenylalanine (Phe) (Figure 1, line 2), an essential aromatic amino acid, which can be taken up by almost every cell via amino acid transporters [,]. In contrast, the uptake of mIBG and related compounds in neuroblastoma cells is essentially restricted via the noradrenaline transporter. In some other cells, catecholamines and mIBG can be taken up by organic cationic transporters (OCTs) [,].
In M, the alkylating entity is bound to the para-position of the aromatic ring of Phe. In order to use the potential of M for neuroblastoma treatment in “conventional” chemotherapy, we synthesised and studied hybrid molecules of BG and M, combining the highly effective alkylating group(s) of the alkylating entity of M with BG (rather than with Phe), with the hope of specific uptake into neuroblastoma cells via the NAT. Conceptually related hybrids between BG und B (namely, mBBG and pBBG; Figure 1, line 3) were already synthesised and studied by our groups [,].
Besides the hybrid of BG with the dialkylating group (Cl-CH2-CH2-)2N- bound in the para-position as in M (pMBG; Figure 1, line 4), we also synthesised a hybrid of BG, which is meta-substituted by the same (Cl-CH2-CH2-)2N- group (mMBG; Figure 1, line 4). Furthermore, two hybrid molecules with monoalkylating (Cl-CH2-CH2-)NH- groups were synthesised: the meta-substituted BG (mM*BG; Figure 1, line 5) and the para-substituted BG (pM*BG; Figure 1, line 5).
The aim of our study was (a) to synthesise the conceptualised four hybrid molecules and (b) to analyse their uptake via the noradrenalin transporter in vitro and their cytotoxic effects.
2. Materials and Methods
2.1. Synthesis of the Hybrid Compounds mMBG, pMBG, mM*BG, and pM*BG
Our syntheses of the MBG hybrids mMBG and pMBG—which we isolated as the corresponding trifluoroacetates mMBG·TFA and pMBG·TFA—proceeded as shown in Scheme 1. The starting materials were the diamines meta- and para-1, which are commercially available. The higher nucleophilicity of their sp3- rather than sp2-bound NH2 groups allowed regioselective amidinylations of the former by the N,N’-di-Boc-protected isothiourea 2, a reagent obtained from S-methylisothiourea hydrogensulfate, as described in []. The N,N’-di-Boc-protected amino-BGs meta-3 and para-3 resulted in yields of 92% and 66%, respectively []. The aniline moiety of these compounds underwent double alkylations with 2-chloroethyl triflate (4), which had been prepared from chloroethanol by a known triflation []. These alkylations provided the (Cl-CH2-CH2-)2N-containing compounds meta-5 (38% yield) and para-5 (51% yield []). The terminating step of the syntheses shown in Scheme 1 was the removal of the two Boc groups. It occurred when we stirred solutions of the respective isomers of compound 5 in trifluoroacetic acid (TFA)/CH2Cl2 (1:1, v:v). Evaporation of all volatiles afforded the (Cl-CH2-CH2-)2N-containing benzylguanidine trifluoroacetates mMBG·TFA and pMBG·TFA. Their syntheses comprised 3 steps in the longest linear sequence and succeeded in total yields of 38% and 27%, respectively.
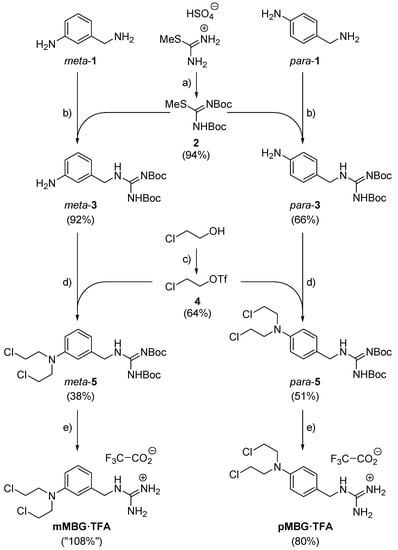
Scheme 1.
Syntheses of our two MBG hybrid molecules. Reagents and conditions: (a) Boc2O (3.1 eq.), aq. NaHCO3 (xs.), room temp., 5 d; 94% (ref. []: 100%). (b) 2 (1.2 eq.), NEt3 (4.2 eq.), CH2Cl2, reflux, 20 h for para-4, 6 h for meta-4. (c) Tf2O (1.1 eq.), pyridine (1.2 eq.), CH2Cl2, 0 °C, 50 min; 64%. (d) 4 (4.0 eq.), K2CO3 (2.2 eq.), CH2Cl2, reflux, 18 h. (e) F3CCOOH/CH2Cl2 (1:1), room temp., 30 min.
Our syntheses of the MBG hybrids mM*BG and pM*BG—which we isolated as the corresponding trifluoroacetates mM*BG·TFA and pM*BG·TFA—started from the commercially available iodotoluenes meta- and para-6, respectively (Scheme 2). Solutions of these compounds in CCl4 were subjected to Wohl-Ziegler brominations. They rendered the corresponding benzyl bromides meta-7 (69% yield) and para-7 (57% yield), respectively. Exposure to solutions of sodium azide in DMF engaged these compounds in SN2 reactions, which afforded the azides meta- and para-8 yields of 58% and 80%, respectively. Each of these compounds is susceptible to a tandem transformation [] beginning with a Staudinger reduction (PPh3; H2O) and ending with an amidinylation with the N,N’-di-Boc-protected thiourea 2 []. This furnished the N,N’-di-Boc-protected benzylguanidines [] meta-9 (= Boc2mIBG; 57%) and para-9 (= Boc2mIBG; 66%). Like ordinary aryl iodides, the last-mentioned compounds and aminoethanol underwent Ullmann-type monoaminations/monoarylations []. They rendered the (HO-CH2-CH2-)NH-containing N,N’-di-Boc-protected BGs [] meta-11 (64% yield) and para-11 (62% yield), respectively. The OH group of these compounds was converted into Cl by treatment with hexachloroethane and PPh3 []. This delivered the (HO-CH2-CH2-)NH-containing N,N’-di-Boc-protected BGs [] meta-12 (59% yield) and para-12 (53% yield). Treatment of these compounds with TFA/CH2Cl2 (1:1, v:v) removed the Boc groups and delivered, after evaporation of all volatiles, the (Cl-CH2-CH2-)NH-containing benzylguanidine trifluoroacetates mM*BG·TFA and pM*BG·TFA in quantitative yields. Overall, their syntheses comprised 6 steps in the longest linear sequence and succeeded in total yields of 7% and 11%, respectively.
