Risk Stratification for Herpes Simplex Virus Pneumonia Using Elastic Net Penalized Cox Proportional Hazard Algorithm with Enhanced Explainability
Abstract
:1. Introduction
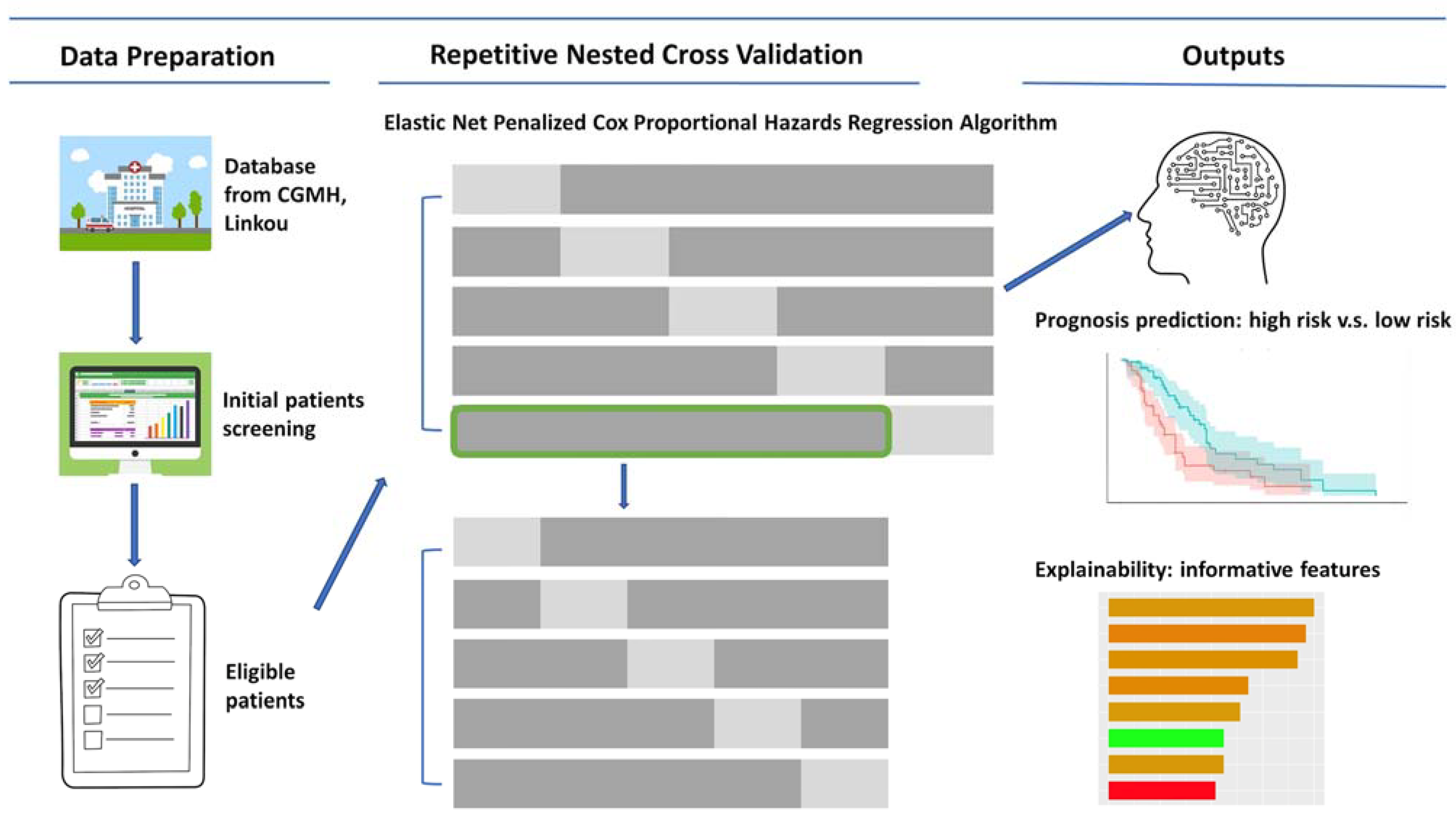
2. Materials and Methods
2.1. Study Design
2.2. Patients Eligibility
2.3. Model Development and Evaluation
2.4. Features Importance
2.5. Statistical Analysis
3. Results
3.1. Baseline Clinical Characteristics of Subjects
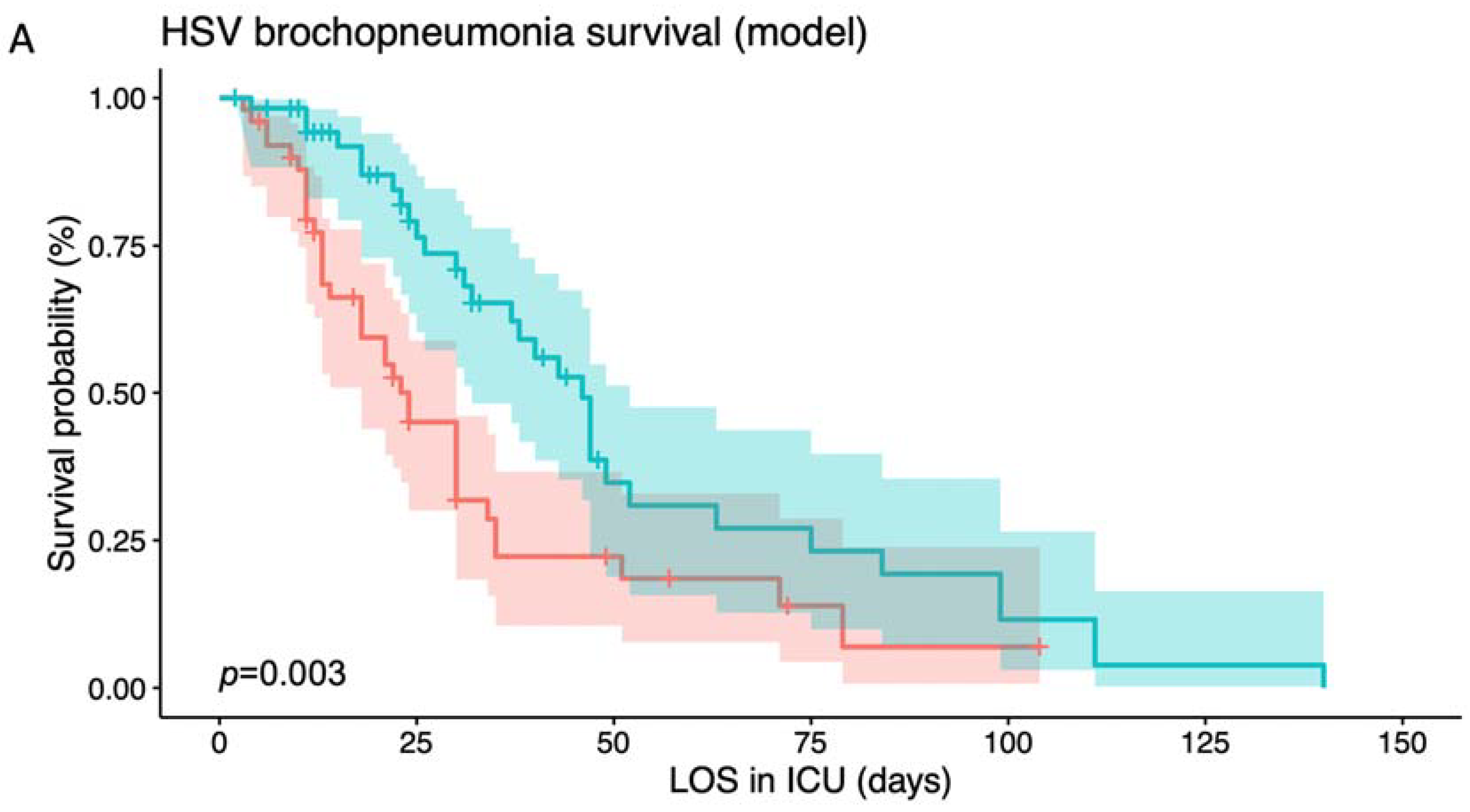
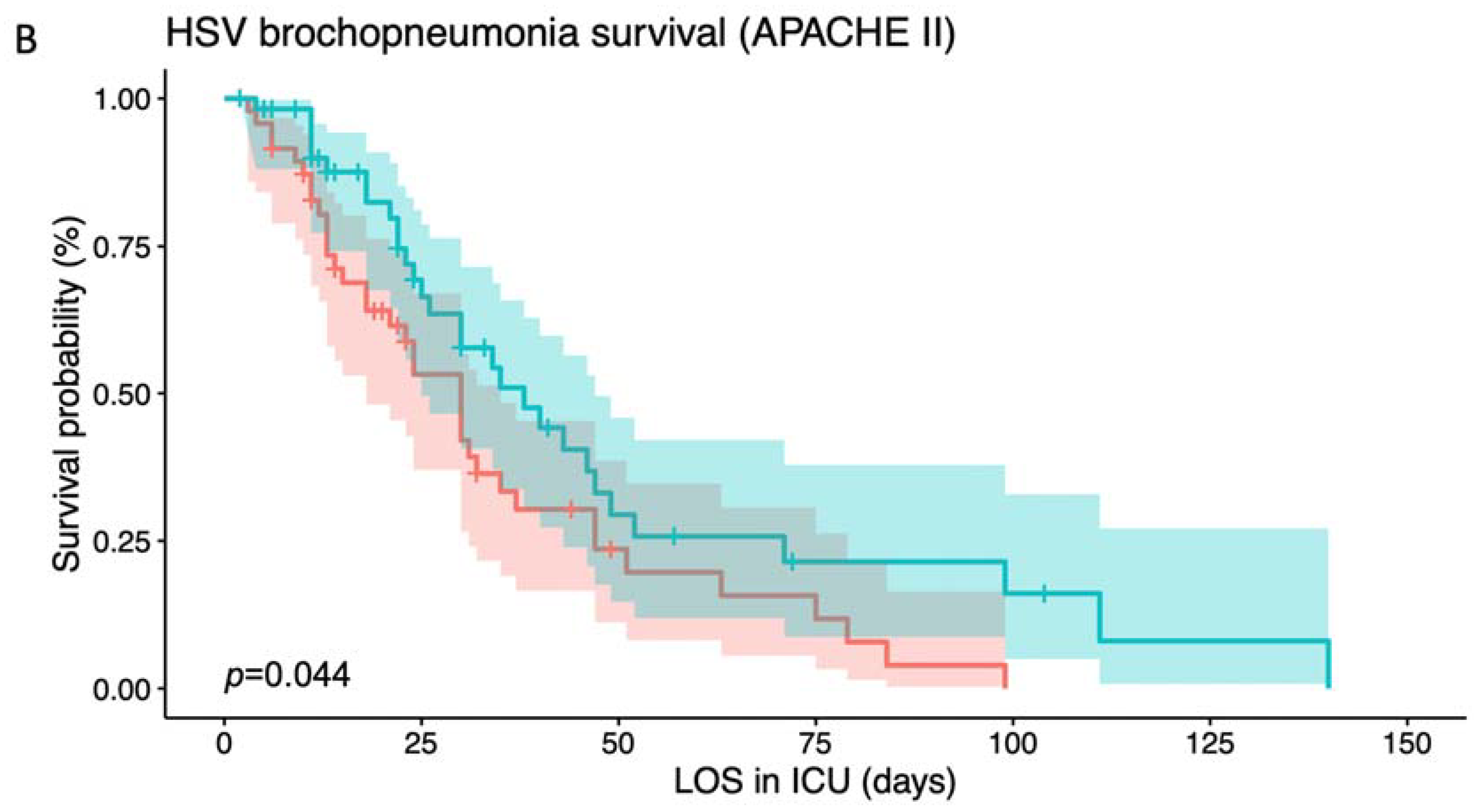
3.2. Risk Stratification Based on Different Indicators
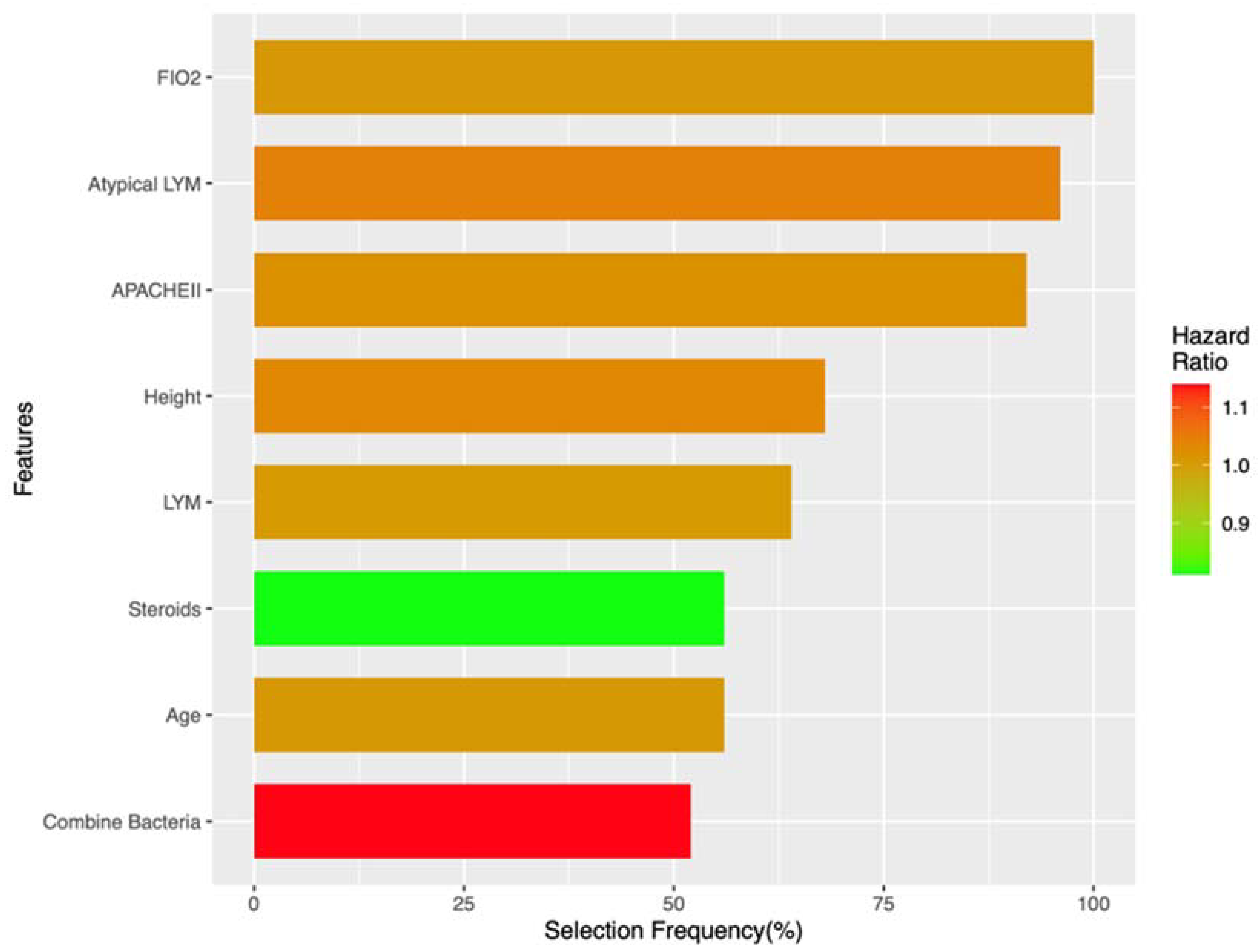
3.3. Risk Factor Identification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Horowitz, R.; Aierstuck, S.; Williams, E.A.; Melby, B. Herpes simplex virus infection in a university health population: Clinical manifestations, epidemiology, and implications. J. Am. Coll. Health 2010, 59, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Ramchandani, M.; Kong, M.; Tronstein, E.; Selke, S.; Mikhaylova, A.; Magaret, A.; Huang, M.L.; Johnston, C.; Corey, L.; Wald, A. Herpes Simplex Virus Type 1 Shedding in Tears and Nasal and Oral Mucosa of Healthy Adults. Sex. Transm. Dis. 2016, 43, 756–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arduino, P.G.; Porter, S.R. Herpes Simplex Virus Type 1 infection: Overview on relevant clinico-pathological features. J. Oral Pathol. Med. 2008, 37, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Aisenberg, G.M.; Torres, H.A.; Tarrand, J.; Safdar, A.; Bodey, G.; Chemaly, R.F. Herpes simplex virus lower respiratory tract infection in patients with solid tumors. Cancer 2009, 115, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Tuxen, D.V.; Cade, J.F.; McDonald, M.I.; Buchanan, M.R.; Clark, R.J.; Pain, M.C. Herpes simplex virus from the lower respiratory tract in adult respiratory distress syndrome. Am. Rev. Respir. Dis. 1982, 126, 416–419. [Google Scholar]
- Luyt, C.E.; Combes, A.; Nieszkowska, A.; Trouillet, J.L.; Chastre, J. Viral infections in the ICU. Curr. Opin. Crit. Care 2008, 14, 605–608. [Google Scholar] [CrossRef]
- Luyt, C.E.; Combes, A.; Deback, C.; Aubriot-Lorton, M.H.; Nieszkowska, A.; Trouillet, J.L.; Capron, F.; Agut, H.; Gibert, C.; Chastre, J. Herpes simplex virus lung infection in patients undergoing prolonged mechanical ventilation. Am. J. Respir. Crit. Care Med. 2007, 175, 935–942. [Google Scholar] [CrossRef]
- Schuierer, L.; Gebhard, M.; Ruf, H.G.; Jaschinski, U.; Berghaus, T.M.; Wittmann, M.; Braun, G.; Busch, D.H.; Hoffmann, R. Impact of acyclovir use on survival of patients with ventilator-associated pneumonia and high load herpes simplex virus replication. Crit. Care 2020, 24, 12. [Google Scholar] [CrossRef] [Green Version]
- Luyt, C.E.; Forel, J.M.; Hajage, D.; Jaber, S.; Cayot-Constantin, S.; Rimmele, T.; Coupez, E.; Lu, Q.; Diallo, M.H.; Penot-Ragon, C.; et al. Acyclovir for Mechanically Ventilated Patients with Herpes Simplex Virus Oropharyngeal Reactivation: A Randomized Clinical Trial. JAMA Intern. Med. 2020, 180, 263–272. [Google Scholar] [CrossRef]
- Gursel, G.; Demirtas, S. Value of APACHE II, SOFA and CPIS scores in predicting prognosis in patients with ventilator-associated pneumonia. Respiration 2006, 73, 503–508. [Google Scholar] [CrossRef]
