The Potential Utility of Tirzepatide for the Management of Polycystic Ovary Syndrome
Abstract
:1. Introduction
2. Common PCOS Features
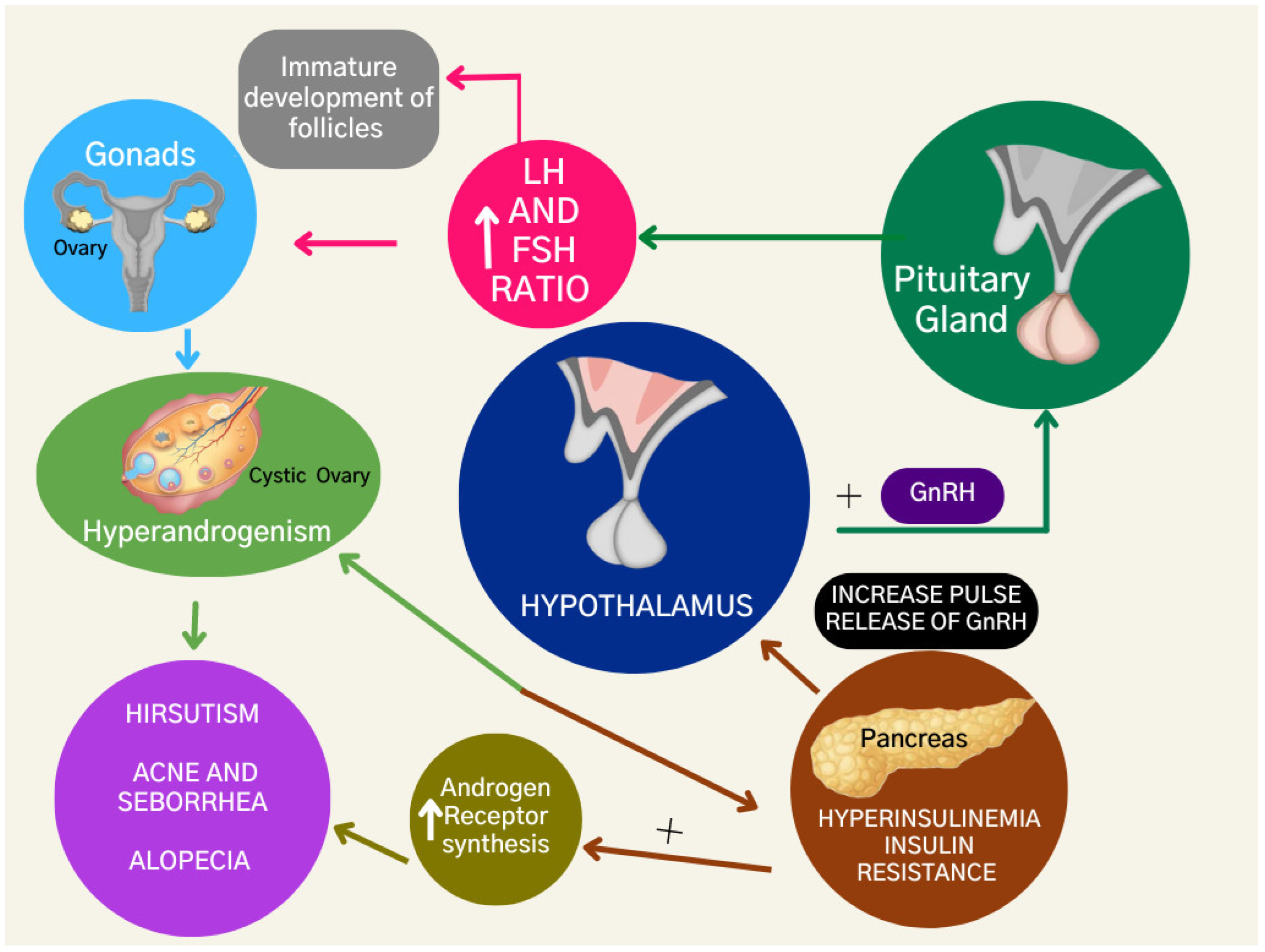
2.1. Hyperandrogenism
2.2. Insulin Resistance
2.3. Hyperinsulinemia
2.4. Obesity
3. PCOS Management
Lifestyle
4. PCOS Pharmacotherapy
4.1. Glucagon-Like Peptide-1 Receptor Agonists
4.2. The Effect of GLP-1R Agonists on Lipid Metabolism
4.3. The Effect of GLP-1R Agonists on Oxidative Stress
4.4. The Effect of GLP-1R Agonists on Inflammation
5. Tirzepatide
5.1. Mechanism of Action
5.2. GIPR Activity
5.3. The Role of GIP in Glucose Metabolism
5.4. Insulin
5.5. Glucagon
5.6. The Role of GIP in Lipid Metabolism
5.7. Pharmacodynamics of Tirzepatide
5.8. Side Effects and Contra-Indications
5.9. Clinical Trials
6. Additional Management Strategies in PCOS
6.1. Orlistat
6.2. Metformin
7. Bariatric Surgery
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Himelein, M.J.; Thatcher, S.S. Polycystic ovary syndrome and mental health: A review. Obstet. Gynecol. Surv. 2006, 61, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Shroff, R.; Kerchner, A.; Maifeld, M.; Van Beek, E.J.; Jagasia, D.; Dokras, A. Young obese women with polycystic ovary syndrome have evidence of early coronary atherosclerosis. J. Clin. Endocrinol. Metab. 2007, 92, 4609–4614. [Google Scholar] [CrossRef] [PubMed]
- Legro, R.S.; Arslanian, S.A.; Ehrmann, D.A.; Hoeger, K.M.; Murad, M.H.; Pasquali, R.; Welt, C.K. Diagnosis and treatment of polycystic ovary syndrome: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2013, 98, 4565–4592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deswal, R.; Narwal, V.; Dang, A.; Pundir, C.S. The Prevalence of Polycystic Ovary Syndrome: A Brief Systematic Review. J. Hum. Reprod. Sci. 2020, 13, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Kouli, C.R.; Bergiele, A.T.; Filandra, F.A.; Tsianateli, T.C.; Spina, G.G.; Zapanti, E.D.; Bartzis, M.I. A survey of the polycystic ovary syndrome in the Greek island of Lesbos: Hormonal and metabolic profile. J. Clin. Endocrinol. Metab. 1999, 84, 4006–4011. [Google Scholar] [CrossRef]