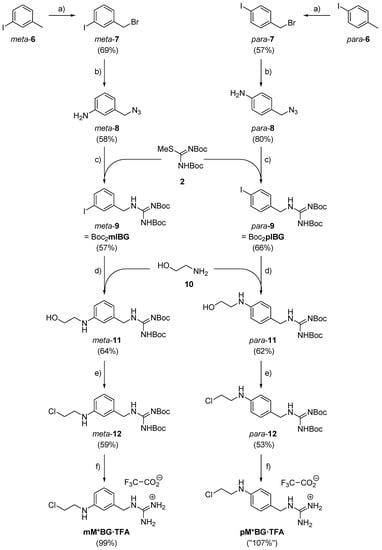
Scheme 2.
Syntheses of our two M*BG hybrid molecules. Reagents and conditions: (a) N-Bromosuccinimide (1.0 eq.), dibenzoyl peroxide (2 mol-%), CCl4, reflux, 20 h. (b) NaN3 (3.0 eq.), DMF, room temp., 5 h. (c) PPh3 (2.1 eq.), acetone, room temp., 1.5 h; then H2O (26 eq.), NEt3 (1.6 eq.), 2 (1.4 eq.), room temp., 2 d. (d) 10 (6.0 eq.), CuI (1.0 eq.), Cs2CO3 (2.0 eq.), isopropanol, room temp., 4 d. (e) PPh3 (1.1 eq.), hexachloroethane (1.1 eq.), CH2Cl2, room temp., 20 h. (f) F3CCOOH/CH2Cl2 1:1, room temp., 30 min.
Remarks to Scheme 1 and Scheme 2, Ref. []: All[MP1] N,N’-di-BOC[M2]-protected guaninides encountered in the present study, i.e., the meta and para isomers of compounds 3, 5, 9, 11, and 12, represented one single of two conceivable tautomers. The 1H-NMR shifts of the two N-bound protons within each of these compounds differed from one another grossly. This let us conclude that these protons are 1 ξ CH2-NH (8.43 ppm ≤ δCH2-NH in CDCl3 solution ≤ 8.65 ppm) plus 1 ξ NHBoc (δNH-Boc in CDCl3 solution = 11.51 or 11.52 ppm), not 2 × NHBoc.
2.2. Cell Culture and Cell Culture Experiments
2.2.1. Human Neuroblastoma Cell Lines
Human Neuroblastoma Cell Lines:
- (a)
- SK-N-SH (American Type Culture Collection, ATCC; MD, USA), Nr. HTB-11 [];
- (b)
- Kelly (German Collection of Microorganisms and Cell Cultures, DSMZ, Braunschweig, Germany), ACC 355 [];
- (c)
- LS (DSMZ, ACC 675); (established in our laboratory) [].
Cells were grown as monolayers in RPMI 1640 medium supplemented with 10% inactivated FCS, L- glutamine (2 mmol/L), and penicillin/streptomycin (100 U/mL; 100 µg/mL) in 250 mL cell culture flasks at 37 °C, 5% CO2 and 100% humidity. For cell experiments, cells were detached by trypsin, washed with PBS++, and resuspended in the respective incubation medium.
2.2.2. Inhibition of [3H]noradrenaline Uptake in Neuroblastoma Cells: Competitive Studies with MBG Hybrid Molecules Compared to mIBG and M
To examine the uptake into the human neuroblastoma cell lines SK-N-SH (high NAT expression), Kelly (moderate NAT expression), and LS (very low NAT expression), competitive uptake experiments with [3H]noradrenaline {[3H]NA} were performed {noradrenaline (Norepinephrine), LL evo-[7-3H]; specific activity: 10-30 Ci (370 GBq-1.11 TBq/mmol); concentration 1mCi/mL; PerkinElmer, Freiburg, Germany}. [3H]noradrenaline is equally taken up by the NAT as radio-labelled mIBG. The competitive uptake studies were carried out in analogy to the experiments described previously with busulfan benzylguanidine (BBG) hybrids []. Briefly, the adherent growing neuroblastoma cell lines were detached with trypsin and, after a short regeneration period, used as suspension culture (usually 500 µL; 1 × 105 cells/mL phosphate buffered saline + Ca++/Mg++ (PBS++) + glucose (5.5 mmol/L)). In addition, 500 µL cell suspensions were incubated with 10 µL noradrenaline (final concentration: 0.1 µmol/L) spiked with [3H]NA for 15 min at 37 °C. After centrifugation, cell pellets were lysed with 500 µL distilled water and mixed with 10 mL of liquid scintillation cocktail (OptiPhase Super Mix, PerkinElmer). The amount of NA (fmoles/106 cells) taken up by cells in controls was calculated by measurement of radioactivity taken up by cells and set as 100%. The uptake of [3H]noradrenaline in the presence of an excess of unlabelled mIBG, M, mM*BG, pM*BG, mMBG, and pMBG was given as % uptake compared to PBS++ controls.
2.2.3. MTS-Test (Viability Test)
Substances were tested in the concentration range from 0.01 µmol/L to 1000 µmol/L using the CellTiter 96® AQueous One Solution Cell Proliferation Assay (MTS) from Promega (Walldorf, Germany). MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulphophenyl)-2H-tetrazolium, inner salt in combination with a reductant (phenazine methosulfate) was used. The test is a colorimetric assay for determining the viable cells by bioreduction to a formazan product. The influence of the respective substances was measured on cell viability. The assay was performed according to manufacturer’s instruction in 96 flat bottom cell culture plates. In addition, 200 µL of cell suspension (SK-N-SH: 2.5 × 104 cells/well; Kelly: 1.5 × 104 cells/well; LS: 1 × 104 cells/well) in cell culture medium was transferred to the wells of a 96-well plate. After 24 h, 10 µL of the respective substances and concentrations was added in triplicates to the wells. The incubation was stopped after 72 h of incubation at 37 °C/5% CO2 and the samples were analysed according to the test protocol. The viability of the cells after treatment with the respective substance concentrations was related to the control samples (incubation in cell culture medium), set as 100% cell viability. Each experiment was carried out in three independent runs.
2.2.4. CELLigence Assay
In order to gain detailed information concerning the effectivity of the hybrid molecules during the proliferation of the neuroblastoma cells, real-time neuroblastoma cancer cell proliferation was determined using the xCELLigence SP system (Roche Applied Science, Mannheim, Germany). In contrast to the MTS assay, the xCELLigence assay allowed us to follow the growth of cells continuously in the absence and presence of the added drugs.