- Kollef, K.E.; Reichley, R.M.; Micek, S.T.; Kollef, M.H. The modified APACHE II score outperforms Curb65 pneumonia severity score as a predictor of 30-day mortality in patients with methicillin-resistant Staphylococcus aureus pneumonia. Chest 2008, 133, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Mirsaeidi, M.; Peyrani, P.; Ramirez, J.A.; Improving Medicine through Pathway Assessment of Critical Therapy of Hospital-Acquired Pneumonia I. Predicting mortality in patients with ventilator-associated pneumonia: The APACHE II score versus the new IBMP-10 score. Clin. Infect. Dis. 2009, 49, 72–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naeini, A.E.; Abbasi, S.; Haghighipour, S.; Shirani, K. Comparing the APACHE II score and IBM-10 score for predicting mortality in patients with ventilator-associated pneumonia. Adv. Biomed. Res. 2015, 4, 47. [Google Scholar]
- Richards, G.; Levy, H.; Laterre, P.F.; Feldman, C.; Woodward, B.; Bates, B.M.; Qualy, R.L. CURB-65, PSI, and APACHE II to assess mortality risk in patients with severe sepsis and community acquired pneumonia in PROWESS. J. Intensive Care Med. 2011, 26, 34–40. [Google Scholar] [CrossRef]
- Wang, H.Y.; Chang, S.C.; Lin, W.Y.; Chen, C.H.; Chiang, S.H.; Huang, K.Y.; Chu, B.Y.; Lu, J.J.; Lee, T.Y. Machine Learning-Based Method for Obesity Risk Evaluation Using Single-Nucleotide Polymorphisms Derived from Next-Generation Sequencing. J. Comput. Biol. 2018, 25, 1347–1360. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.J.; Wang, H.Y.; Lin, T.W.; Lu, J.J.; Hsieh, C.H.; Liao, C.T. Development of a Machine Learning Model for Survival Risk Stratification of Patients With Advanced Oral Cancer. JAMA Netw. Open 2020, 3, e2011768. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.Y.; Chen, C.H.; Tseng, Y.J.; Tsai, Y.T.; Chang, C.Y.; Wang, H.Y.; Chen, C.K. Predicting post-stroke activities of daily living through a machine learning-based approach on initiating rehabilitation. Int. J. Med. Inform. 2018, 111, 159–164. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.Y.; Chung, C.R.; Horng, J.T.; Lu, J.J.; Lee, T.Y. Large-scale mass spectrometry data combined with demographics analysis rapidly predicts methicillin resistance in Staphylococcus aureus. Brief. Bioinform. 2021, 22, bbaa293. [Google Scholar] [CrossRef] [PubMed]
- Force, A.D.T.; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar]
- Chang, W.J.; Wang, H.Y.; Huang, Y.C.; Lin, C.Y.; Leu, S.W.; Hsieh, M.J.; Huang, C.C. Outcomes of Herpes Simplex Virus Pneumonitis in Critically Ill Patients. Viruses 2022, 14, 205. [Google Scholar] [CrossRef]
- Harrell, F.E.; Lee, K.L.; Mark, D.B. Multivariable Prognostic Models: Issues in Developing Models, Evaluating Assumptions and Adequacy, and Measuring and Reducing Errors. Stat. Med. 1996, 15, 361–387. [Google Scholar] [CrossRef]
- Sacks, D.; Baxter, B.; Campbell, B.C.V.; Carpenter, J.S.; Cognard, C.; Dippel, D.; Eesa, M.; Fischer, U.; Hausegger, K.; Hirsch, J.A.; et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int. J. Stroke 2018, 13, 612–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Wang, J.; Zhang, B.; Li, X.; Liu, Y. Diabetes Mellitus and Cause-Specific Mortality: A Population-Based Study. Diabetes Metab. J. 2019, 43, 319–341. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Christiani, D.C.; Thompson, B.T.; Bajwa, E.K.; Gong, M.N. Role of diabetes in the development of acute respiratory distress syndrome. Crit. Care Med. 2013, 41, 2720–2732. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.B.; Keniston, A.; Gajic, O.; Trillo Alvarez, C.A.; Medvedev, S.; Douglas, I.S. Diabetes mellitus does not adversely affect outcomes from a critical illness. Crit. Care Med. 2010, 38, 16–24. [Google Scholar] [CrossRef]
- Jellinge, M.E.; Hansen, F.; Coia, J.E.; Song, Z. Herpes Simplex Virus Type 1 Pneumonia—A Review. J. Intensive Care Med. 2021, 36, 1398–1402. [Google Scholar] [CrossRef]
- Ufuk, F.; Demirci, M.; Sagtas, E.; Akbudak, I.H.; Ugurlu, E.; Sari, T. The prognostic value of pneumonia severity score and pectoralis muscle Area on chest CT in adult COVID-19 patients. Eur. J. Radiol. 2020, 131, 109271. [Google Scholar] [CrossRef]
- Bak, S.H.; Kwon, S.O.; Han, S.S.; Kim, W.J. Computed tomography-derived area and density of pectoralis muscle associated disease severity and longitudinal changes in chronic obstructive pulmonary disease: A case control study. Respir. Res. 2019, 20, 226. [Google Scholar] [CrossRef] [Green Version]
- Cury, S.S.; de Moraes, D.; Freire, P.P.; de Oliveira, G.; Marques, D.V.P.; Fernandez, G.J.; Dal-Pai-Silva, M.; Hasimoto, E.N.; Dos Reis, P.P.; Rogatto, S.R.; et al. Tumor Transcriptome Reveals High Expression of IL-8 in Non-Small Cell Lung Cancer Patients with Low Pectoralis Muscle Area and Reduced Survival. Cancers 2019, 11, 1251. [Google Scholar] [CrossRef] [Green Version]
| Feature Name | Level | Alive (n = 38) | Non-Survival (n = 66) | p |
|---|---|---|---|---|
| Gender (%) | F | 11 (28.9) | 17 (25.8) | 0.902 |
