Transient Ischemic Attack Outpatient Clinic: Past Journey and Future Adventure
Abstract
:1. Introduction
2. Models of Care for TIA Patients
3. Challenges of Outpatient TIA Management and Possible Solutions
3.1. Definition, Triage, and Diagnosis
3.2. System Dynamics of Outpatient TIA Clinics
3.3. Outcome Measures and Risk of Life-Threatening Complications
4. Tele-Stroke Centers
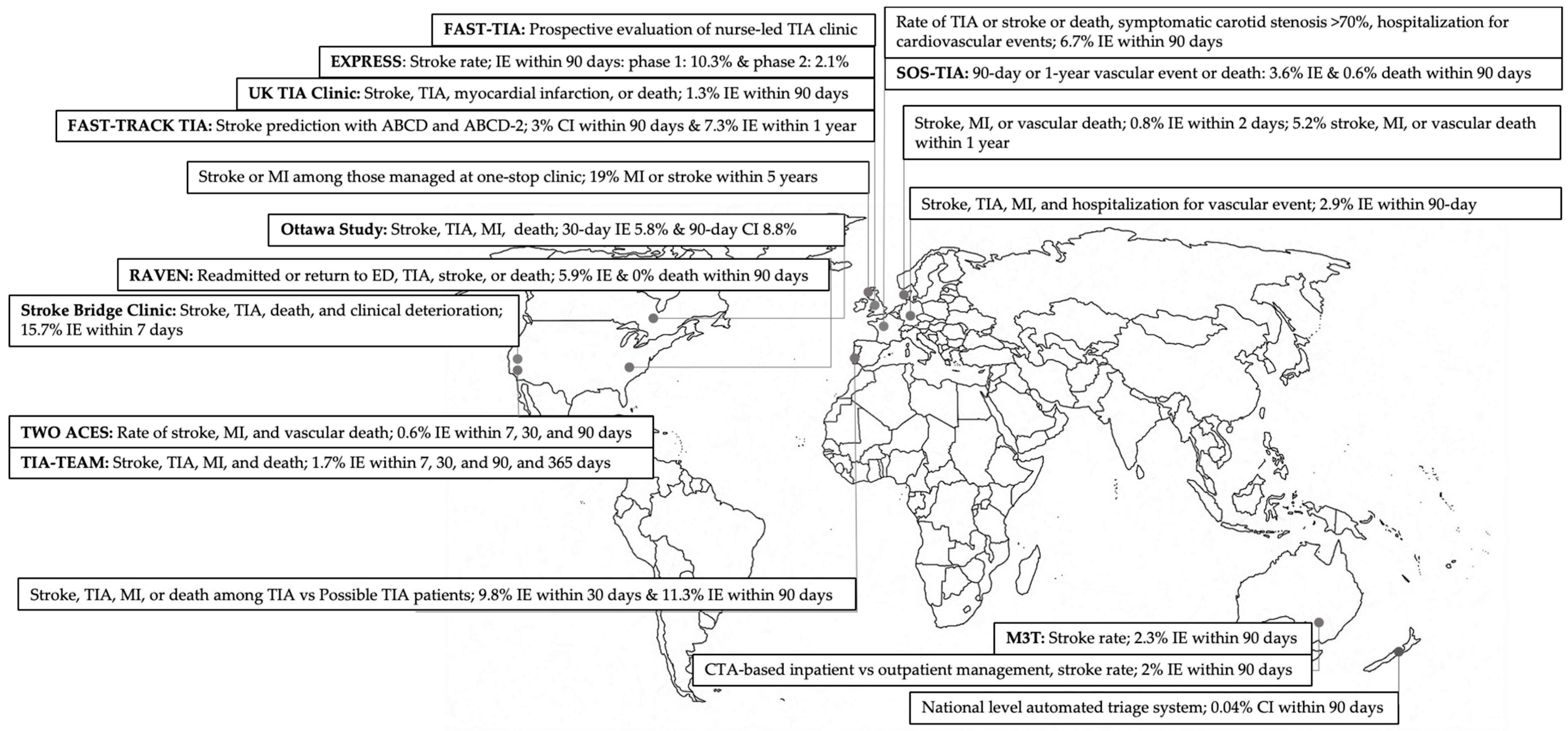
5. Ongoing Clinical Trials for Outpatient TIA Management
6. Future Direction
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Panuganti, K.K.; Tadi, P.; Lui, F. Transient Ischemic Attack; StatPearls Publishing: Tampa, FL, USA, 2019. [Google Scholar]
- Rothwell, P.M.; Warlow, C.P. Timing of TIAs Preceding Stroke Time Window for Prevention Is Very Short. Neurology 2005, 64, 817–820. [Google Scholar] [CrossRef]
- Rothwell, P.M.; Giles, M.F.; Chandratheva, A.; Marquardt, L.; Geraghty, O.; Redgrave, J.N.E.; Lovelock, C.E.; Binney, L.E.; Bull, L.M.; Cuthbertson, F.C.; et al. Effect of Urgent Treatment of Transient Ischaemic Attack and Minor Stroke on Early Recurrent Stroke (EXPRESS Study): A Prospective Population-Based Sequential Comparison. Lancet 2007, 370, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Sanders, L.M.; Srikanth, V.K.; Jolley, D.J.; Sundararajan, V.; Psihogios, H.; Wong, K.; Ramsay, D.; Phan, T.G. Monash Transient Ischemic Attack Triaging Treatment: Safety of a Transient Ischemic Attack Mechanism-Based Outpatient Model of Care. Stroke 2012, 43, 2936–2941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavallée, P.C.; Meseguer, E.; Abboud, H.; Cabrejo, L.; Olivot, J.-M.M.; Simon, O.; Mazighi, M.; Nifle, C.; Niclot, P.; Lapergue, B.; et al. A Transient Ischaemic Attack Clinic with Round-the-Clock Access (SOS-TIA): Feasibility and Effects. Lancet Neurol. 2007, 6, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Lavallée, P.; Amarenco, P. TIA Clinic: A Major Advance in Management of Transient Ischemic Attacks. Front. Neurol. Neurosci. 2013, 33, 30–40. [Google Scholar] [CrossRef]
- Chang, B.P.; Rostanski, S.; Willey, J.; Miller, E.C.; Shapiro, S.; Mehendale, R.; Kummer, B.; Navi, B.B.; Elkind, M.S.V. Safety and Feasibility of a Rapid Outpatient Management Strategy for Transient Ischemic Attack and Minor Stroke: The Rapid Access Vascular Evaluation-Neurology (RAVEN) Approach. Ann. Emerg. Med. 2019, 74, 562–571. [Google Scholar] [CrossRef]