Neuroblastoma cells were seeded in 96-well plates (E-Plate 96, ACEA Biosciences, San Diego, CA, USA), 20,000 cells/well. Real-time dynamic cell proliferation was monitored in 30 min intervals for 96 h at 37 °C. 24 h after seeding, and cells were treated with M and the MBG hybrids, as indicated. Cell index values were calculated using the RTCA Software (2.0). All curves were normalised to the time point after drug treatment was conducted (~24 h after seeding) applying the RTCA software [,].
3. Results
3.1. Synthesis of Our Four MBG Hybrid Compounds
The syntheses of the four hybrid compounds are described in detail in Section 2.1. The syntheses of mMBG·TFA and pMBG·TFA comprised 3 steps in the longest linear sequence and succeeded in total yields of 38% and 27%, respectively. The syntheses of mM*BG·TFA and pM*BG·TFA comprised 6 steps in the longest linear sequence and succeeded in total yields of 7% and 11%, respectively.
3.2. Stability of the Newly Synthesised MBG Hybrid Compounds
Once any of our MBG hybrids, i.e., mMBG, pMBG, mM*BG, and pM*BG, had been synthesised as the respective trifluoroacetate mMBG·TFA, pMBG·TFA, mM*BG·TFA, or pM*BG·TFA, they were stored in the freezer at −20 °C. Trifluoroacetate (TFA) was chosen as a counter ion for each respective benzylguanidinium moiety because the corresponding salts were highly soluble in aqueous media. Storage at this temperature was possible for several to many weeks, neither affecting the integrity nor the homogeneity of the respective compound. In order to estimate these compounds’ stabilities under the conditions of our cell culture experiments (see below), an aqueous solution of the compound of interest was incubated at 37 °C. After 1 h or 5 h of such exposure, 1H-NMR spectroscopy (300.1 MHz) in DMSO-d6 solution revealed to which extent the respective MBG hybrid had survived the heat stress (Table 1). The least stable compound was the dialkylating agent mMBG·TFA, whereas the most stable compounds were the monoalkylating agents mM*BG·TFA and pM*BG·TFA.

Table 1.
Fraction of originally intact MBG hybrid molecules (formulas: Figure 1) remaining unaltered after having been maintained at +37 °C in aqueous solutions a.
3.3. Interaction of Our MBG Hybrid Compounds with Neuroblastoma Cell Lines with Different NAT Expressions
3.3.1. Inhibition of [3H]noradrenaline Uptake in Neuroblastoma Cells: Competitive Studies with MBG Hybrid Molecules Compared to mIBG and M
Competitive uptake experiments of [3H]noradrenaline {[3H]NA} in the presence of mIBG, M, and the MBG hybrids were performed with neuroblastoma cell lines with high NAT expression (SK-N-SH), moderate NAT expression (Kelly), and no NAT expression (LS) (for method, see Section 2.2.2). As expected, mIBG is the most potent inhibitor of [3H]NA uptake via the NAT []. Figure 2 shows that the hybrids—in contrast to M—significantly inhibited the uptake of [3H]NA in the NAT-expressing neuroblastoma cell lines SK-N-SH and Kelly, but not LS cells.
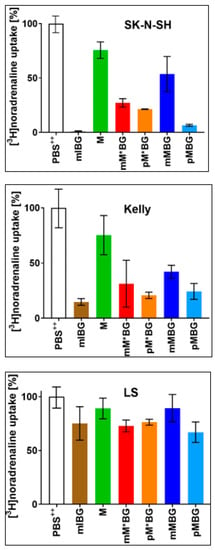
Figure 2.
Uptake of [3H]noradrenaline (c = 0.1 µmol/L) within 15 min at 37 °C into neuroblastoma cells with different expressions of the NAT (SK-N-SH > Kelly >> LS). This uptake was measured in a PBS++ (+5.5 mmol/L glucose) control, designated as “100% uptake” and in the presence of a molar excess of one of the following potential competitors (c = 100 µmol/L): mIBG·HCl (■), M (■), mM*BG·TFA (■), pM*BG·TFA (■), mMBG·TFA (■), and pMBG·TFA (■). Mean value ± S.D based on 3 independent sets of experiments, each of which was performed in triplicate.
3.3.2. Viability Analyses (MTS Assays) of MBG Hybrid Compounds
Cell viability assays measure the reduction of MTS by NADPH and/or NADH, which are generated by dehydrogenases in metabolically active cells. Potentially toxic drugs can lead to impaired activity, associated with reduced cell proliferation and finally to cell death. In the experiments shown in Figure 3, the effects of monoalkylating MBGs (mM*BG and pM*BG) on cell viability were compared with the effects of mIBG and M (dialkylating MBGs were not obtainable at this time). In later experiments shown in Figure 4, the effects of dialkylating MBGs (mMBG and pMBG) were directly compared to the effects of monoalkylating MBGs (mM*BG and pM*BG).
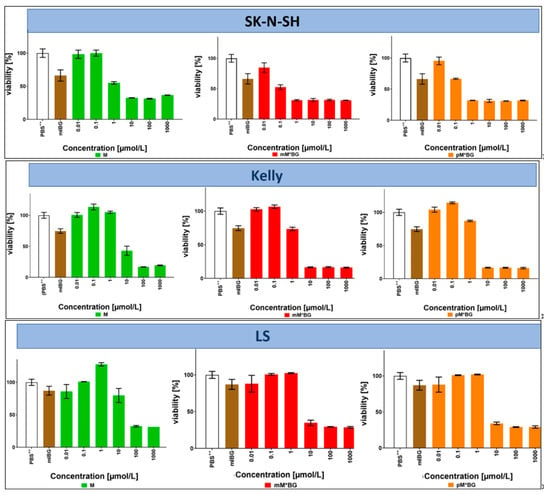
Figure 3.
MTS viability assays with the neuroblastoma cell lines SK-N-SH, Kelly, and LS after incubations with the indicated compounds at 37 °C for 72 h: Effects of M (left: ■), mM*BG·TFA (centre: ■) and pM*BG·TFA (right; ■) administered in concentrations of 0.01, 0.1, 1, 10, 100, and 1000 µmol/L (corresponding to logarithmic horizontal scales). The extreme left of each diagram depicts the corresponding PBS++ (+ 5.5 mmol/L glucose) control (whose MTS assay served to define “100% viability”) and a reference treatment with mIBG·HCl (■), administered only in a concentration of 10 µmol/L. Mean ± S.D; 3 independent experiments, each in triplicate.