- Chaney, P. Are the Rotterdam Criteria Still Relevant in PCOS Diagnoses? Available online: https://www.volusonclub.net/empowered-womens-health/are-the-rotterdam-criteria-still-relevant-in-pcos-diagnoses-weighing-the-consensus-current-relevance/ (accessed on 19 July 2022).
- Teede, H.; Deeks, A.; Moran, L. Polycystic ovary syndrome: A complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010, 8, 41. [Google Scholar] [CrossRef] [Green Version]
- Smet, M.E.; McLennan, A. Rotterdam criteria, the end. Australas. J. Ultrasound Med. 2018, 21, 59–60. [Google Scholar] [CrossRef]
- Torpy, J.M.; Lynm, C.; Glass, R.M. Polycystic Ovary Syndrome. JAMA 2007, 297, 554. [Google Scholar] [CrossRef] [Green Version]
- Rosenfield, R.L.; Ehrmann, D.A. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr. Rev. 2016, 37, 467–520. [Google Scholar] [CrossRef]
- Cleveland Clinic. Androgens: Function, Measurement and Related Disorders. Available online: https://my.clevelandclinic.org/health/articles/22002-androgens (accessed on 3 July 2022).
- Goodarzi, M.O.; Carmina, E.; Azziz, R. DHEA, DHEAS and PCOS. J. Steroid Biochem. Mol. Biol. 2015, 145, 213–225. [Google Scholar] [CrossRef]
- Legro, R.S. Evaluation and Treatment of Polycystic Ovary Syndrome; MDText.com, Inc.: South Dartmouth, MA, USA, 2015. [Google Scholar]
- Ashraf, S.; Nabi, M.; Rasool, S.U.A.; Rashid, F.; Amin, S. Hyperandrogenism in polycystic ovarian syndrome and role of CYP gene variants: A review. Egypt. J. Med. Hum. Genet. 2019, 20, 25. [Google Scholar] [CrossRef] [Green Version]
- Gainder, S.; Sharma, B. Update on Management of Polycystic Ovarian Syndrome for Dermatologists. Indian Derm. Online J. 2019, 10, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Cooney, L.G.; Lee, I.; Sammel, M.D.; Dokras, A. High prevalence of moderate and severe depressive and anxiety symptoms in polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. 2017, 32, 1075–1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diamanti-Kandarakis, E.; Dunaif, A. Insulin resistance and the polycystic ovary syndrome revisited: An update on mechanisms and implications. Endocr. Rev. 2012, 33, 981–1030. [Google Scholar] [CrossRef]
- Galan, N. Symptoms and Screening of Insulin Resistance with PCOS. Available online: https://www.verywellhealth.com/pcos-and-insulin-resistance-2616319 (accessed on 4 July 2022).