| M | 27 (71.1) | 49 (74.2) | ||
| Age (mean (SD)) | 66.58 (13.16) | 66.70 (15.50) | 0.968 | |
| Height (mean (SD)) | 162.26 (5.90) | 162.45 (6.30) | 0.882 | |
| BW (mean (SD)) | 60.25 (11.42) | 60.90 (12.43) | 0.792 | |
| BMI (mean (SD)) | 22.93 (4.40) | 23.08 (4.67) | 0.876 | |
| Smoking (%) | NO | 12 (31.6) | 18 (27.3) | 0.809 |
| YES | 26 (68.4) | 48 (72.7) | ||
| Oral Ulcer (%) | NO | 18 (47.4) | 27 (40.9) | 0.664 |
| YES | 20 (52.6) | 39 (59.1) | ||
| APACHE II (mean (SD)) | 25.00 (8.23) | 30.86 (5.70) | <0.001 | |
| CRP (mean (SD)) | 116.74 (82.02) | 122.11 (108.60) | 0.792 | |
| WBC (mean (SD)) | 10,828.95 (5247.49) | 12,342.42 (8524.54) | 0.324 | |
| Lymphocyte percentage (mean (SD)) | 8.21 (5.43) | 8.78 (14.10) | 0.812 | |
| Lymphocyte count (mean (SD)) | 817.43 (614.34) | 618.85 (587.21) | 0.106 | |
| Atypical lymphocyte percentage (mean (SD)) | 0.31 (0.66) | 1.21 (4.64) | 0.24 | |
| HSV alone (%) | NO | 28 (73.7) | 57 (86.4) | 0.178 |
| YES | 10 (26.3) | 9 (13.6) | ||
| Bacteria combined (%) | NO | 16 (42.1) | 24 (36.4) | 0.711 |
| YES | 22 (57.9) | 42 (63.6) | ||
| Fungus combined (%) | NO | 32 (84.2) | 48 (72.7) | 0.273 |
| YES | 6 (15.8) | 18 (27.3) | ||
| Aspergillosis (%) | NO | 37 (97.4) | 58 (87.9) | 0.195 |
| YES | 1 (2.6) | 8 (12.1) | ||
| Combine Virus (%) | NO | 30 (78.9) | 42 (63.6) | 0.159 |
| YES | 8 (21.1) | 24 (36.4) | ||
| PJP (%) | NO | 35 (92.1) | 53 (80.3) | 0.185 |
| YES | 3 (7.9) | 13 (19.7) | ||
| Mycobacterium spp. (%) | NO | 38 (100.0) | 64 (97.0) | 0.732 |
| YES | 0 (0.0) | 2 (3.0) | ||
| Organ failure (%) | NO | 32 (84.2) | 43 (65.2) | 0.063 |
| YES | 6 (15.8) | 23 (34.8) | ||
