The Magnitude of Contralateral Suppression of Otoacoustic Emissions Is Ear- and Age-Dependent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Apparatus
2.3. Procedure
2.4. Data Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pickles, J.O. Auditory pathways: Anatomy and physiology. Handb. Clin. Neurol. 2015, 129, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Seldana, E. All the way from the cortex: A review of auditory cortico-subcollicular pathways. Cerebellum 2015, 14, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Terreros, G.; Delano, H.P. Corticofugal modulation of peripheral auditory responses. Front. Syst. Neurosci. 2015, 9, 134. [Google Scholar] [CrossRef] [Green Version]
- Winer, J.A. Decoding of the auditory corticofugal systems. Hear. Res. 2005, 207, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Warr, W.B.; Guinan, J.J., Jr.; White, J.S. Organization of efferent fibers: The lateral and medial olivocochlear systems. In Neurobiology of Hearing: The Cochlea; Altshuler, R.A., Hoffman, D.W., Bobbin, R.P., Eds.; Raven Press: New York, NY, USA, 1986; pp. 333–348. [Google Scholar]
- Lopez-Poveda, E.A. Olivocochlear efferents in animals and humans: From anatomy to clinical relevance. Front. Neurol. 2018, 9, 197. [Google Scholar] [CrossRef] [Green Version]
- Bajo, V.M.; Moore, D.R. Descending projections from the auditory cortex to the inferior colliculus in the gerbils, Meriones unguiculatus. J. Comp. Neurol. 2005, 1586, 101–116. [Google Scholar] [CrossRef]
- Jäger, K.; Kössl, M. Corticofugal modulation of DPOAEs in gerbils. Hear. Res. 2016, 332, 61–72. [Google Scholar] [CrossRef]
- Ponton, C.; Eggermont, J.J.; Khosla, D.; Kwong, B.; Don, M. Maturation of human central auditory system activity: Separating auditory evoked potentials by dipole source modeling. Clin. Neurophysiol. 2002, 113, 407–420. [Google Scholar] [CrossRef]
- Sharma, A.; Martin, K.; Rol, P.; Bauer, P.; Sweeney, M.H.; Gilley, P.; Dorman, M. P1 latency as a biomarker for central auditory development in children with hearing impairment. J. Am. Acad. Audiol. 2005, 16, 564–573. [Google Scholar] [CrossRef]
- Seither-Preisler, A.; Johnson, L.; Krumbholz, K.; Nobbe, A.; Patterson, R.; Seither, S.; Lütkenhöner, B. Tone sequences with conflicting fundamental pitch and timbre changes are heard differently by musicians and nonmusicians. J. Exp. Psychol. Hum. Percept. Perform. 2007, 33, 743–751. [Google Scholar] [CrossRef] [Green Version]
- Dehaene-Lambertz, G.; Spelke, E.S. The Infancy of the Human Brain. Neuron 2015, 88, 93–109. [Google Scholar] [CrossRef] [Green Version]