- Olivot, J.M.; Wolford, C.; Castle, J.; Mlynash, M.; Schwartz, N.E.; Lansberg, M.G.; Kemp, S.; Albers, G.W. TWO ACES: Transient Ischemic Attack Work-up as Outpatient Assessment of Clinical Evaluation and Safety. Stroke 2011, 42, 1839–1843. [Google Scholar] [CrossRef] [Green Version]
- Shahjouei, S.; Li, J.; Koza, E.; Abedi, V.; Sadr, A.V.; Chen, Q.; Mowla, A.; Griffin, P.; Ranta, A.; Zand, R. Risk of Subsequent Stroke among Patients Receiving Outpatient vs Inpatient Care for Transient Ischemic Attack: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2022, 5, e2136644. [Google Scholar] [CrossRef]
- Harrison, J.K.; Sloan, B.; Dawson, J.; Lees, K.R.; Morrison, D.S. The ABCD and ABCD2 as Predictors of Stroke in Transient Ischemic Attack Clinic Outpatients: A Retrospective Cohort Study over 14 Years. QJM Int. J. Med. 2010, 103, 679–685. [Google Scholar] [CrossRef] [Green Version]
- Luengo-Fernandez, R.; Li, L.; Silver, L.; Gutnikov, S.; Beddows, N.C.; Rothwell, P.M. Long-Term Impact of Urgent Secondary Prevention after Transient Ischemic Attack and Minor Stroke: Ten-Year Follow-Up of the EXPRESS Study. Stroke 2022, 53, 488–496. [Google Scholar] [CrossRef]
- Banerjee, S.; Natarajan, I.; Biram, R.; Sutton, K.; Ekeng, G.; Ames, D.; Chataway, J. FAST-TIA: A Prospective Evaluation of a Nurse-Led Anterior Circulation TIA Clinic. Postgrad. Med. J. 2009, 85, 637–642. [Google Scholar] [CrossRef]
- Cheong, E.; Toner, P.; Dowie, G.; Jannes, J.; Kleinig, T. Evaluation of a CTA-Triage Based Transient Ischemic Attack Service: A Retrospective Single Center Cohort Study. J. Stroke Cerebrovasc. Dis. 2018, 27, 3436–3442. [Google Scholar] [CrossRef]
- Correia, M.; Fonseca, A.C.; Canhão, P. Short-Term Outcome of Patients with Possible Transient Ischemic Attacks: A Prospective Study. BMC Neurol. 2015, 15, 78. [Google Scholar] [CrossRef] [Green Version]
- Dutta, D. Diagnosis of TIA (DOT) Score—Design and Validation of a New Clinical Diagnostic Tool for Transient Ischaemic Attack. BMC Neurol. 2016, 16, 20. [Google Scholar] [CrossRef] [Green Version]
- Lavallée, P.C.; Sissani, L.; Labreuche, J.; Meseguer, E.; Cabrejo, L.; Guidoux, C.; Klein, I.F.; Touboul, P.-J.J.; Amarenco, P.; Lavallee, P.C.; et al. Clinical Significance of Isolated Atypical Transient Symptoms in a Cohort with Transient Ischemic Attack. Stroke 2017, 48, 1495–1500. [Google Scholar] [CrossRef]
- Majidi, S.; Leon Guerrero, C.R.; Burger, K.M.; Rothrock, J.F. Inpatient versus Outpatient Management of TIA or Minor Stroke: Clinical Outcome. J. Vasc. Interv. Neurol. 2017, 9, 49–53. [Google Scholar]