| Diabetes mellitus (%) | NO | 23 (60.5) | 63 (95.5) | <0.001 |
| YES | 15 (39.5) | 3 (4.5) | ||
| Immunocompromise (%) | NO | 22 (57.9) | 27 (40.9) | 0.142 |
| YES | 16 (42.1) | 39 (59.1) | ||
| Sepsis (%) | NO | 23 (60.5) | 53 (80.3) | 0.05 |
| YES | 15 (39.5) | 13 (19.7) | ||
| Cardiovascular Crisis (%) | NO | 35 (92.1) | 65 (98.5) | 0.271 |
| YES | 3 (7.9) | 1 (1.5) | ||
| CXR.GGO (%) | NO | 33 (86.8) | 59 (89.4) | 0.941 |
| YES | 5 (13.2) | 7 (10.6) | ||
| CXR.Interstitial (%) | NO | 28 (73.7) | 45 (68.2) | 0.713 |
| YES | 10 (26.3) | 21 (31.8) | ||
| CXR.Consolidation (%) | NO | 15 (39.5) | 31 (47.0) | 0.592 |
| YES | 23 (60.5) | 35 (53.0) | ||
| CT.GGO (%) | NO | 37 (97.4) | 63 (95.5) | 1 |
| YES | 1 (2.6) | 3 (4.5) | ||
| CT.Interstitial (%) | NO | 33 (86.8) | 57 (86.4) | 1 |
| YES | 5 (13.2) | 9 (13.6) | ||
| CT.Consolidation (%) | NO | 26 (68.4) | 50 (75.8) | 0.56 |
| YES | 12 (31.6) | 16 (24.2) | ||
| Bronchoscopy (%) | NO | 12 (31.6) | 20 (30.3) | 1 |
| YES | 26 (68.4) | 46 (69.7) | ||
| ARDS (%) | NO | 30 (78.9) | 20 (30.3) | <0.001 |
| YES | 8 (21.1) | 46 (69.7) | ||
| AKI (%) | NO | 25 (65.8) | 14 (21.2) | <0.001 |
| YES | 13 (34.2) | 52 (78.8) | ||
| Steroids (%) | NO | 19 (50.0) | 26 (39.4) | 0.398 |
| YES | 19 (50.0) | 40 (60.6) | ||
| Treatment (%) | NO | 30 (78.9) | 49 (74.2) | 0.762 |
| YES | 8 (21.1) | 17 (25.8) | ||
| Treat enough (%) | NO | 32 (84.2) | 50 (75.8) | 0.443 |
| YES | 6 (15.8) | 16 (24.2) | ||
| PEEP (mean (SD)) | 9.32 (1.76) | 9.36 (1.76) | 0.894 | |
| delta P (mean (SD)) | 16.29 (5.02) | 18.61 (6.08) | 0.049 | |
| PIP (mean (SD)) | 25.87 (5.79) | 27.82 (6.46) | 0.127 | |
| TV (mean (SD)) | 486.13 (88.36) | 468.61 (104.29) | 0.386 | |