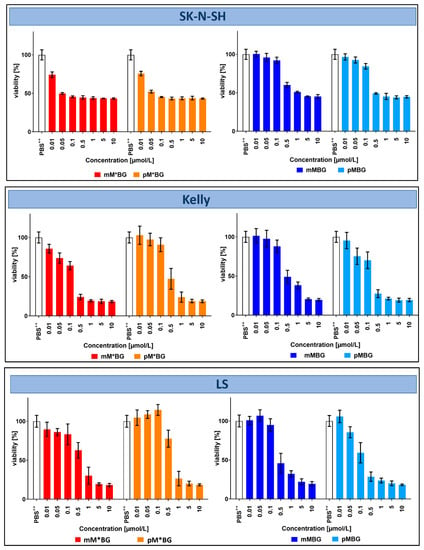
Figure 4.
MTS viability assays with the neuroblastoma cell lines SK-N-SH, Kelly, and LS after incubations with the indicated compounds at 37 °C for 72 h: Effects of mM*BG·TFA (■) and pM*BG·TFA (■) (left), compared to mMBG·TFA (■) and pMBG· TFA(■) (right), administered in concentrations of 0.01–0.05–0.1–0.5–1–5–10 µmol/L (corresponding to near logarithmic horizontal scale). The extreme left of each diagram depicts the corresponding PBS++ (+ 5.5 mmol/L glucose) control (whose MTS assay served to define “100% viability”). Mean ± S.D; 3 independent experiments, each in triplicate.
After an incubation period of 72 h, clear effects were observed from about 10 µmol/L, in SK-N-H cells already at 1 µmol/L. Both M*BGs were more effective than M and mIBG·HCl, the isomers mM*BG·TFA and pM*BG·TFA being similarly effective.
Taken together, the effects of BG hybrids with only one alkylating group (mM*BG/pM*BG) were similar or even a little bit better than those with two alkylating groups (mMBG/pMBG).
3.3.3. Proliferation Kinetics of Neuroblastoma Cells (xCELLigence Assay) of MBG Hybrid Compounds
In order to gain detailed information concerning the effectivity of the hybrid molecules during the proliferation of the neuroblastoma cells, real-time neuroblastoma cancer cell proliferation was determined using the xCELLigence SP system (Roche Applied Science, Mannheim, Germany). In contrast to the MTS assay, the xCELLigence assays allowed us to follow the growth of cells continuously in the absence and presence of the added drugs.
In screening experiments, the effects of M and the MBGs were first investigated in the concentration range of 0.1–100 µmol/L in order to estimate acceptable concentrations for further experiments: 1–10 µmol/L (final concentration in the cell cultures) proved to be well suitable. The following experiments shown in Figure 5 and Figure 6 were carried out using a drug concentration of 10 µmol/L.
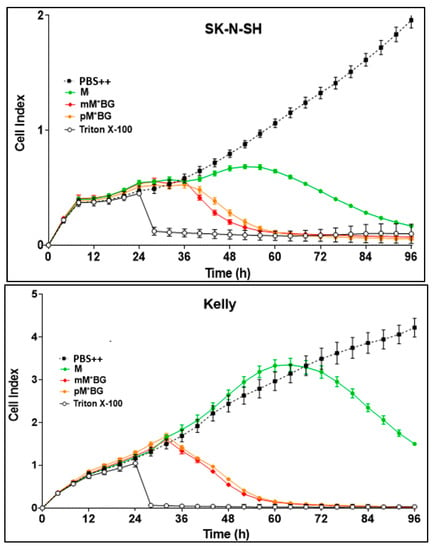
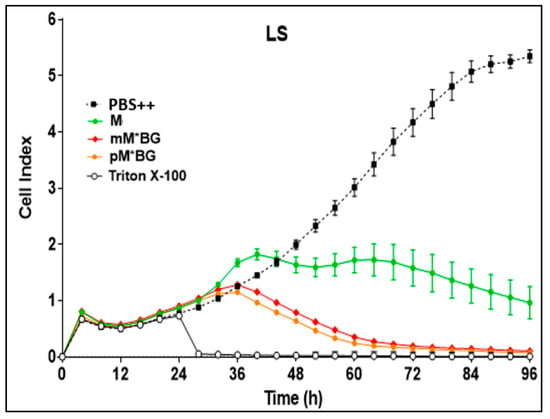
Figure 5.
Real-time proliferation monitoring over 96 h in the presence of M (green line), mM*BG·TFA (red line), and pM*BG·TFA (orange line) compared to controls (PBS ++ + 5.5 mmol/L glucose, black line). Following the initial tumour cell seeding (at hour 0), the neuroblastoma cells SK-N-SH, Kelly, and LS were treated 24 h later with 10 µmol/L (final concentration) of M and the M*BG hybrids. Treatment with Triton X-100 1%, inducing maximum tumour cell lysis, was used as a negative control. The cell index was normalised after 24 h when treatment had been accomplished. Mean ± S.D.; 3 independent experiments each in triplicate.
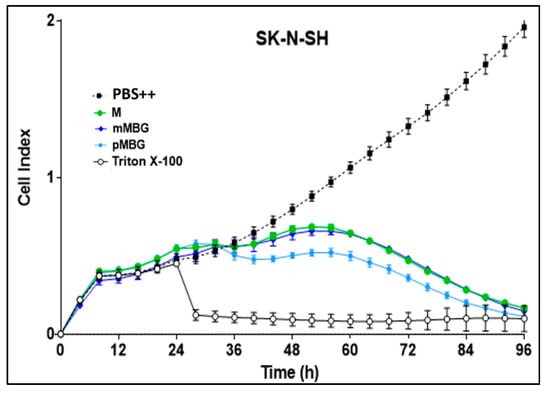
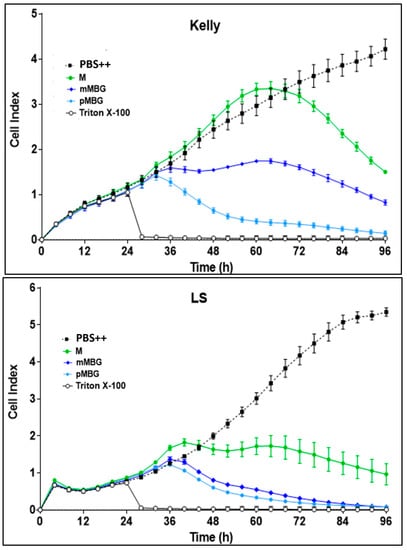
Figure 6.
Real-time proliferation monitoring over 96 h in the presence of M (green line), mMBG·TFA (dark blue line), and pMBG·TFA (light blue line) compared to controls (PBS ++ + 5.5 mmol/L glucose, black line). Following the initial tumour cell seeding (at hour 0), the neuroblastoma cells SK-N-SH, Kelly, and LS were treated 24 h later with 10 µmol/L (final concentration) of M and the MBG hybrids. Treatment with Triton X-100 1%, inducing maximum tumour cell lysis, was used as a negative control. The cell index was normalised after 24 h when treatment had been accomplished. Mean ± S.D.; 3 independent experiments each in triplicate.