- Montassier, E.; Lim, T.X.; Goffinet, N.; Guillon, B.; Segard, J.; Martinage, A.; Potel, G.; Le Conte, P. Results of an Outpatient Transient Ischemic Attack Evaluation: A 90-Day Follow-up Study. J. Emerg. Med. 2013, 44, 970–975. [Google Scholar] [CrossRef]
- Vora, N.; Tung, C.E.; Mlynash, M.; Garcia, M.; Kemp, S.; Kleinman, J.; Zaharchuk, G.; Albers, G.; Olivot, J.-M. TIA Triage in Emergency Department Using Acute MRI (TIA-TEAM): A Feasibility and Safety Study. Int. J. Stroke 2015, 10, 343–347. [Google Scholar] [CrossRef]
- Wasserman, J.; Perry, J.; Dowlatshahi, D.; Stotts, G.; Stiell, I.; Sutherland, J.; Symington, C.; Sharma, M. Stratified, Urgent Care for Transient Ischemic Attack Results in Low Stroke Rates. Stroke 2010, 41, 2601–2605. [Google Scholar] [CrossRef] [Green Version]
- Weitzel-Mudersbach, P.V.; Johnsen, S.P.; Andersen, G. Low Risk of Vascular Events Following Urgent Treatment of Transient Ischaemic Attack: The Aarhus TIA Study. Eur. J. Neurol. 2011, 18, 1285–1290. [Google Scholar] [CrossRef]
- Dutta, D.; Bowen, E.; Foy, C. Four-Year Follow-up of Transient Ischemic Attacks, Strokes, and Mimics. Stroke 2015, 46, 1227–1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, C.; Bailey, D.; Hart, S.; Hutchison, A.; Sandercock, P.; Doubal, F.; Sudlow, C.; Farrall, A.; Wardlaw, J.; Dennis, M.; et al. Clinical Diagnosis of TIA or Minor Stroke and Prognosis in Patients with Neurological Symptoms: A Rapid Access Clinic Cohort. PLoS ONE 2019, 14, e0210452. [Google Scholar] [CrossRef] [PubMed]
- Raposo, N.; Albucher, J.F.; Rousseau, V.; Acket, B.; Chollet, F.; Olivot, J.M. ED Referral Dramatically Reduces Delays of Initial Evaluation in a French TIA Clinic. Front. Neurol. 2018, 9, 914. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, E.; Priglinger, M.L.; Bertmar, C.; Day, S.; Borsodi, C.; Herkes, G.; Krause, M. Rapid Access Point of Care Clinic for Transient Ischemic Attacks and Minor Strokes. J. Clin. Neurosci. 2016, 23, 106–110. [Google Scholar] [CrossRef]
- Hermanson, S.; Vora, N.; Blackmore, C.C.; Williams, B.; Isenberg, N. Feasibility and Safety of a Rapid-Access Transient Ischemic Attack Clinic. J. Am. Assoc. Nurse Pract. 2022, 34, 550–556. [Google Scholar] [CrossRef]
- Shapiro, S.D.; Boehme, A.K.; Chang, B.P.; Miller, E.C.; Willey, J.; Elkind, M.S.V. Safety and Hospital Costs Averted Using a Rapid Outpatient Management Strategy for Transient Ischemic Attack and Minor Strokes: The RAVEN Clinic. Neurohospitalist 2021, 11, 107–113. [Google Scholar] [CrossRef]