- Hall, J.W. New Handbook of Auditory Evoked Responses; Pearson: Boston, MA, USA, 2007. [Google Scholar]
- Spitzer, E.; White-Schwoch, T.; Woodruff Carr, K.; Skoe, E.; Kraus, N. Continued maturation of the click-evoked auditory brainstem response in preschoolers. J. Am. Acad. Audiol. 2015, 26, 30–35. [Google Scholar] [CrossRef] [Green Version]
- Krizman, J.; Tierney, A.; Fitzroy, A.B.; Skoe, E.; Amar, J.; Kraus, N. Continued maturation of auditory brainstem function during adolescence: A longitudinal approach. Clin. Neurophysiol. 2015, 126, 2348–2355. [Google Scholar] [CrossRef] [Green Version]
- Eggermont, J.J. Development of Central Auditory Nervous System. In Handbook of Central Auditory Processing Disorder; Plural Publishing: San Diego, CA, USA, 2014; Volume 1, pp. 59–88. [Google Scholar]
- Moore, J.K.; Linthicum, F.H., Jr. The human auditory system: A timeline of development. Int. J. Audiol. 2007, 46, 460–478. [Google Scholar] [CrossRef]
- Elliott, L.L. Performance of children aged 9 to 17 years on a test of speech intelligibility in noise using sentence material with controlled word predictability. J. Acoust. Soc. Am. 1979, 66, 12–21. [Google Scholar] [CrossRef]
- Hensch, T.K. Critical period plasticity in local circuits. Nat. Rev. Neurosci. 2005, 6, 877–888. [Google Scholar] [CrossRef]
- Kemp, D.T. Stimulated otoacoustic emissions from within the human auditory system. J. Acoust. Soc. Am. 1978, 64, 1386–1391. [Google Scholar] [CrossRef]
- Kemp, D.T. Otoacoustic emissions, their origin in cochlear function, and use. Br. Med. Bull. 2002, 63, 223–241. [Google Scholar] [CrossRef]
- Collet, L.; Kemp, D.T.; Veuillet, E.; Duclaux, R.; Moulin, A.; Morgon, A. Effect of contralateral auditory stimuli on active cochlear micromechanical properties in human subjects. Hear. Res. 1990, 43, 25162. [Google Scholar] [CrossRef]
- Veuillet, E.; Collet, L.; Duclaux, R. Effect of contralateral acoustic stimulation on active cochlear micromechanical properties in human subjects: Dependence on stimulus variables. J. Neurophysiol. 1991, 65, 724–735. [Google Scholar] [CrossRef]
- Dragicevic, C.D.; Aedo, C.; Leon, A.; Bowen, M.; Jara, N.; Terreros, G.; Robles, L.; Delano, P.H. The olivocochlear reflex strength and cochlear sensitivity are independently modulated by auditory cortex microstimulation. J. Assoc. Res. Otolaryngol. 2015, 16, 223–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aedo, C.; Terreros, G.; Leon, A.; Delano, P.H. The corticofugal effects of auditory cortex microstimulaton on auditory nerve and superior olivary complex responses are mediated via alpha-9 nicotinic receptor subunit. PLoS ONE 2016, 11, e0155991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schofield, B.R. Structural organization of the descending auditory pathway. In The Oxford Handbook of Auditory Science: The Auditory Brain; Oxford University Press: Oxford, UK, 2010; pp. 43–64. [Google Scholar]
