Lymphatic Complications Following Sentinel Node Biopsy or Pelvic Lymphadenectomy for Endometrial Cancer
Abstract
1. Introduction
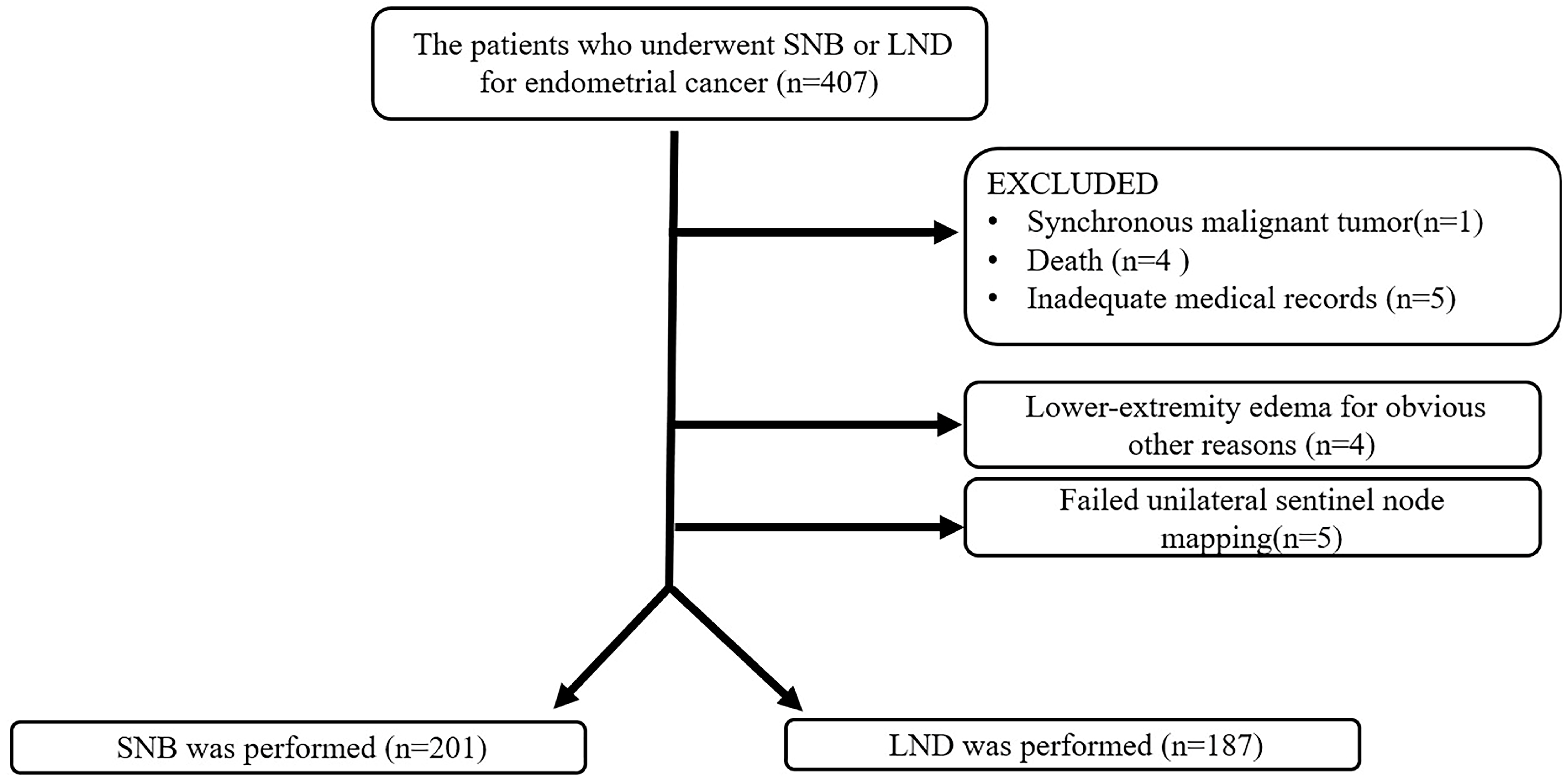
2. Materials and Methods
2.1. Participants
2.2. Sentinel Node Biopsy
2.3. Diagnosis of the Lower-Extremity Lymphedema and Pelvic Lymphocele
2.4. Statistical Analysis
3. Results
3.1. Characteristics
3.2. Occurrence Rates of LEL and PL
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chan, J.K.; Kapp, D.S.; Cheung, M.K.; Osann, K.; Shin, J.Y.; Cohn, D.; Seid, P.L. The impact of the absolute number and ratio of positive lymph nodes on survival of endometrioid uterine cancer patients. Br. J. Cancer 2007, 97, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Morrow, C.P.; Bundy, B.N.; Kurman, R.J.; Creasman, W.T.; Heller, P.; Homesley, H.D.; Graham, J.E. Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: A Gynecologic Oncology Group study. Gynecol. Oncol. 1991, 40, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kim, H.S.; Kim, T.J.; Chang, S.J.; Kim, D.Y.; Ryu, S.Y.; Kim, B.G.; Kim, Y.T.; Bae, D.S.; Ryu, H.S.; et al. Survival impact based on the thoroughness of pelvic lymphadenectomy in intermediate- or high-risk groups of endometrioid-type endometrial cancer: A multi-center retrospective cohort analysis. Gynecol. Oncol. 2016, 141, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.C.; Macdonald, O.K.; Lee, C.M.; Gaffney, D.K. Survival impact of lymph node dissection in endometrial adenocarcinoma: A surveillance, epidemiology, and end results analysis. Int. J. Gynecol. Cancer 2008, 18, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Trimble, E.L.; Kosary, C.; Park, R.C. Lymph node sampling and survival in endometrial cancer. Gynecol. Oncol. 1998, 71, 340–343. [Google Scholar] [CrossRef]
- Chan, J.K.; Cheung, M.K.; Huh, W.K.; Osann, K.; Husain, A.; Teng, N.N.; Kapp, D.S. Therapeutic role of lymph node resection in endometrioid corpus cancer: A study of 12,333 patients. Cancer 2006, 107, 1823–1830. [Google Scholar] [CrossRef]
- Zhao, L.; Li, L.; Ye, Y.; Han, X.; Fu, X.; Yu, Y.; Luo, J. Lymphadenectomy and prognosis for elderly females with stage I endometrioid endometrial cancer. Arch. Gynecol. Obstet. 2019, 300, 683–691. [Google Scholar] [CrossRef]
- Kilgore, L.C.; Partridge, E.E.; Alvarez, R.D.; Austin, J.M.; Shingleton, H.M.; Noojin, F., 3rd; Conner, W. Adenocarcinoma of the endometrium: Survival comparisons of patients with and without pelvic node sampling. Gynecol. Oncol. 1995, 56, 29–33. [Google Scholar] [CrossRef]