- Hastrup, S.; Johnsen, S.P.; Jensen, M.; von Weitzel-Mudersbach, P.; Simonsen, C.Z.; Hjort, N.; Møller, A.T.; Harbo, T.; Poulsen, M.S.; Iversen, H.K.; et al. Specialized Outpatient Clinic vs Stroke Unit for TIA and Minor Stroke. Neurology 2021, 96, e1096–e1109. [Google Scholar] [CrossRef]
- Hörer, S.; Schulte-Altedorneburg, G.; Haberl, R.L. Management of Patients with Transient Ischemic Attack Is Safe in an Outpatient Clinic Based on Rapid Diagnosis and Risk Stratification. Cerebrovasc. Dis. 2011, 32, 504–510. [Google Scholar] [CrossRef]
- Australian Stroke Foundation. Clinical Guidelines for Stroke Management—Chapter 2: Early Assessment and Diagnosis; Stroke Foundation: Melbourne, Australia, 2021. [Google Scholar]
- Ranta, A.; Dovey, S.; Weatherall, M.; O’Dea, D.; Gommans, J.; Tilyard, M. Cluster Randomized Controlled Trial of TIA Electronic Decision Support in Primary Care. Neurology 2015, 84, 1545–1551. [Google Scholar] [CrossRef]
- Easton, J.D.; Saver, J.L.; Albers, G.W.; Alberts, M.J.; Chaturvedi, S.; Feldmann, E.; Hatsukami, T.S.; Higashida, R.T.; Johnston, S.C.; Kidwell, C.S.; et al. Definition and Evaluation of Transient Ischemic Attack: A Scientific Statement for Healthcare Professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardio. Stroke 2009, 40, 2276–2293. [Google Scholar] [CrossRef] [Green Version]
- Mlynash, M.; Olivot, J.-M.; Tong, D.C.; Lansberg, M.G.; Eyngorn, I.; Kemp, S.; Moseley, B.M.E.; Albers, G.W. Yield of Combined Perfusion and Diffusion MR Imaging in Hemispheric TIA. Neurology 2009, 72, 1127–1133. [Google Scholar] [CrossRef]
- Sacco, R.L.; Kasner, S.E.; Broderick, J.P.; Caplan, L.R.; Connors, J.J.; Culebras, A.; Elkind, M.S.V.; George, M.G.; Hamdan, A.D.; Higashida, R.T.; et al. An Updated Definition of Stroke for the 21st Century: A Statement for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 2064–2089. [Google Scholar] [CrossRef] [Green Version]
- Fisher, C.M. Intermittent Cerebral Ischemia. In Cerebral Vascular Disease; Grune & Stratton: New York, NY, USA, 1958; pp. 81–97. [Google Scholar]
- Sadighi, A.; Stanciu, A.; Banciu, M.; Abedi, V.; El Andary, N.; Holland, N.; Zand, R. Rate and Associated Factors of Transient Ischemic Attack Misdiagnosis. eNeurologicalSci 2019, 15, 100193. [Google Scholar] [CrossRef]
- Stanciu, A.; Banciu, M.; Sadighi, A.; Marshall, K.A.; Marshall, K.A.; Holland, N.R.; Holland, N.R.; Abedi, V.; Abedi, V.; Zand, R. A Predictive Analytics Model for Differentiating between Transient Ischemic Attacks (TIA) and Its Mimics. BMC Med. Inform. Decis. Mak. 2020, 20, 112. [Google Scholar] [CrossRef]