- Suthakar, K.; Ryugo, D.K. Descending projections from the inferior colliculus to medial olivocochlear efferents: Mice with normal hearing, early onset hearing loss, and congenital deafness. Hear. Res. 2017, 343, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Khalfa, S.; Veuillet, E.; Collet, L. Influence of handedness on peripheral auditory asymmetry. Eur. J. Neurosci. 1998, 10, 2731–2737. [Google Scholar] [CrossRef]
- Bidelman, G.M.; Bhagat, S.P. Right-ear advantage drives the link between olivocochlear efferent “antimasking” and speech-in-noise listening benefits. Neuroreport 2015, 26, 483–487. [Google Scholar] [CrossRef]
- Veuillet, E.; Georgieff, N.; Philibert, B.; Dallery, J.; Marie-Cardine, M.; Collet, L. Abnormal peripheral auditory asymmetry in schizophrenia. J. Neurol. Neurosurg. Psychiatry 2001, 70, 88–94. [Google Scholar] [CrossRef] [Green Version]
- Khalfa, S.; Bougeard, R.; Morand, N.; Veuillet, E.; Isnard, J.; Guenot, M.; Ryvlin, P.; Fischer, C.; Collet, L. Evidence of peripheral auditory activity modulation by the auditory cortex in humans. Neuroscience 2001, 104, 347–358. [Google Scholar] [CrossRef]
- Perrot, X.; Ryvlin, P.; Isnard, J.; Guénot, M.; Catenoix, H.; Fischer, C.; Mauguière, F.; Collet, L. Evidence for corticofugal modulation of peripheral auditory activity in humans. Cereb. Cortex 2006, 16, 941–948. [Google Scholar] [CrossRef] [Green Version]
- Yakunina, N.; Tae, W.S.; Kim, S.S.; Nam, E.C. Functional MRI evidence of the cortico-olivary efferent pathway during active auditory target processing in humans. Hear. Res. 2019, 379, 1–11. [Google Scholar] [CrossRef]
- Morlet, T.; Hamburger, A.; Kuint, J.; Ari-Even Roth, D.; Gartner, M.; Muchnik, C.; Collet, L.; Hildesheimer, M. Assessement of medial olivocochlear system function in pre-term and fullterm newborns using a rapid test of transient optoacoustic emissions. Clin. Otolaryngol. 2004, 29, 183–190. [Google Scholar] [CrossRef]
- Carvallo, R.M.M.; Sanches, S.G.G.; Ibidi, S.M.; Soares, J.C.; Durante, A.S. Efferent inhibition of optoacoustic emissions in preterm neonates. Braz. J. Otorhinolaryngol. 2015, 81, 491–497. [Google Scholar] [CrossRef]
- Morlet, T.; Goforth, L.; Hood, L.J.; Ferber, C.; Duclaux, R.; Berlin, C.I. Development of human cochlear active mechanism asymmetry: Involvement of the medial olivocochlear system? Hear. Res. 1999, 134, 153–162. [Google Scholar] [CrossRef]