- Goodarzi, M.O.; Korenman, S.G. The importance of insulin resistance in polycystic ovary syndrome. Fertil. Steril. 2003, 80, 255–258. [Google Scholar] [CrossRef]
- Wilcox, G. Insulin and insulin resistance. Clin. Biochem. Rev. 2005, 26, 19–39. [Google Scholar]
- Sinha, S.K.; Chan, K.K.-C.; Sadeghi-Nejad, A. Hyperinsulinism: Background, Pathophysiology, Etiology; Medscape: New York, NY, USA, 2022. [Google Scholar]
- Baptiste, C.G.; Battista, M.C.; Trottier, A.; Baillargeon, J.P. Insulin and hyperandrogenism in women with polycystic ovary syndrome. J. Steroid Biochem. Mol. Biol. 2010, 122, 42–52. [Google Scholar] [CrossRef] [Green Version]
- Cena, H.; Chiovato, L.; Nappi, R.E. Obesity, Polycystic Ovary Syndrome, and Infertility: A New Avenue for GLP-1 Receptor Agonists. J. Clin. Endocrinol. Metab. 2020, 105, e2695–e2709. [Google Scholar] [CrossRef]
- Legro, R.S.; Castracane, V.D.; Kauffman, R.P. Detecting insulin resistance in polycystic ovary syndrome: Purposes and pitfalls. Obs. Gynecol. Surv. 2004, 59, 141–154. [Google Scholar] [CrossRef]
- Barber, T.M.; Hanson, P.; Weickert, M.O.; Franks, S. Obesity and Polycystic Ovary Syndrome: Implications for Pathogenesis and Novel Management Strategies. Clin. Med. Insights Reprod. Health 2019, 13, 1179558119874042. [Google Scholar] [CrossRef] [Green Version]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum. Reprod. 2018, 33, 1602–1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, M.; Shankar, R.; Pathak, K.; Yadav, R. Polycystic ovarian syndrome: A review covering phytoconstituents for its outstrip management. Pharmacol. Res. Mod. Chin. Med. 2021, 1, 100011. [Google Scholar] [CrossRef]
- Fraison, E.; Kostova, E.; Moran, L.J.; Bilal, S.; Ee, C.C.; Venetis, C.; Costello, M.F. Metformin versus the combined oral contraceptive pill for hirsutism, acne, and menstrual pattern in polycystic ovary syndrome. Cochrane Database Syst. Rev. 2020, 8, Cd005552. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Ajabiya, J.; Teli, D.; Bojarska, J.; Apostolopoulos, V. Tirzepatide, a New Era of Dual-Targeted Treatment for Diabetes and Obesity: A Mini-Review. Molecules 2022, 27, 4315. [Google Scholar] [CrossRef]
- Sloop, K.W.; Briere, D.A.; Emmerson, P.J.; Willard, F.S. Beyond Glucagon-like Peptide-1: Is G-Protein Coupled Receptor Polypharmacology the Path Forward to Treating Metabolic Diseases? ACS Pharm. Transl. Sci. 2018, 1, 3–11. [Google Scholar] [CrossRef]
- Norman, R.J.; Teede, H.J. A new evidence-based guideline for assessment and management of polycystic ovary syndrome. Med. J. Aust. 2018, 209, 299–300. [Google Scholar] [CrossRef]
- Kim, C.H.; Lee, S.H. Effectiveness of Lifestyle Modification in Polycystic Ovary Syndrome Patients with Obesity: A Systematic Review and Meta-Analysis. Life 2022, 12, 308. [Google Scholar] [CrossRef]
- Papavasiliou, K.; Papakonstantinou, E. Nutritional support and dietary interventions for women with polycystic ovary syndrome. Nutr. Diet. Suppl. 2017, 9, 63–85. [Google Scholar] [CrossRef] [Green Version]
- Wong, J.M.; Gallagher, M.; Gooding, H.; Feldman, H.A.; Gordon, C.M.; Ludwig, D.S.; Ebbeling, C.B. A randomized pilot study of dietary treatments for polycystic ovary syndrome in adolescents. Pediatr. Obes. 2016, 11, 210–220. [Google Scholar] [CrossRef] [Green Version]