- Yenen, M.C.; Dilek, S.; Dede, M.; Goktolga, U.; Deveci, M.S.; Aydogu, T. Pelvic-paraaortic lymphadenectomy in clinical Stage I endometrial adenocarcinoma: A multicenter study. Eur. J. Gynaecol. Oncol. 2003, 24, 327–329. [Google Scholar]
- Kitchener, H.; Swart, A.M.; Qian, Q.; Amos, C.; Parmar, M.K. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): A randomised study. Lancet 2009, 373, 125–136. [Google Scholar]
- Benedetti Panici, P.; Basile, S.; Maneschi, F.; Alberto Lissoni, A.; Signorelli, M.; Scambia, G.; Angioli, R.; Tateo, S.; Mangili, G.; Katsaros, D.; et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: Randomized clinical trial. J. Natl. Cancer Inst. 2008, 100, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- Frost, J.A.; Webster, K.E.; Bryant, A.; Morrison, J. Lymphadenectomy for the management of endometrial cancer. Cochrane Database Syst. Rev. 2017, 10, CD007585. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.; Huang, H.Q.; Armer, J.; Carlson, J.W.; Lockwood, S.; Nolte, S.; Kauderer, J.; Hutson, A.; Walker, J.L.; Fleury, A.C.; et al. GOG 244—The Lymphedema and Gynecologic cancer (LeG) study: The impact of lower-extremity lymphedema on quality of life, psychological adjustment, physical disability, and function. Gynecol. Oncol. 2021, 160, 244–251. [Google Scholar] [CrossRef]
- Bowman, C.; Piedalue, K.A.; Baydoun, M.; Carlson, L.E. The Quality of Life and Psychosocial Implications of Cancer-Related Lower-Extremity Lymphedema: A Systematic Review of the Literature. J. Clin. Med. 2020, 9, 3200. [Google Scholar] [CrossRef] [PubMed]
- Dessources, K.; Aviki, E.; Leitao, M.M., Jr. Lower extremity lymphedema in patients with gynecologic malignancies. Int. J. Gynecol. Cancer 2020, 30, 252–260. [Google Scholar] [CrossRef]
- Lindqvist, E.; Wedin, M.; Fredrikson, M.; Kjolhede, P. Lymphedema after treatment for endometrial cancer—A review of prevalence and risk factors. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 211, 112–121. [Google Scholar] [CrossRef]
- Carlson, J.W.; Kauderer, J.; Hutson, A.; Carter, J.; Armer, J.; Lockwood, S.; Nolte, S.; Stewart, B.R.; Wenzel, L.; Walker, J.; et al. GOG 244-The lymphedema and gynecologic cancer (LEG) study: Incidence and risk factors in newly diagnosed patients. Gynecol. Oncol. 2020, 156, 467–474. [Google Scholar] [CrossRef]
- Pigott, A.; Obermair, A.; Janda, M.; Vagenas, D.; Ward, L.C.; Reul-Hirche, H.; Hayes, S.C. Incidence and risk factors for lower limb lymphedema associated with endometrial cancer: Results from a prospective, longitudinal cohort study. Gynecol. Oncol. 2020, 158, 375–381. [Google Scholar] [CrossRef]