- Gkoritsa, E.; Tsakanikos, M.; Korres, S.; Dellagrammaticas, H.; Apostolopoulos, N.; Ferekidis, E. Transient otoacoustic emissions in the detection of olivocochlear bundle maturation. Int. J. Pediatr. Otorhinolaryngol. 2006, 70, 671–676. [Google Scholar] [CrossRef]
- Chabert, R.; Guitton, M.J.; Amram, D.; Uziel, A.; Pujol, R.; Lallemant, J.G.; Puel, J.L. Early maturation of evoked optoacoustic emissions and medial olivocochlear reflex in preterm neonates. Pediatr. Res. 2006, 59, 305–308. [Google Scholar] [CrossRef] [Green Version]
- Jedrzejczak, W.W.; Pilka, E.; Skarzynski, P.H.; Skarzynski, H. Contralateral suppression of otoacoustic emissions in pre-school children. Int. J. Pediatr. Otorhinolaryngol. 2020, 132, 109915. [Google Scholar] [CrossRef]
- Clarke, E.M.; Ahmmed, A.; Parker, D.; Adams, C. Contralateral suppression of otoacoustic emissions in children with specific language impairment. Ear Hear. 2006, 27, 153–160. [Google Scholar] [CrossRef]
- Sanchez, S.G.G.; Carvallo, R.M. Contralateral suppression of transient evoked otoacoustic emissions in children with auditory processing disorder. Audiol. Neurotol. 2006, 11, 366–372. [Google Scholar] [CrossRef]
- Veuillet, E.; Magnan, A.; Ecalle, J.; Thai-Van, H.; Collet, L. Auditory processing disorder in children with reading disabilities: Effect of audiovisual training. Brain 2007, 130, 2915–2928. [Google Scholar] [CrossRef] [Green Version]
- Akbari, M.; Panahi, R.; Valadbeigi, A.; Nahrani, M.H. Speech-in-noise perception ability can be related to auditory efferent pathway function: A comparative study in reading impaired and normal reading children. Braz. J. Otorhinolaryngol. 2020, 86, 209–216. [Google Scholar] [CrossRef]
- Burguetti, F.A.R.; Carvallo, R.M.M. Efferent auditory system: Its effect on auditory processing. Braz. J. Otorhinolaryngol. 2008, 74, 737–745. [Google Scholar] [CrossRef]
- Yalçinkaya, F.; Yilmaz, S.T.; Muluk, N.B. Transient evoked otoacoustic emissions and contralateral suppressions in children with auditory listening problems. Auris Nasus Larynx 2010, 37, 47–54. [Google Scholar] [CrossRef]
- Angeli, M.L.d.S.A.; De Almeida, C.I.R.; Sens, P.M. Comparative study between school performance on first grade children and suppression of otoacoustic transient emission. Braz. J. Otorhinolaryngol. 2008, 74, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Lefavrais, P. Test de l’Alouette; ECPA: Paris, France, 1965. [Google Scholar]