- Ranta, A.; Cariga, P. Who Should Manage Transient Ischemic Attacks? A Comparison between Stroke Experts, Generalists, and Electronic Decision Support. J. N. Z. Med. Assoc. NZMJ 2013, 126, 8716. [Google Scholar]
- Ranta, A.; Dovey, S.; Gommans, J.; Tilyard, M.; Weatherall, M. Impact of General Practitioner Transient Ischemic Attack Training on 90-Day Stroke Outcomes: Secondary Analysis of a Cluster Randomized Controlled Trial. J. Stroke Cerebrovasc. Dis. 2018, 27, 2014–2018. [Google Scholar] [CrossRef]
- Johnston, S.C.; Amarenco, P.; Denison, H.; Evans, S.R.; Himmelmann, A.; James, S.; Knutsson, M.; Ladenvall, P.; Molina, C.A.; Wang, Y. Ticagrelor and Aspirin or Aspirin Alone in Acute Ischemic Stroke or TIA. N. Engl. J. Med. 2020, 383, 207–217. [Google Scholar] [CrossRef]
- Johnston, S.C.; Easton, J.D.; Farrant, M.; Barsan, W.; Conwit, R.A.; Elm, J.J.; Kim, A.S.; Lindblad, A.S.; Palesch, Y.Y. Clopidogrel and Aspirin in Acute Ischemic Stroke and High-Risk TIA. N. Engl. J. Med. 2018, 379, 215–225. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Zhao, X.; Liu, L.; Wang, D.; Wang, C.; Wang, C.; Li, H.; Meng, X.; Cui, L.; et al. Clopidogrel with Aspirin in Acute Minor Stroke or Transient Ischemic Attack. N. Engl. J. Med. 2013, 369, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, J.; Hill, M.D.; Ryckborst, K.J.; Eliasziw, M.; Demchuk, A.M.; Buchan, A.M. Fast Assessment of Stroke and Transient Ischaemic Attack to Prevent Early Recurrence (FASTER): A Randomised Controlled Pilot Trial. Lancet Neurol. 2007, 6, 961–969. [Google Scholar] [CrossRef]
- Jauch, E.C.; Saver, J.L.; Adams, H.P.; Bruno, A.; Connors, J.J.B.; Demaerschalk, B.M.; Khatri, P.; McMullan, P.W.; Qureshi, A.I.; Rosenfield, K.; et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 870–947. [Google Scholar] [CrossRef] [PubMed]
- Joshi, J.K.; Ouyang, B.; Prabhakaran, S. Should TIA Patients Be Hospitalized or Referred to a Same-Day Clinic? A Decision Analysis. Neurology 2011, 77, 2082–2088. [Google Scholar] [CrossRef] [PubMed]