- Wedin, M.; Stalberg, K.; Marcickiewicz, J.; Ahlner, E.; Akesson, A.; Lindahl, G.; Kjolhede, P.; LASEC Study Group. Incidence of lymphedema in the lower limbs and lymphocyst formation within one year of surgery for endometrial cancer: A prospective longitudinal multicenter study. Gynecol. Oncol. 2020, 159, 201–208. [Google Scholar] [CrossRef]
- Rebegea, L.F.; Stoleriu, G.; Manolache, N.; Serban, C.; Craescu, M.; Lupu, M.N.; Voinescu, D.C.; Firescu, D.; Ciobotaru, O.R. Associated risk factors of lower limb lymphedema after treatment of cervical and endometrial cancer. Exp. Ther. Med. 2020, 20, 181. [Google Scholar] [CrossRef]
- Marchocki, Z.; Cusimano, M.C.; Clarfield, L.; Kim, S.R.; Fazelzad, R.; Espin-Garcia, O.; Bouchard-Fortier, G.; Rossi, E.C.; Stewart, K.I.; Soliman, P.T.; et al. Sentinel lymph node biopsy in high-grade endometrial cancer: A systematic review and meta-analysis of performance characteristics. Am. J. Obstet. Gynecol. 2021, 225, 367.e1–367.e39. [Google Scholar] [CrossRef]
- Rossi, E.C.; Kowalski, L.D.; Scalici, J.; Cantrell, L.; Schuler, K.; Hanna, R.K.; Method, M.; Ade, M.; Ivanova, A.; Boggess, J.F. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): A multicenter, prospective, cohort study. Lancet Oncol. 2017, 18, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Hagen, B.; Valla, M.; Aune, G.; Ravlo, M.; Abusland, A.B.; Araya, E.; Sundset, M.; Tingulstad, S. Indocyanine green fluorescence imaging of lymph nodes during robotic-assisted laparoscopic operation for endometrial cancer. A prospective validation study using a sentinel lymph node surgical algorithm. Gynecol. Oncol. 2016, 143, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Holloway, R.W.; Gupta, S.; Stavitzski, N.M.; Zhu, X.; Takimoto, E.L.; Gubbi, A.; Bigsby, G.E.; Brudie, L.A.; Kendrick, J.E.; Ahmad, S. Sentinel lymph node mapping with staging lymphadenectomy for patients with endometrial cancer increases the detection of metastasis. Gynecol. Oncol. 2016, 141, 206–210. [Google Scholar] [CrossRef] [PubMed]
- How, J.A.; O’Farrell, P.; Amajoud, Z.; Lau, S.; Salvador, S.; How, E.; Gotlieb, W.H. Sentinel lymph node mapping in endometrial cancer: A systematic review and meta-analysis. Minerva Ginecol. 2018, 70, 194–214. [Google Scholar] [CrossRef]
- Bogani, G.; Ditto, A.; Chiappa, V.; Raspagliesi, F. Sentinel node mapping in endometrial cancer. Transl. Cancer Res. 2019, 8, 2218–2219. [Google Scholar] [CrossRef] [PubMed]