Figure 5 shows that the hybrids with one alkylating group (mM*BG·TFA and pM*BG·TFA) proved to be more effective than M to all three cell lines.
The hybrids with two alkylating groups (mMBG TFA and pMBG·TFA) were more toxic than M in Kelly and LS cells, but less pronounced in SK-N-SH cells.
4. Discussion
To an important extent, the chemotherapy of cancer relies on the in vivo alkylation of biomolecules by alkylating agents [,]. The earliest used examples were the compounds (Cl-CH2-CH2-)3N and (Cl-CH2-CH2-)2N-Me [,,]. The last-mentioned compound is the simplest representative of a class of compounds containing the structural motif (Cl-CH2-CH2-)2N-. They possess high alkylating power, which makes them akin to, and possibly even superior to, the alkylating agent (Cl-CH2-CH2-)2S. The latter was dubbed “mustard gas” during WWI warfare. This is why compounds containing the motif (Cl-CH2-CH2-)2N- are commonly referred to as “N-mustards”. N-mustards—not only M []—played a pivotal role in the development of chemotherapy for cancer [,,,,,]. Their development is continuing at a considerable pace [,,,,,]. The present study contributes its share to this field.
As already described, the alkylating chemotherapeutics busulfan (B) and melphalan (M) have been successfully introduced into the therapy of high-risk neuroblastoma before stem cell transplantation []. Due to their toxicity, especially on hematopoietic stem cells, both substances are not used in conventional chemotherapy of neuroblastoma without stem cell transplantation. Therefore, we decided to synthesise hybrid molecules of benzylguanidine (with the aim of more specific uptake into neuroblastoma cells) and the alkylating groups of these cytostatic drugs B and M. In a preceding paper, we reported the effects of mBBG and pBBG (busulfan benzylguanidine hybrids) compared to B {busulfan, 5-[(methanesulfonyl)oxy]pentyl methanesulfonate} []. In the actual manuscript, we present our results concerning the effects of MBGs (melphalan benzylguanidine hybrids) on neuroblastoma cells.
As already mentioned in the introduction, it is obvious that the alkylating cytostatic drug M has a fundamental handicap: the carrier structure of its alkylating group is Phe, which can probably be taken up by almost all cell types by amino acid transporters [,]. This is probably the reason for the unspecific side effects of M. Nevertheless, M proved to be very effective in the conditioning regime in high-risk neuroblastoma for the destruction of neuroblastoma cells and hematopoietic cells before transplantation [,]. In order to reduce the side effects observed during treatment with M, our aim was to synthesise hybrid molecules consisting of the high effective alkylating group of M and a carrier group (benzylguanidine), which can be taken up by the NAT of neuroblastoma cells. However, their uptake is not limited to neuroblastoma cells: mIBG (and probably the MBGs) can also be incorporated by cells expressing organic cation transporters (OCTs), such as kidney and liver cells, but uptake via these transporters can be reduced by clinically approved corticosteroids []. Therefore, a combination of corticosteroids with [123I]mIBG/[131I]mIBG or with MBGs should enhance their preferred uptake via the NAT and thereby improve diagnosis and therapy of neuroblastoma.
For synthesis, the alkylating group (Cl-CH2-CH2-)2N- of M binds to benzylguanidine in the meta- (instead of iodine in mIBG) and para-position. Furthermore, hybrids with only one alkylating group, (Cl-CH2-CH2-)NH-, bound in the meta- and para-position, were also synthesised. All four M hybrids were at least equally as effective, but in most cases more effective, on neuroblastoma cell cultures than M. Surprisingly, hybrids containing only one alkylating group were at least equally as effective as those with two alkylating groups. In M, the two activated alkylating, positively charged groups can crosslink the strands of DNA by reacting with the nucleophilic amino groups comprising guanine N-7 and adenine N-3 [,,]. Concerning the M hybrids mM*BG and pM*BG with only one alkylating group, they are definitely unable to crosslink DNA. However, their alkylating power towards the mentioned nucleophilic amino groups containing guanine N-7 and adenine N-3 should be essentially the same as the monoalkylation power of their bifunctional analogues mMBG and pMBG. This allows us to speculate that the more pronounced effects of the hybrids mM*BG and pM*BG with only one alkylating group sometimes observed may be, at least partly, associated their higher thermal stability (Table 1).
Comparing the three neuroblastoma cell lines with different NAT expression, it was conspicuous that LS cells (very low NAT expression) were similarly sensitive against the M hybrids than SK-N-SH and Kelly cells. This aspect needs to be investigated in more detail with other cells lacking NAT expression. We suppose that the presence of OCTs in LS cells is responsible for the uptake of the M hybrids (not yet investigated).
5. Conclusions
Our newly synthesised M hybrids mMBG and pMBG containing the alkylating entity (Cl-CH2-CH2-)2N- of M—two 2-chloroethyl groups bound to one N—proved to be more effective against neuroblastoma cell lines as the equally (Cl-CH2-CH2-)2N-containing drug M, indicating their potential as an improved chemotherapeutic drug in neuroblastoma therapy. Although our original intention was to only synthesise molecules with strict homologies to M, we found that the less closely related, M-inspired (Cl-CH2-CH2-)NH-containing benzylguanidine hybrids mM*BG and pM*BG—with only one 2-chloroethyl group bound to the N atom—were also effective in neuroblastoma cells. This may be due to—either altogether or in part—mM*BG and pM*BG being more thermally stable than mMBG and pMBG during incubation at 37 °C.
All four hybrid molecules were at least equally as cytotoxic, but in most cases even more cytotoxic against the neuroblastoma cells than melphalan in vitro. Based on these data, we conclude that all four hybrids should essentially be suitable for neuroblastoma therapy. However, further experiments in vitro and in vivo are necessary in order to support this concept. Next, in vitro experiments will be done with an expanded set of well characterised, commercially available neuroblastoma cell lines together with non-neuroblastoma cells (kidney, liver, fibroblast, and hematopoietic stem cells) in order to comparatively analyse the cytotoxicity and uptake of the MBGs and melphalan by the noradrenaline transporter, amino acid transporter, and organic cation transporter in detail. The influence of glucocorticoides for a more selective uptake via the noradrenaline transporter will also be analysed. Subsequently, mouse experiments (the toxicity and treatment of the neuroblastoma-bearing mouse) should be performed with melphalan and the most suitable MBGs.