| FIO2 (mean (SD)) | 44.47 (13.35) | 54.55 (20.01) | 0.007 | |
| A-a gradient (mean (SD)) | 178.96 (106.36) | 230.33 (133.89) | 0.046 |
| Feature Type | Feature Name | Selection Frequency (%) | HR (SD) |
|---|---|---|---|
| Risky factor | FIO2 | 100 | 1.01 (0.01) |
| Risky factor | Atypical lymphocyte percentage | 96 | 1.04 (0.03) |
| Risky factor | APACHEII | 92 | 1.02 (0.02) |
| Risky factor | Height | 68 | 1.03 (0.05) |
| Risky factor | Lymphocyte percentage | 64 | 1.01 (0.01) |
| Risky factor | Age | 56 | 1.01 (0.01) |
| Protective factor | Steroids | 56 | 0.81 (0.14) |
| Risky factor | Bacteria combined | 52 | 1.14 (0.21) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-C.; Lin, W.-Y.; Tseng, Y.-J.; Fu, Y.; Li, W.; Huang, Y.-C.; Wang, H.-Y. Risk Stratification for Herpes Simplex Virus Pneumonia Using Elastic Net Penalized Cox Proportional Hazard Algorithm with Enhanced Explainability. J. Clin. Med. 2023, 12, 4489. https://doi.org/10.3390/jcm12134489
Wang Y-C, Lin W-Y, Tseng Y-J, Fu Y, Li W, Huang Y-C, Wang H-Y. Risk Stratification for Herpes Simplex Virus Pneumonia Using Elastic Net Penalized Cox Proportional Hazard Algorithm with Enhanced Explainability. Journal of Clinical Medicine. 2023; 12(13):4489. https://doi.org/10.3390/jcm12134489
Chicago/Turabian StyleWang, Yu-Chiang, Wan-Ying Lin, Yi-Ju Tseng, Yiwen Fu, Weijia Li, Yu-Chen Huang, and Hsin-Yao Wang. 2023. "Risk Stratification for Herpes Simplex Virus Pneumonia Using Elastic Net Penalized Cox Proportional Hazard Algorithm with Enhanced Explainability" Journal of Clinical Medicine 12, no. 13: 4489. https://doi.org/10.3390/jcm12134489