- Geppert, B.; Lonnerfors, C.; Bollino, M.; Persson, J. Sentinel lymph node biopsy in endometrial cancer-Feasibility, safety and lymphatic complications. Gynecol. Oncol. 2018, 148, 491–498. [Google Scholar] [CrossRef]
- Polan, R.M.; Rossi, E.C.; Barber, E.L. Extent of lymphadenectomy and postoperative major complications among women with endometrial cancer treated with minimally invasive surgery. Am. J. Obstet. Gynecol. 2019, 220, 263.e1–263.e8. [Google Scholar] [CrossRef] [PubMed]
- Casarin, J.; Multinu, F.; Tortorella, L.; Cappuccio, S.; Weaver, A.L.; Ghezzi, F.; Cilby, W.; Kumar, A.; Langstraat, C.; Glaser, G.; et al. Sentinel lymph node biopsy for robotic-assisted endometrial cancer staging: Further improvement of perioperative outcomes. Int. J. Gynecol. Cancer 2020, 30, 41–47. [Google Scholar] [CrossRef]
- Leitao, M.M., Jr.; Zhou, Q.C.; Gomez-Hidalgo, N.R.; Iasonos, A.; Baser, R.; Mezzancello, M.; Chang, K.; Ward, J.; Chi, D.S.; Long Roche, K.; et al. Patient-reported outcomes after surgery for endometrial carcinoma: Prevalence of lower-extremity lymphedema after sentinel lymph node mapping versus lymphadenectomy. Gynecol. Oncol. 2020, 156, 147–153. [Google Scholar] [CrossRef]
- Accorsi, G.S.; Paiva, L.L.; Schmidt, R.; Vieira, M.; Reis, R.; Andrade, C. Sentinel Lymph Node Mapping vs Systematic Lymphadenectomy for Endometrial Cancer: Surgical Morbidity and Lymphatic Complications. J. Minim. Invasive Gynecol. 2020, 27, 938–945.e2. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, V.A.; Riemma, G.; Rosati, A.; Vargiu, V.; Granese, R.; Ercoli, A.; Cianci, S. Surgical complications occurring during minimally invasive sentinel lymph node detection in endometrial cancer patients. A systematic review of the literature and metanalysis. Eur. J. Surg. Oncol. 2021, 47, 2142–2149. [Google Scholar] [CrossRef] [PubMed]
- Chiu, W.K.; Kwok, S.T.; Wang, Y.; Luk, H.M.; Chan, A.H.Y.; Tse, K.Y. Applications and Safety of Sentinel Lymph Node Biopsy in Endometrial Cancer. J. Clin. Med. 2022, 11, 6462. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Terai, Y.; Fujiwara, S.; Tanaka, Y.; Sasaki, H.; Tsunetoh, S.; Yamamoto, K.; Yamada, T.; Ohmichi, M. The detection of sentinel lymph nodes in laparoscopic surgery can eliminate systemic lymphadenectomy for patients with early stage endometrial cancer. Int. J. Clin. Oncol. 2018, 23, 305–313. [Google Scholar] [CrossRef]
- Tanaka, T.; Terai, Y.; Yamamoto, K.; Yamada, T.; Ohmichi, M. The diagnostic accuracy of fluorodeoxyglucose-positron emission tomography/computed tomography and sentinel node biopsy in the prediction of pelvic lymph node metastasis in patients with endometrial cancer: A retrospective observational study. Medicine 2018, 97, e12522. [Google Scholar] [CrossRef]