Author Contributions
Conceptualization: G.B., R.H. (Hybrid Concept and Neuroblastoma-Screening) and R.B. (Hybrid Concept and Syntheses). Methodology: R.B., M.L., M.B. and C.K. Validation: G.B. (Tuebingen)/R.B. (Freiburg). Writing: R.B. and G.B. Supervision: R.B. and R.H. Funding Acquisition: R.B. and R.H. Experiments: M.L., C.K. and M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deutsche Forschungsgemeinschaft (grants HA 2386/2-1 und BR 881/9-1). We are indebted for this generous support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
(a) Synthesis of the MBG hybrids: Lischka, M. Synthese cytotoxischer Hybridmoleküle aus Benzylguanidin und Alkylantien zur Therapie Kupplung: Synthese 5.5′-verbrückter 1,1′-Biphenyl-2,2′-diphosphane und deren Einsatz in katalytisch-asymmetrischen 1,4-Additionen von Arylboronsäuren an cyclische Enone. Doctoral Thesis, Albert-Ludwigs-Universität Freiburg, Freiburg im Breisgau, Germany, 2019. (https://www.zvab.com/Synthese-cytotoxischer-Hybridmolekule-Benzylguanidin-Alkylantien-Therapie/31007703557/bd accessed on 11 May 2023). (b)Experiments using three neuroblastoma cell lines, obtained from: SK-N-SH (American Type Culture Collection, ATCC; MD, USA), Nr. HTB-11; Kelly (German Collection of Microorganisms and Cell Cultures, DSMZ, Braunschweig, Germany), ACC 355; LS (DSMZ, ACC 675).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ray, S.K. (Ed.) Neuroblastoma. Molecular Mechanisms and Therapeutic Interventions; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2019; ISBN 978-0-12-812005-7. [Google Scholar]
- Abbas, A.A.; Samkari, A.M.N. High-Risk Neuroblastoma: Poor outcomes despite aggressive multimodal therapy. Curr. Canc. Ther. Rev. 2022, 18, 14–40. [Google Scholar] [CrossRef]
- LaBross, E.H.; Comoy, E.; Bohoun, C.; Zucker, J.M.; Schweisguth, O. Catecholamine metabolism in neuroblastoma. J. Natl. Cancer Inst. 1976, 57, 633–638. [Google Scholar] [CrossRef]
- Pacholczyk, T.; Blakely, R.D.; Amara, S.G. Expression cloning of a cocaine-and antidepressant-sensitive human noradrenaline transporter. Nature 1991, 350, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Glowniak, J.V.; Kilty, J.E.; Amara, S.G.; Hoffman, B.J.; Turner, F.E. Evaluation of meta-iodobenzylguanidine uptake by the norepinephrine, dopamine and serotonine transporters. J. Nucl. Med. 1993, 34, 1140–1146. [Google Scholar]
- Schmidt, M.; Hero, B.; Simon, T. I-131-mIBG therapy in neuroblastoma: Established role and prospective application (Review). Clin. Transl. Imaging 2016, 4, 87–101. [Google Scholar] [CrossRef]
- Kimmig, B.; Brandeis, W.E.; Eisenhut, M.; Bubeck, B.; Hermann, H.J.; zum Winkel, K. Scintigraphy of a neuroblastoma with I-131 meta-Iodobenzylguanidine. J. Nucl. Med. 1984, 25, 773–775. [Google Scholar]
- Treuner, J.; Feine, U.; Niethammer, D.; Müller-Schauenburg, W.; Meinke, J.; Eibach, E.; Dopfer, R.; Klingebiel, T.; Grumbach, S. Scintigraphic Imaging of Neuroblastoma with m-[131]iodobenzylguanidine. J. Nucl. Med. 1984, 25, 333–334. Available online: https://www.osti.gov/biblio/6639263 (accessed on 11 May 2023).
- Wieland, D.M.; Brown, L.E.; Tobes, M.C.; Rogers, W.L.; Marsh, D.D.; Mangner, D.J.; Swanson, D.P.; Beierwaltes, B.H. Imaging of primate adrenal medulla with [123I] and [131I]meta-Iodobenzylguanidine: Concise Communication. J. Nucl. Med. 1981, 22, 358–364. [Google Scholar] [PubMed]
- Treuner, J.; Klingebiel, T.; Feine, U.; Buck, J.; Bruchelt, G.; Dopfer, R.; Girgert, R.; Müller-Schauenburg, W.; Meinke, J.; Kaiser, W.; et al. Clinical experiences in the treatment of neuroblastoma with I-131-metaiodobenzylguanidine. Ped. Hem. Oncol. 1986, 3, 205–216. [Google Scholar] [CrossRef]
- Wieland, D.M.; Swanson, D.P.; Brown, L.E.; Beierwaltes, W.H. Imaging the adrenal medulla with an I-131-labeled antiadrenergic agent. J. Nucl. Med. 1979, 20, 155–158. [Google Scholar]
- Wieland, D.M.; Wu, J.-l.; Brown, L.E.; Mangner, T.J.; Swanson, D.P.; Beierwaltes, W.H. Radiolabeled adrenergic neuron-blocking agents: Adrenomedullary imaging with [131]iodobenzylguanidine. J. Nucl. Med. 1980, 21, 349–353. [Google Scholar]
- Ehninger, G.; Klingebiel, T.; Kumbier, I.; Schuler, U.; Feine, U.; Treuner, J.; Waller, H.D. Stability and pharmacokinetics of [131I] meta-iodobenzylguanidine in patients. Cancer Res. 1999, 47, 6147–6149. [Google Scholar]
- Bergel, F.; Stock, J.A. First synthesis and cytotoxicity study of melphalan Cyto-active Amino-acid and Peptide Derivatives. Substituted Phenylalanines. J. Chem. Soc. 1954, 14, 2409–2417. [Google Scholar] [CrossRef]
- Galton, D.A. Myleran in chronic myeloic leukemia: Results of treatment. Lancet 1953, 264, 208–213. [Google Scholar] [CrossRef]
- Ladenstein, R.; Pötschger, U.; Pearson, A.D.J.; Brock, P.; Luksch, R.; Castel, V.; Yaniv, I.; Papadakis, V.; Laureys, G.; Malis, J.; et al. (SIOPEN, SIOP Europe Neuroblastoma Group): Busulfan and melphalan versus carboplatin, etoposide, and melphalan as high-dose chemotherapy for high-risk neuroblastoma (HR-NBL1/SIOPEN): An international, randomized, multi-arm, open-label, phase 3 trial. Lancet Oncol. 2017, 18, 500–514. [Google Scholar] [CrossRef] [PubMed]