- Jarhult, S.J.; Howell, M.L.; Barnaure-Nachbar, I.; Chang, Y.; White, B.A.; Amatangelo, M.; Brown, D.F.; Singhal, A.B.; Schwamm, L.H.; Silverman, S.B.; et al. Implementation of a Rapid, Protocol-Based TIA Management Pathway. West. J. Emerg. Med. 2018, 19, 216–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benavente, L.; Calleja, S.; Larrosa, D.; Vega, J.; Mauri, G.; Pascual, J.; Lahoz, C.H. Long Term Evolution of Patients Treated in a TIA Unit. Int. Arch. Med. 2013, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, P.M.; Parikh, N.S.; Heier, L.A.; Ruiz, E.; Fink, M.E.; Navi, B.B.; White, H. Evaluation of Transient Ischemic Attack and Minor Stroke: A Rapid Outpatient Model for the COVID-19 Pandemic and Beyond. Neurohospitalist 2022, 12, 38–47. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke a Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2019, 50, E344–E418. [Google Scholar]
- Lim, A.; Singhal, S.; Lavallee, P.; Amarenco, P.; Rothwell, P.M.; Albers, G.; Sharma, M.; Brown, R.; Ranta, A.; Maddula, M.; et al. An International Report on the Adaptations of Rapid Transient Ischaemic Attack Pathways During the COVID-19 Pandemic. J. Stroke Cerebrovasc. Dis. 2020, 29, 105228. [Google Scholar] [CrossRef]
- Poupore, N.; Strat, D.; Mackey, T.; Brown, K.; Snell, A.; Nathaniel, T.I. Thrombolytic Therapy in Ischemic Stroke Patients with a Preceding Transient Ischemic Attack in Telestroke and Non-telestroke Settings. Neurol. Clin. Neurosci. 2020, 8, 298–308. [Google Scholar] [CrossRef]
- D’Anna, L.; Ellis, N.; Bentley, P.; Brown, Z.; Halse, O.; Jamil, S.; Jenkins, H.; Malik, A.; Kalladka, D.; Kwan, J.; et al. Delivering Telemedicine Consultations for Patients with Transient Ischaemic Attack during the COVID-19 Pandemic in a Comprehensive Tertiary Stroke Centre in the United Kingdom. Eur. J. Neurol. 2021, 28, 3456–3460. [Google Scholar] [CrossRef]
- Mann, D.M.; Chen, J.; Chunara, R.; Testa, P.A.; Nov, O. COVID-19 Transforms Health Care through Telemedicine: Evidence from the Field. J. Am. Med. Inform. Assoc. 2020, 27, 1132–1135. [Google Scholar] [CrossRef]
- University Hospital, Grenoble. Evaluation of the Effectiveness of a City Hospital Care Network for the Care of Patients with Transient Ischemic Accident (TIA). Available online: https://clinicaltrials.gov/ct2/show/NCT05216198 (accessed on 5 April 2023).
- University Hospital, Toulouse. Feasibility Study on the Medical and Economic Consequences of Outpatient Management of TIAs and Minor Strokes (MEDECO-AIT). Available online: https://clinicaltrials.gov/ct2/show/NCT03605355 (accessed on 5 April 2023).
- University of Minnesota Telestroke for Comprehensive Transient Ischemic Attack Care in Acute Stroke Ready Hospitals (TELECAST-TIA). Available online: https://clinicaltrials.gov/ct2/show/NCT03724110 (accessed on 5 April 2023).