- Garinis, A.C.; Glattke, T.; Cone-Wesson, B.K. TEOAE suppression in adults with learning disabilities. Int. J. Audiol. 2008, 47, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.; Kemp, D.T. The influence of evoking stimulus level on the neural suppression of transient evoked otoacoustic emissions. Hear. Res. 1996, 94, 140–147. [Google Scholar] [CrossRef]
- Veuillet, E.; Bazin, F.; Collet, L. Objective evidence of peripheral auditory disorders in learning-impaired children. J. Audiol. Med. 1999, 8, 18–29. [Google Scholar]
- O-Uchi, T.; Kanzaki, J.; Satoh, Y.; Yoshihara, S.; Ogata, A.; Inoue, Y.; Mashino, H. Age-related changes in evoked otoacoustic emission in normal-hearing ears. Acta Otolaryngol. Suppl. 1994, 514, 89–94. [Google Scholar] [CrossRef]
- Collet, L.; Moulin, A.; Gartner, M.; Morgon, A. Age-related changes in evoked otoacoustic emissions. Ann. Otol. Rhinol. Laryngol. 1990, 99, 993–997. [Google Scholar] [CrossRef]
- Moore, J.K.; Guan, Y.L. Cytoarchitectural and axonal maturation in human auditory cortex. J. Assoc. Res. Otolaryngol. 2001, 2, 297–311. [Google Scholar] [CrossRef] [Green Version]
- Moncrieff, D.W. Dichotic listening in children: Age-related changes in direction and magnitude of ear advantage. Brain Cogn. 2011, 76, 316–322. [Google Scholar] [CrossRef]
- Jerger, J.; Martin, J. Hemispheric asymmetry of the right ear advantage in dichotic listening. Hear. Res. 2004, 198, 125–136. [Google Scholar] [CrossRef]
- Mcfadden, D.; Martin, G.K.; Stagner, B.B.; Maloney, M.M. Sex differences in distortion-product and transient-evoked otoacoustic emissions compared. J. Acoust. Soc. Am. 2009, 125, 239–246. [Google Scholar] [CrossRef]
- Abdollahi, F.Z.; Lotfi, Y. Gender difference in TEOAEs and contralateral suppression of TEOAEs in normal hearing adults. Iran Rehabil. J. 2011, 9, 22–25. [Google Scholar]
- Nisha, K.V.; Loganathan, M.K.; Prabhu, P. Gender differences in contralateral suppression of spontaneous otoacoustic emissions in individuals with auditory neuropathy spectrum disorders. Eur. Arch. Otorhinolaryngol. 2023, 280, 1493–1499. [Google Scholar] [CrossRef]
- Stuart, A.; Cobb, K.M. Reliability of measures of transient evoked otoacoustic emissions with contralateral suppression. J. Commun. Disord. 2015, 58, 35–42. [Google Scholar] [CrossRef]
- Jedrzejczak, W.W.; Pilka, E.; Pastucha, M.; Kochanek, K.; Skarzynski, H. The reliability of contralateral suppression of otoacoustic emissions is greater in women than in men. Audiol. Res. 2022, 12, 79–86. [Google Scholar] [CrossRef]
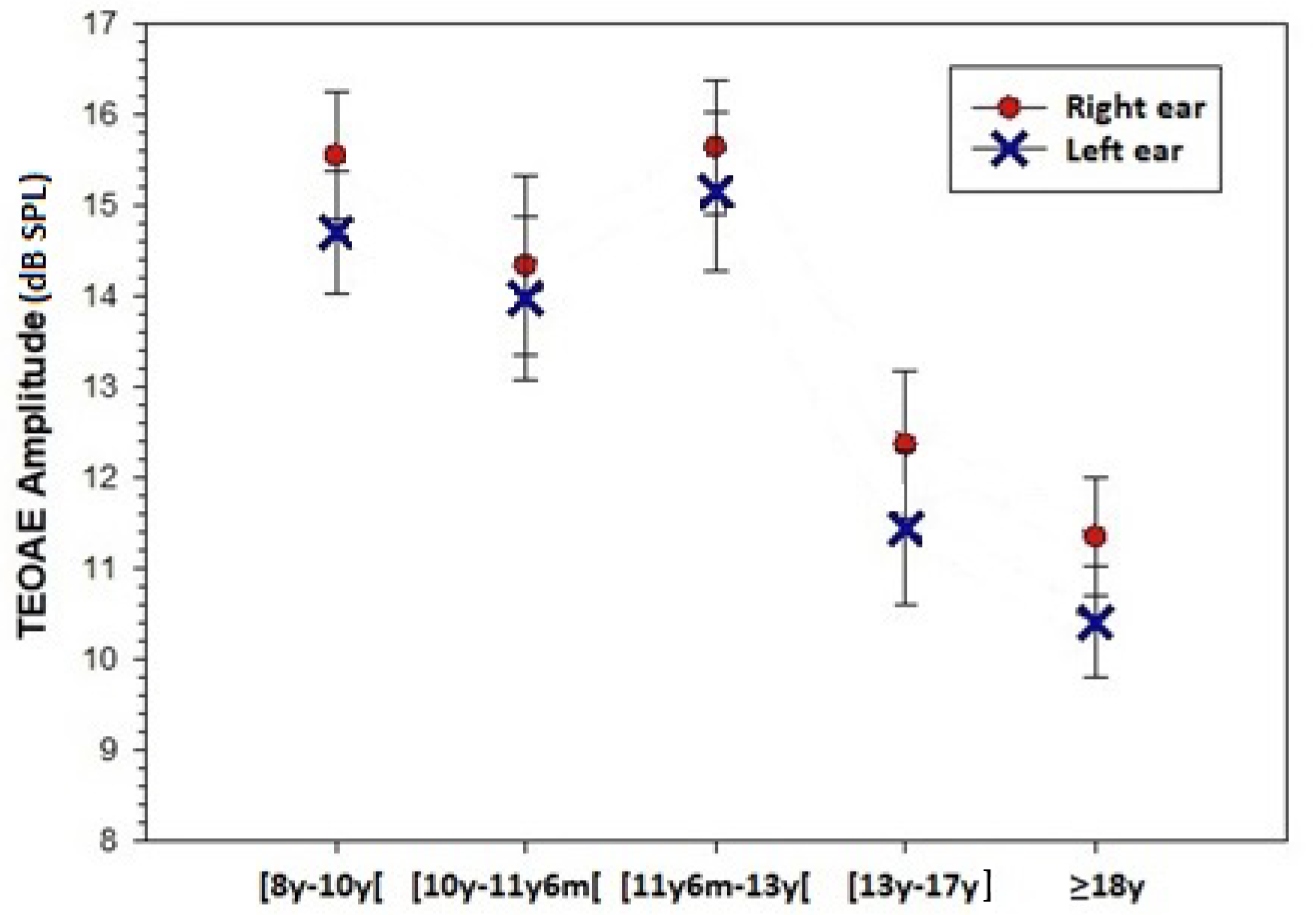



| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | ||
|---|---|---|---|---|---|---|
| Age (months) | Min | 96 | 120 | 139 | 157 | 219 |