- Begleiter, A.; Lam, H.Y.; Grover, J.; Froese, E.; Goldenberg, G.J. Evidence for Active Transport of Melphalan by Two Amino Acid Carriers in L5178Y Lymphoblasts In Vitro. Cancer Res. 1979, 39, 353–359. Available online: https://aacrjournals.org/cancerres/article/39/2_Part_1/353/483470/Evidence-for-Active-Transport-of-Melphalan-by-Two (accessed on 11 May 2023). [PubMed]
- Goldenberg, G.J.; Lam, H.Y.; Begleiter, A. Active carrier-mediated transport of melphalan by two separate amino acid transport systems in LPC-1 plasmacytoma cells in vitro. J. Biol. Chem. 1979, 254, 1064. [Google Scholar] [CrossRef]
- Gründemann, D.; Schechinger, B.; Rappold, G.A.; Schömig, E. Molecular identification of the corticosterone sensitive extraneuronal catecholamine transporter. Nat. Neurosci. 1998, 1, 349–351. [Google Scholar] [CrossRef]
- Bayer, M.; Kuci, Z.; Schömig, E.; Gründemann, D.; Dittmann, H.; Handgretinger, R.; Bruchelt, G. Uptake of mIBG and catecholamines in noradrenaline and organic cation transporter expressing cells: Potential use of corticosterone for a preffered uptake in neuroblastoma and- pheochromocytoma cells. Nuc. Med. Bio. 2009, 36, 287–294. [Google Scholar] [CrossRef]
- Hampel, T.; Bruns, M.; Bayer, M.; Handgretinger, R.; Bruchelt, G.; Brückner, R. Synthesis and biological effects of new hybrid compounds composed of benzylguanidines and the alkylating group of busulfan on neuroblastoma cells. Bioorg. Med. Chem. Lett. 2014, 24, 2728–2733. [Google Scholar] [CrossRef]
- Bruns, M.; Hampel, T.; Brückner, R.; Bayer, M.; Handgretinger, R.; Bruchelt, G. Synthesis of para and meta-4-(Guanidinomethylphenoxyl)-Butylmethansulfonate (pBBG, mBBG) and their effects on neuroblastoma cells compared to mIBG and Busulfan. In Advances in Neuroblastoma Research; Abstract Booklet: Cologne, Germany, 2014; p. 126 (OR044). [Google Scholar]
- Liu, C.; Guo, W.; Shi, X.; Kaium, M.A.; Gu, X.; Zhu, Y.Z. Leonurine-cysteine analog conjugates as a new class of multifunctional anti-myocardial ischemia agent. Eur. J. Med. Chem. 2011, 46, 3996–4009. [Google Scholar] [CrossRef] [PubMed]
- Lischka, M. Synthese Cytotoxischer Hybridmoleküle aus Benzylguanidin und Alkylantien zur Therapie Kupplung: Synthese 5.5‘-Verbrückter 1,1‘-Biphenyl-2,2‘-Diphosphane und Deren Einsatz in katalytisch-Asymmetrischen 1,4-Additionen von Arylboronsäuren an Cyclische Enone. Doctoral Thesis, Albert-Ludwigs-Universität Freiburg, Freiburg im Breisgau, Germany, 2019. Available online: https://www.zvab.com/Synthese-cytotoxischer-Hybridmolekule-Benzylguanidin-Alkylantien-Therapie/31007703557/bd (accessed on 11 May 2023).
- Falzon, C.L.; Ackermann, U.; Spratt, N.; Tochon-Danguy, H.J.; White Howells, J.D.; Scott, A.M. F-18 labelled N,N-bis-haloethylamino-phenylsulfoxides–A new class of compounds for the imaging of hypoxic tissue. J. Label. Compd. Radiopharm. Off. J. Int. Isot. Soc. 2006, 49, 1089–1103. [Google Scholar] [CrossRef]
- Chiang, Y.L.; Russak, J.A.; Carrillo, N.; Bode, J.W. Synthesis of Enantiomerically Pure Isoxazolidine Monomers for the Preparation of b3-Oligopeptides by Iterative α-Keto Acid-Hydroxylamine (KAHA) Ligations. Helv. Chim. Acta 2012, 95, 2481–2501. [Google Scholar] [CrossRef]
- Job, G.E.; Buchwald, S.L. Copper-Catalyzed Arylation of β-Amino Alcohols. Org. Lett. 2002, 4, 3703–3706. [Google Scholar] [CrossRef]
- Baker, R.T.; Gordon, J.C.; Hamilton, C.W.; Henson, N.J.; Lin, O.H.; Maguire, S.; Murugesu, M.; Scott, B.L.; Smythe, N.C. Iron Complex-Catalyzed Ammonia–Borane Dehydrogenation. A Potential Route toward B–N-Containing Polymer Motifs Using Earth-Abundant Metal Catalysts. J. Am. Chem. Soc. 2012, 134, 5598–5609. [Google Scholar] [CrossRef]
- Biedler, J.L.; Helson, L.; Spengler, B.A. Morphology and growth, tumorgenicity and cytogenetics of human neuroblastma cells in continuous culture. Cancer Res. 1973, 33, 2643–2652. [Google Scholar]
- Mercatelli, D.; Balboni, N.; Palma, A.; Aleo, E.; Sana, P.P.; Perini, G.; Giorgi, F.M. Single-cell gene network analysis and transcriptional landscape of MYCN-amplified neuroblastoma cell lines. Biomolecules 2021, 11, 177. [Google Scholar] [CrossRef]
- Rudolf, G.; Schilbach-Stückle, K.; Handgretinger, R.; Kaiser, P.; Hameister, H. Cytogenetics and molecular characterization of newly-established neuroblastoma cell line LS. Hum.Genet. 1991, 86, 562–566. [Google Scholar] [CrossRef]
- Abassi, Y.A.; Xi, B.; Zhang, W.; Ye, P.; Kirstein, S.L.; Gaylord, M.R.; Feinstein, S.C.; Wang, X.; Xu, X. Kinetic cell-based morphological screening: Prediction of mechanism of compound action and off-target effects. Chem. Biol. 2009, 16, 712–723. [Google Scholar] [CrossRef]
- Weiland, T.; Berger, A.; Essmann, F.; Lauer, U.M.; Bitzer, M.; Venturelli, S. Kinetic tracking of therapy-induced senescence using the real-time cell analyzer single plate system. Assay Drug Dev. Technol. 2012, 10, 289–295. [Google Scholar] [CrossRef]
- Buck, J.; Bruchelt, G.; Girgert, R.; Treuner, J.; Niethammer, D. Specific uptake of m-125I iodobenzylguanidine in the human neuroblastoma cell line SK-N-SH. Cancer Res. 1985, 45, 6366–6370. [Google Scholar] [PubMed]