| Study, Publication Year; Country | Recruitment | Study Design | Included Patients | Definition of mIS | Definition of TIA | Sample Size (N) | 2-Days Stroke Risk (%) | 7-Days Stroke Risk (%) | 30-Days Stroke Risk (%) | 90-Days Stroke Risk (%) | Mean Age (Year), Men (%) | Hypertension (%) | Diabetes (%) | Myocardial Infarction (%) | Ischemic Heart Disease (%) | Dyslipidemia (%) | Atrial Fibrillation (%) | Carotid Stenosis (%) | Smoking (%) | Prior TIA (%) | Prior Stroke (%) | ABCD2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chang et al. (RAVEN), [7] 2019; USA | 2016–2018 | R | TIA andmIS | - | Clsc | 101 | - | - | - | 0.9 | -, - | - | - | - | - | - | - | - | - | - | - | - |
| Cheong et al., [13] 2019; Australia | 2012–2016 | P | TIA | NA | T. B. | 306 | 0.0 | 0.3 | 0.3 | 1.3 | 68, - | 58.0 | 18.0 | - | 12.0 | 48.0 | 10.0 | - | 17.0 | - | - | Median: 4 [3–5] |
| Correia et al., [14] 2015; Portugal | 2004–2009 | P | TIA | NA | T. B. | 258 | - | - | 3.1 | 4.0 | 68, 59.7 | 73.5 | 17.1 | - | 9.7 | 47.3 | 8.9 | 76.7 | 67.7 | 8.5 | 2.3 | >4:72% |
| Dutta et al., [15] 2016; UK | 2010–2012 | R | TIA and mIS | <24 h, Pos Imaging | Clsc | 529 | - | - | - | 1.3 | -, - | - | - | - | - | - | - | - | - | - | - | >4:31.5% |
| Harrison et al. (Fast-Track TIA), [10] 2010; UK | 1992–2004 | R | TIA | NA | Clsc | 795 | - | - | - | 3.0 | 76, 43.0 | 29.2 | 8.2 | - | - | - | - | - | - | - | - | - |
| Lavallée et al. (SOS-TIA), [16] 2017; France | 2003–2008 | P | TIA and mIS | >24 h | T. B. | 1850 | - | - | - | 3.6 | 63, 52.0 | 65.1 | 10.7 | 9.3 | 9.3 | 39.6 | 7.9 | 10.4 | 21.4 | 22.0 | 4.4 | >4:48.2% |
| Majidi et al. (Stroke Bridge Clinic), [17] 2017; USA | 2013–2014 | R | TIA and mIS | NIHSS ≤ 3 | Clsc | 22 | - | 4.5 | - | - | -, - | - | - | - | - | - | - | - | - | - | - | - |
| Montassier et al., [18] 2013; France | 2009–2009 | P | TIA | NA | T. B. | 62 | 1.7 | 1.7 | 1.7 | 1.7 | 73.1, 46.7 | 45.0 | 11.7 | - | 8.3 | 21.7 | 8.3 | - | 6.7 | 8.3 | 1.7 | >4:28.3% |
| Olivot et al. (TWO ACES), [8] 2011; USA | 2007–2009 | P | TIA and mIS | ≥24 h | Clsc | 157 | - | 0.6 | 0.6 | 0.6 | 67, 55.0 | 49.0 | 11.0 | 3.0 | - | 51.0 | 16.0 | 0.7 | 6.0 | - | 15.0 | Median: 3 [3,4] |
| Sanders et al. (M3T), [4] 2012; Australia | 2004–2007 | P | TIA | NA | Clsc | 301 | - | - | - | 2.4 | 67.7, 58.1 | 67.4 | 26.6 | - | - | 59.5 | 14.6 | 14.3 | - | - | - | - |
| Vora et al. (TIA-TEAM), [19] 2015; USA | 2010–2011 | P | TIA | NA | Clsc | 58 | 0.0 | 1.7 | 1.7 | 1.7 | -, - | - | - | - | - | - | - | - | - | - | - | Median: 3 [2–4] |
| Wasserman et al. (Ottawa), [20] 2010; Canada | 2007–2009 | P | TIA | NA | Clsc | 982 | 1.0 | 1.9 | 2.6 | 3.2 | 67, 50.9 | 58.1 | 19.9 | - | 16.6 | 32.5 | 8.6 | 45.0 | 13.3 | - | 11.7 | >4:67% |
| Weitzel-Mudersbach et al., [21] 2011; Denmark | 2007–2008 | P | TIA | NA | Clsc | 107 | 0.9 | - | - | - | -, - | - | - | - | - | - | - | - | - | - | - | - |
| Study and Outcome | Patient Stratification Strategy | Management | Clinic Details | Considerations |
|---|---|---|---|---|