- Tanaka, T.; Miyamoto, S.; Terada, S.; Kogata, Y.; Fujiwara, S.; Tanaka, Y.; Taniguchi, K.; Komura, K.; Yamamoto, K.; Yamada, T.; et al. The Diagnostic Accuracy of an Intraoperative Frozen Section Analysis and Imprint Cytology of Sentinel Node Biopsy Specimens from Patients with Uterine Cervical and Endometrial Cancer: A Retrospective Observational Study. Pathol. Oncol. Res. 2020, 26, 2273–2279. [Google Scholar] [CrossRef]
- Abu-Rustum, N.R.; Khoury-Collado, F.; Pandit-Taskar, N.; Soslow, R.A.; Dao, F.; Sonoda, Y.; Levine, D.A.; Brown, C.L.; Chi, D.S.; Barakat, R.R.; et al. Sentinel lymph node mapping for grade 1 endometrial cancer: Is it the answer to the surgical staging dilemma? Gynecol. Oncol. 2009, 113, 163–169. [Google Scholar] [CrossRef]
- Executive, C. The Diagnosis and Treatment of Peripheral Lymphedema: 2016 Consensus Document of the International Society of Lymphology. Lymphology 2016, 49, 170–184. [Google Scholar]
- Glaser, G.; Dinoi, G.; Multinu, F.; Yost, K.; Al Hilli, M.; Larish, A.; Kumar, A.; McGree, M.; Weaver, A.L.; Cheville, A.; et al. Reduced lymphedema after sentinel lymph node biopsy versus lymphadenectomy for endometrial cancer. Int. J. Gynecol. Cancer 2021, 31, 85–91. [Google Scholar] [CrossRef]
- Hareyama, H.; Hada, K.; Goto, K.; Watanabe, S.; Hakoyama, M.; Oku, K.; Hayakashi, Y.; Hirayama, E.; Okuyama, K. Prevalence, classification, and risk factors for postoperative lower extremity lymphedema in women with gynecologic malignancies: A retrospective study. Int. J. Gynecol. Cancer 2015, 25, 751–757. [Google Scholar] [CrossRef]
- Watanabe, Y.; Koshiyama, M.; Seki, K.; Nakagawa, M.; Ikuta, E.; Oowaki, M.; Sakamoto, S.I. Development and Themes of Diagnostic and Treatment Procedures for Secondary Leg Lymphedema in Patients with Gynecologic Cancers. Healthcare 2019, 7, 101. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.; Huang, H.Q.; Armer, J.; Carlson, J.W.; Lockwood, S.; Nolte, S.; Stewart, B.R.; Kauderer, J.; Hutson, A.; Walker, J.L.; et al. GOG 244—The LymphEdema and Gynecologic cancer (LEG) study: The association between the gynecologic cancer lymphedema questionnaire (GCLQ) and lymphedema of the lower extremity (LLE). Gynecol. Oncol. 2019, 155, 452–460. [Google Scholar] [CrossRef]