- Chabner, B.A.; Roberts, T.G. Chemotherapy and the war on cancer. Nat. Rev. Cancer 2005, 5, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Karati, D.; Mahadik, K.R.; Trivedi, P.; Kumar, D. Alkylating Agents, the Road Less Traversed, Changing Anticancer Therapy. Anticancer Agents Med. Chem. 2022, 8, 1478–1495. [Google Scholar] [CrossRef]
- Gilman, A.; Philips, F.S. The biological actions and therapeutic applications of the β-chloroethyl amines and sulfides. Science 1946, 103, 409–436. [Google Scholar] [CrossRef]
- Goodman, L.S.; Wintrobe, M.M.; Dameshek, W.; Goodman, M.J.; Gilman, A.; McLennan, M.T. Nitrogen mustard therapy; use of methyl-bis (β-chloroethyl) amine hydrochloride and tris (β-chloroethyl) amine hydrochloride for Hodgkin’s disease, lymphosarcoma, leukemia and certain allied and miscellaneous disorders. J. Am. Med. Assoc. 1946, 132, 126–132. [Google Scholar] [CrossRef]
- Jacobson, L.O.; Spurr, C.L.; Barron, E.S.G.; Smith, T.; Lushbaugh, C.; Dick, C.F. Nitrogen mustard therapy. J. Am. Med. Assoc. 1946, 13, 2263–2271. [Google Scholar] [CrossRef]
- Gilman, A. The initial clinical trial of nitrogen mustard. Am. J. Surg. 1963, 105, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Diethelm-Varela, B.; Ai, Y.; Liang, D.; Xue, F. Nitrogen Mustards as Anticancer Chemotherapies: Historic Perspective, Current Developments and Future Trends. Curr. Top. Med. Chem. 2019, 19, 691–712. [Google Scholar] [CrossRef]
- More, G.S.; Thomas, A.B.; Chitlange, S.S.; Nanda, R.K.; Gajbhiye, R.L. Nitrogen Mustards as Alkylating Agents: A Review on Chemistry, Mechanism of Action and Current USFDA Status of Drugs. Anticancer. Agents Med. Chem. 2019, 19, 1080–1102. [Google Scholar] [CrossRef]
- Chen, Y.; Jia, Y.; Song, W.; Zhang, L. Therapeutic potential of nitrogen mustard based hybrid molecules. Front. Pharmacol. 2018, 9, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Kumar, S.; Prasad, D.N.; Bhardwaj, T.R. Therapeutic journey of nitrogen mustard as alkylating anticancer agents: Historic to future perspectives. Eur. J. Med. Chem. 2018, 151, 401–433. [Google Scholar] [CrossRef] [PubMed]
- Deka, B.C.; Bhattacharyya, P.K. Nitrogen Mustards: The Novel DNA Alkylator. Clin. Cancer Drugs 2017, 4, 10–46. [Google Scholar] [CrossRef]
- Gao, X.; Li, J.; Wang, M.; Xu, S.; Liu, W.; Zag, L.; Li, Z.; Hua, H.; Xu, J.; Li, D. Novel enmein-type diterpenoid hybrids coupled with nitrogen mustards: Synthesis of promising candidates for anticancer therapeutics. Eur. J. Med. Chem. 2018, 146, 588–598. [Google Scholar] [CrossRef]
- Sun, J.; Wang, J.; Wang, X.; Hu, X.; Cao, H.; Bai, J.; Li, D.; Hua, H. Design and synthesis of b-carboline derivatives with nitrogen mustard moieties against breast cancer. Bioorg. Med. Chem. 2021, 45, 116341. [Google Scholar] [CrossRef]
- Antoni, F.; Bernhardt, G. Derivatives of nitrogen mustard anticancer agents with improved cytotoxicity. Arch. Pharm. 2021, 354, e2000366. [Google Scholar] [CrossRef]
- Cheptea, C.; Sunel, V.; Morosanu, A.C.; Dimitriu, D.G.; Dulcescu-Oprea, M.M.; Angheluta, M.D.; Miron, M.; Nechifor, C.D.; Dorohoi, D.O.; Malancus, R.N. Optimized Synthesis of New N-Mustards Based on 2-Mercaptobenzoxazole Derivatives with Antitumor Activity. Biomedicines 2021, 9, 476. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, W.; Tian, Y.; Ma, L.; Zhou, L.; Sun, H.; Ma, Y.; Hou, H.; Wang, X.; Ye, J.; et al. Design, synthesis and biological evaluation of novel diosgenin-benzoic acid mustard hybrids with potential anti-proliferative activities in human hepatoma HepG2 cells. J. Enzyme Inhib. Med. Chem. 2022, 37, 1299–1314. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Mu, J.; Wang, S.; Jia, C.; Li, D.; Hua, H.; Cao, H. Design and synthesis of chromone-nitrogen mustard derivatives and evaluation of anti-breast cancer activity. J. Enz. Inhib. Med. Chem. 2022, 37, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Granger, M.; Naranjo, A.; Bagatell, R.; DuBois, S.G.; McCune, J.S.; Tenney, S.C.; Hogarty, M.D.; Mills, D.; Panoff, J.E.; Chang, J.H.-H.; et al. Myeloablative Buslfan/Melphalan(BuMel) consolidation following induction chemotherapy for patients with newly diagnosed high-risk neuroblastoma: Children’s Oncology Group (COG) trial ANBL12P1. Transplant. Cell. Ther. 2021, 27, e1–e490. [Google Scholar] [CrossRef] [PubMed]
- Bayer, M.; Schmitt, J.; Dittmann, H.; Handgretinger, R.; Bruchelt, G.; Sauter, A.W. Improved selectivity of mIBG uptake into neuroblastoma cells in vitro and in vivo by inhibition of organic cationic transporter 3 uptake using clinically approved corticosteroids. Nuc. Med. Biol. 2016, 43, 543–551. [Google Scholar] [CrossRef]
- Osborne, M.R.; Lawley, P.D. Alkylation of DNA by melphalan with special reference to adenine derivatives and adenine-guanine-cross-linking. Chem. Biol. Interact. 1993, 89, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Hansson, J.; Lewensohn, R.; Ringborg, U.; Nilsson, B. Formation and removal of DNA cross-links induced by melphalan and nitrogen mustard in relation to drug-induced cytotoxicity in human melanoma cells. Cancer Res. 1987, 47, 2631–2637. [Google Scholar] [PubMed]
- Chen, W.; Han, Y.; Peng, X. Aromatic nitrogen mustard-based prodrugs: Activity, selectivity, and the mechanism of DNA cross-linking. Chem. Eur. J. 2014, 20, 7410–7418. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).