| Chang et al. (RAVEN), [7] 2019; USA Outcome: RAVEN feasibility and safety; 90-day readmission or return to the ED; and risk of TIA, stroke, or death. | Clinic:
| ED:
|
|
|
| Cheong et al., [13] 2019; Australia Outcome: 90-day stroke risk. | Stroke Unit:
| ED:
|
|
|
| Correia et al., [14] 2015; PortugalOutcome: 30- and 90-day risk of stroke, TIA, MI, or vascular death among TIA versus possible TIA patients. |
| ED:
|
|
|
| Dutta et al., [15] 2016; UK Outcome: 90-day, 1-year, 2-year, and end-of-follow-up risk of stroke, MI, any vascular event (TIA, stroke, or MI), and all-cause death. |
| Clinic:
|
|
|
| Harrison et al. (Fast-Track TIA), [10] 2010; UK Outcome: Evaluate the prognostic value of ABCD and ABCD2 in subsequent stroke prediction up to 14 years. |
| Details are not available. |
|
|
| Lavallée et al. (SOS-TIA), [16] 2017; France Outcome: 90-day and 1-year risk of stroke, MI, or vascular death; compare investigational findings between typical and atypical transient symptoms. | Clinic:
| Clinic: Based on a vascular neurologist’s opinion:
|
|
|
| Majidi et al. (stroke bridge clinic), 2017; [17] USA Outcome: 7-day clinically detectable stroke, TIA, death, and clinical deterioration in the minor stroke subset. |
| ED:
|
|
|
| Montassier et al., [18] 2013; France Outcome: 90-day TIA, stroke, death, and risk of stroke using ABCD2, symptomatic carotid stenosis > 70%, hospitalization for cardiovascular events. | Clinic:
| ED:
|
|
|
| Olivot et al. (TWO ACES), [8] 2011; USA Outcome: 7-, 30-, and 90-day stroke, MI, and vascular death; program evaluation. |
| ED:
|
|
|
| Sanders et al. (M3T), [4] 2012; Australia Outcome: 90-day stroke. |
| ED:
|
|
|
| Vora et al. (TIA-TEAM), [19] 2015; USA Outcome: 7-, 90-, and 365-day risk of TIA, stroke, MI, or death, and compare with DWI and ABCD2 scores for stroke prediction rates. | Inpatient:
| ED:
|
|
|
| Wasserman et al. (Ottawa), [20] 2010; Canada Outcome: 2-, 7-, 30-, and 90-day risk of stroke, TIA, MI, and death. | Inpatient:
| ED:
|
|
|
| Weitzel-Mudersbach et al., [21] 2011; Denmark Outcome: 1-year combined risk of stroke, MI, or vascular death; 1-year secondary prevention compliance; 7- and 90-day and 1-year cumulated stroke risk. | Inside the primary coverage area:
| Stroke Unit: First outpatient visits or 24 h in the stroke unit:
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahjouei, S.; Seyedmirzaei, H.; Abedi, V.; Zand, R. Transient Ischemic Attack Outpatient Clinic: Past Journey and Future Adventure. J. Clin. Med. 2023, 12, 4511. https://doi.org/10.3390/jcm12134511
Shahjouei S, Seyedmirzaei H, Abedi V, Zand R. Transient Ischemic Attack Outpatient Clinic: Past Journey and Future Adventure. Journal of Clinical Medicine. 2023; 12(13):4511. https://doi.org/10.3390/jcm12134511
Chicago/Turabian StyleShahjouei, Shima, Homa Seyedmirzaei, Vida Abedi, and Ramin Zand. 2023. "Transient Ischemic Attack Outpatient Clinic: Past Journey and Future Adventure" Journal of Clinical Medicine 12, no. 13: 4511. https://doi.org/10.3390/jcm12134511