- Zikan, M.; Fischerova, D.; Pinkavova, I.; Slama, J.; Weinberger, V.; Dusek, L.; Cibula, D. A prospective study examining the incidence of asymptomatic and symptomatic lymphoceles following lymphadenectomy in patients with gynecological cancer. Gynecol. Oncol. 2015, 137, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Kim, J.W.; Kim, S.H.; Kim, Y.T.; Kim, J.H. An analysis of the risk factors and management of lymphocele after pelvic lymphadenectomy in patients with gynecologic malignancies. Cancer Res. Treat. 2004, 36, 377–383. [Google Scholar] [CrossRef]
- Yoo, B.; Ahn, H.; Kim, M.; Suh, D.H.; Kim, K.; No, J.H.; Kim, Y.B. Nomogram predicting risk of lymphocele in gynecologic cancer patients undergoing pelvic lymph node dissection. Obstet. Gynecol. Sci. 2017, 60, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Kim, B.W. The incidence of postoperative symptomatic lymphocele after pelvic lymphadenectomy between abdominal and laparoscopic approach: A systemic review and meta-analysis. Surg. Endosd. 2022, 36, 7114–7125. [Google Scholar] [CrossRef]
- Jansen, A.; de Jong, A.; Hoogendam, J.P.; Baeten, I.G.T.; Jürgenliemk-Schulz, I.M.; Zweemer, R.P.; Gerestein, C.G. Lymphocele following lymph node dissection in cervical and endometrial cancer: A systematic review and meta-analysis. Gynecol. Oncol. 2023, 170, 273–281. [Google Scholar] [CrossRef]
- Tanaka, T.; Ueda, S.; Miyamoto, S.; Terada, S.; Konishi, H.; Kogata, Y.; Fujiwara, S.; Tanaka, Y.; Taniguchi, K.; Komura, K.; et al. Oncologic outcomes for patients with endometrial cancer who received minimally invasive surgery: A retrospective observational study. Int. J. Clin. Oncol. 2020, 25, 1985–1994. [Google Scholar] [CrossRef]

| SNB | LND | p Value | |
|---|---|---|---|
| Number of patients | 201 | 187 | |
| Age (years) * | 56.4 ± 10.6 | 57.4 ± 11.1 | 0.35 |
| BMI * | 24.6 ± 5.4 | 23.3 ± 6.8 | 0.03 |
| FIGO stage (%) | |||
| I | 199 (99.0) | 175 (93.6) | <0.01 |
| II | 0 (0) | 0 (0) | |
| III | 2 (1.0) | 12 (6.4) | |
| Histological type (%) | 0.89 | ||
| Endometrioid grade 1 or 2 | 196 (97.5) | 170 (90.9) | |
| Endometrioid grade 3 | 3 (1.5) | 8 (4.3) | |
| Serous carcinoma | 1 (0.5) | 5 (2.7) | |
| Carcinosarcoma | 1 (0.5) | 1 (0.5) | |
| Other | 0 | 3 (1.6) | |
| Surgical Approach (%) | <0.01 | ||
| Laparoscopy | 96 (47.8) | 177 (94.7) | |
| Robotic | 105 (52.2) | 10 (5.3) | |
| Number of LNs removed * | 3.0 ± 1.2 | 33.8 ± 13 | <0.01 |
| Lymph node metastasis (%) | 0 | 3 (1.6) | 0.07 |
| Adjuvant therapy (%) | |||
| Chemotherapy | 22 (10.9) | 37 (19.8) | <0.02 |
| Radiation | 0 | 1 (0.6) | 0.5 |
| Follow-up, median months (IQR) | 28 (16–40) | 73 (58–89) | <0.01 |
| SNB | LND | p Value | |
|---|---|---|---|
| Number of patients | 201 | 187 | |
| LEL (%) | 4 (2.0) | 40 (21.3) | <0.01 |