| Max | 118 | 138 | 156 | 213 | 425 | |
| Mean (SE) | 108.3 (1.5) | 130 (1.2) | 147.2 (1.2) | 181.3 (3.6) | 279.5 (12.4) | |
| Sex | 12♀/12♂ | 12♀/12♂ | 12♀/12♂ | 10♀/14♂ | 11♀/13♂ | |
| Manual laterality (%) | Min | 71.4 | 80 | 77.8 | 71.4 | 80 |
| Max | 100 | 100 | 100 | 100 | 100 | |
| Mean (SE) | 91.5 (2.3) | 95.1 (1.7) | 93 (2.1) | 92.9 (2.1) | 97.3 (1.3) |
| Variables | Age Group | N | Mean | Lower | Upper | Median | SD | IQR |
|---|---|---|---|---|---|---|---|---|
| EAright | [8y–10y] | 24 | −3.67 | −4.48 | −2.86 | −3.46 | 1.92 | 1.88 |
| [10y–11y6m] | 24 | −3.88 | −4.59 | −3.18 | −3.61 | 1.67 | 2.88 | |
| [11y6m–13y] | 24 | −2.97 | −3.62 | −2.31 | −2.59 | 1.55 | 1.77 | |
| [13y–17] | 24 | −3.60 | −4.49 | −2.70 | −3.31 | 2.12 | 2.50 | |
| ≥18y | 24 | −2.97 | −3.60 | −2.35 | −2.96 | 1.48 | 2.04 | |
| EAleft | [8y–10y] | 24 | −3.27 | −3.99 | −2.56 | −3.10 | 1.70 | 2.56 |
| [10y–11y6m] | 24 | −3.18 | −3.82 | −2.54 | −3.48 | 1.51 | 2.26 | |
| [11y6m–13y] | 24 | −2.42 | −2.97 | −1.87 | −2.40 | 1.30 | 1.22 | |
| [13y–17] | 24 | −2.62 | −3.28 | −1.96 | −2.23 | 1.57 | 2.27 | |
| ≥18y | 24 | −1.90 | −2.31 | −1.48 | −1.84 | 0.99 | 1.25 | |
| AI | [8y–10y] | 24 | −0.40 | −1.14 | 0.35 | −0.36 | 1.76 | 2.02 |
| [10y–11y6m] | 24 | −0.70 | −1.48 | 0.07 | −0.57 | 1.85 | 2.15 | |
| [11y6m–13y] | 24 | −0.55 | −1.07 | −0.03 | −0.59 | 1.22 | 1.58 | |
| [13y–17] | 24 | −0.97 | −1.45 | −0.50 | −0.86 | 1.12 | 0.92 | |
| ≥18y | 24 | −1.08 | −1.60 | −0.56 | −0.77 | 1.23 | 1.58 |
| Variables | Sex | N | Mean | Lower | Upper | Median | SD | IQR |
|---|---|---|---|---|---|---|---|---|
| EA right | F | 57 | −3.54 | −4.00 | −3.07 | −3.42 | 1.74 | 2.24 |
| M | 63 | −3.31 | −3.77 | −2.85 | −2.88 | 1.82 | 2.23 | |
| EA left | F | 57 | −2.80 | −3.25 | −2.36 | −2.58 | 1.68 | 2.30 |
| M | 63 | −2.57 | −2.90 | −2.23 | −2.40 | 1.32 | 1.93 | |
| AI | F | 57 | −0.73 | −1.13 | −0.34 | −0.71 | 1.48 | 1.47 |
| M | 63 | −0.75 | −1.11 | −0.38 | −0.60 | 1.47 | 1.63 |
| Variables | Age Group | Sex | N | Mean | Lower | Upper | Median | SD | IQR |
|---|---|---|---|---|---|---|---|---|---|
| EAright | [8y–10y] | F | 12 | −4.16 | −5.07 | −3.24 | −3.74 | 1.44 | 1.18 |
| M | 12 | −3.18 | −4.62 | −1.74 | −2.40 | 2.27 | 1.71 | ||
| [10y–11y6m] | F | 12 | −3.81 | −4.78 | −2.84 | −3.59 | 1.53 | 2.11 | |
| M | 12 | −3.95 | −5.14 | −2.77 | −3.79 | 1.86 | 3.14 | ||
| [11y6m–13y] | F | 12 | −3.15 | −4.25 | −2.04 | −2.63 | 1.74 | 1.56 | |
| M | 12 | −2.79 | −3.68 | −1.90 | −2.59 | 1.40 | 2.37 | ||
| [13y–17] | F | 10 | −3.64 | −5.24 | −2.04 | −2.91 | 2.24 | 2.53 | |
| M | 14 | −3.56 | −4.79 | −2.34 | −3.62 | 2.12 | 2.30 | ||