| Grade 1 | 4 (2.0) | 32 (17.1) | <0.01 |
| Grade 2 | 0 | 8 (4.3) | <0.01 |
| Grade 3 | 0 | 0 | |
| Median months to LEL development (IQR) | 10 (5–20) | 18 (8–37) | 0.2 |
| PL (%) | 0 | 4 (2.1) |
| Authors | Number of Patients | Follow Up | Method | Incidence of Lower Extremity Lymphedema | Incidence of Pelvic Lymphocele | p Value |
|---|---|---|---|---|---|---|
| Geppert et al. (2018) [27] | SNB (n = 76) LND* (n = 83) | 12 months (12–32) | CTC version 3.0 | 1 (1.3%) 15 (18.1%) | 2 (2.6%) 11 (13.3%) | <0.01 |
| Leitao et al. (2019) [30] | SNB (n = 180) LND** (n = 352) | 63 months (44–101) 93 months (44–131) | LELPRO survey | 49 (27.2%) 144 (40.9%) | NR | <0.01 |
| Accorsi et al. (2018) [31] | SNB (n = 61) LND** (n = 89) | 90 days | MSKCCSSEGS | 0 (0%) 9 (10.1%) | NR | <0.01 |
| Glaser et al. (2020) [39] | SNB (n = 127) LND** (n = 41) | 25 months (21–29) 51 months (32–72) | LEL screening questions | 33 (26.0%) 41 (39.0%) | NR | <0.01 |
| Our study | SNB (n = 201) LND*** (n = 247) | 28 months (16–40) 73 months (49–94) | ISL classification | 4 (2.0%) 40 (21.3%) | 0 (0%) 4 (2.1%) | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terada, S.; Tanaka, T.; Murakami, H.; Tsuchihashi, H.; Toji, A.; Daimon, A.; Miyamoto, S.; Nishie, R.; Ueda, S.; Hashida, S.; et al. Lymphatic Complications Following Sentinel Node Biopsy or Pelvic Lymphadenectomy for Endometrial Cancer. J. Clin. Med. 2023, 12, 4540. https://doi.org/10.3390/jcm12134540
Terada S, Tanaka T, Murakami H, Tsuchihashi H, Toji A, Daimon A, Miyamoto S, Nishie R, Ueda S, Hashida S, et al. Lymphatic Complications Following Sentinel Node Biopsy or Pelvic Lymphadenectomy for Endometrial Cancer. Journal of Clinical Medicine. 2023; 12(13):4540. https://doi.org/10.3390/jcm12134540
Chicago/Turabian StyleTerada, Shinichi, Tomohito Tanaka, Hikaru Murakami, Hiromitsu Tsuchihashi, Akihiko Toji, Atsushi Daimon, Shunsuke Miyamoto, Ruri Nishie, Shoko Ueda, Sousuke Hashida, and et al. 2023. "Lymphatic Complications Following Sentinel Node Biopsy or Pelvic Lymphadenectomy for Endometrial Cancer" Journal of Clinical Medicine 12, no. 13: 4540. https://doi.org/10.3390/jcm12134540
APA StyleTerada, S., Tanaka, T., Murakami, H., Tsuchihashi, H., Toji, A., Daimon, A., Miyamoto, S., Nishie, R., Ueda, S., Hashida, S., Morita, N., Maruoka, H., Konishi, H., Kogata, Y., Taniguchi, K., Komura, K., & Ohmichi, M. (2023). Lymphatic Complications Following Sentinel Node Biopsy or Pelvic Lymphadenectomy for Endometrial Cancer. Journal of Clinical Medicine, 12(13), 4540. https://doi.org/10.3390/jcm12134540