| ≥18y | F | 11 | −2.89 | −4.06 | −1.72 | −2.17 | 1.74 | 2.04 | |
| M | 13 | −3.04 | −3.82 | −2.26 | −3.04 | 1.30 | 1.64 | ||
| EAleft | [8y–10y] | F | 12 | −3.72 | −4.99 | −2.46 | −3.69 | 1.98 | 2.71 |
| M | 12 | −2.83 | −3.65 | −2.00 | −2.79 | 1.30 | 1.45 | ||
| [10y–11y6m] | F | 12 | −3.10 | −4.09 | −2.12 | −3.48 | 1.55 | 2.30 | |
| M | 12 | −3.25 | −4.23 | −2.27 | −3.24 | 1.54 | 2.10 | ||
| [11y6m–13y] | F | 12 | −2.42 | −3.37 | −1.47 | −2.43 | 1.49 | 1.22 | |
| M | 12 | −2.42 | −3.15 | −1.69 | −2.28 | 1.15 | 1.05 | ||
| [13y–17y] | F | 10 | −2.95 | −4.24 | −1.66 | −2.64 | 1.81 | 2.02 | |
| M | 14 | −2.39 | −3.19 | −1.59 | −1.99 | 1.39 | 1.83 | ||
| ≥18y | F | 11 | −1.75 | −2.39 | −1.12 | −1.58 | 0.94 | 1.25 | |
| M | 13 | −2.02 | −2.65 | −1.38 | −1.92 | 1.05 | 0.94 | ||
| AI | [8y–10y] | F | 12 | −0.43 | −1.67 | 0.80 | −0.83 | 1.94 | 2.53 |
| M | 12 | −0.36 | −1.40 | 0.68 | −0.02 | 1.64 | 1.68 | ||
| [10y–11y6m] | F | 12 | −0.71 | −1.88 | 0.47 | −0.46 | 1.85 | 2.16 | |
| M | 12 | −0.70 | −1.93 | 0.52 | −0.69 | 1.93 | 2.16 | ||
| [11y6m–13y] | F | 12 | −0.72 | −1.31 | −0.14 | −0.81 | 0.93 | 1.46 | |
| M | 12 | −0.37 | −1.32 | 0.57 | −0.31 | 1.49 | 1.49 | ||
| [13y–17y] | F | 10 | −0.69 | −1.29 | −0.09 | −0.77 | 0.84 | 0.65 | |
| M | 14 | −1.17 | −1.91 | −0.43 | −0.93 | 1.28 | 1.08 | ||
| ≥18y | F | 11 | −1.14 | −2.19 | −0.08 | −0.71 | 1.57 | 1.31 | |
| M | 13 | −1.02 | −1.58 | −0.47 | −0.83 | 0.91 | 1.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thai-Van, H.; Veuillet, E.; Le Normand, M.-T.; Damien, M.; Joly, C.-A.; Reynard, P. The Magnitude of Contralateral Suppression of Otoacoustic Emissions Is Ear- and Age-Dependent. J. Clin. Med. 2023, 12, 4553. https://doi.org/10.3390/jcm12134553
Thai-Van H, Veuillet E, Le Normand M-T, Damien M, Joly C-A, Reynard P. The Magnitude of Contralateral Suppression of Otoacoustic Emissions Is Ear- and Age-Dependent. Journal of Clinical Medicine. 2023; 12(13):4553. https://doi.org/10.3390/jcm12134553
Chicago/Turabian StyleThai-Van, Hung, Evelyne Veuillet, Marie-Thérèse Le Normand, Maxime Damien, Charles-Alexandre Joly, and Pierre Reynard. 2023. "The Magnitude of Contralateral Suppression of Otoacoustic Emissions Is Ear- and Age-Dependent" Journal of Clinical Medicine 12, no. 13: 4553. https://doi.org/10.3390/jcm12134553
APA StyleThai-Van, H., Veuillet, E., Le Normand, M.-T., Damien, M., Joly, C.-A., & Reynard, P. (2023). The Magnitude of Contralateral Suppression of Otoacoustic Emissions Is Ear- and Age-Dependent. Journal of Clinical Medicine, 12(13), 4553. https://doi.org/10.3390/jcm12134553