- Moran, L.J.; Hutchison, S.K.; Norman, R.J.; Teede, H.J. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst. Rev. 2011, 7, Cd007506. [Google Scholar] [CrossRef]
- Patel, S. Polycystic ovary syndrome (PCOS), an inflammatory, systemic, lifestyle endocrinopathy. J. Steroid Biochem. Mol. Biol. 2018, 182, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Kose, O.; Arabaci, T.; Kara, A.; Yemenoglu, H.; Kermen, E.; Kizildag, A.; Gedikli, S.; Ozkanlar, S. Effects of Melatonin on Oxidative Stress Index and Alveolar Bone Loss in Diabetic Rats With Periodontitis. J. Periodontol. 2016, 87, e82–e90. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Smith, C.A.; Costello, M.F.; MacMillan, F.; Moran, L.; Ee, C. Barriers and facilitators to weight management in overweight and obese women living in Australia with PCOS: A qualitative study. BMC Endocr. Disord. 2019, 19, 106. [Google Scholar] [CrossRef] [Green Version]
- Domecq, J.P.; Prutsky, G.; Mullan, R.J.; Hazem, A.; Sundaresh, V.; Elamin, M.B.; Phung, O.J.; Wang, A.; Hoeger, K.; Pasquali, R.; et al. Lifestyle modification programs in polycystic ovary syndrome: Systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2013, 98, 4655–4663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, J.O.; Wyatt, H.R.; Peters, J.C. The Importance of Energy Balance. Eur. Endocrinol. 2013, 9, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Dalla Man, C.; Micheletto, F.; Sathananthan, A.; Rizza, R.A.; Vella, A.; Cobelli, C. A model of GLP-1 action on insulin secretion in nondiabetic subjects. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E1115–E1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, A.; Marso, S.P.; Neeland, I.J. Liraglutide for weight management: A critical review of the evidence. Obes. Sci. Prac. 2017, 3, 3–14. [Google Scholar] [CrossRef]
- Dailey, M.J.; Moran, T.H. Glucagon-like peptide 1 and appetite. Trends Endocrinol. Metab. 2013, 24, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Sisley, S.; Gutierrez-Aguilar, R.; Scott, M.; D’Alessio, D.A.; Sandoval, D.A.; Seeley, R.J. Neuronal GLP1R mediates liraglutide’s anorectic but not glucose-lowering effect. J. Clin. Investig. 2014, 124, 2456–2463. [Google Scholar] [CrossRef] [Green Version]
- Knudsen, L.B.; Lau, J. The Discovery and Development of Liraglutide and Semaglutide. Front. Endocrinol. 2019, 10, 155. [Google Scholar] [CrossRef] [Green Version]
- van Bloemendaal, L.; Ten Kulve, J.S.; la Fleur, S.E.; Ijzerman, R.G.; Diamant, M. Effects of glucagon-like peptide 1 on appetite and body weight: Focus on the CNS. J. Endocrinol. 2014, 221, T1-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bednarz, K.; Kowalczyk, K.; Cwynar, M.; Czapla, D.; Czarkowski, W.; Kmita, D.; Nowak, A.; Madej, P. The Role of Glp-1 Receptor Agonists in Insulin Resistance with Concomitant Obesity Treatment in Polycystic Ovary Syndrome. Int. J. Mol. Sci. 2022, 23, 4334. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhu, C.; Lu, M.; Chen, C.; Nie, X.; Abudukerimu, B.; Zhang, K.; Ning, Z.; Chen, Y.; Cheng, J.; et al. The key role of a glucagon-like peptide-1 receptor agonist in body fat redistribution. J. Endocrinol. 2019, 240, 271–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishii, S.; Nagai, Y.; Sada, Y.; Fukuda, H.; Nakamura, Y.; Matsuba, R.; Nakagawa, T.; Kato, H.; Tanaka, Y. Liraglutide Reduces Visceral and Intrahepatic Fat Without Significant Loss of Muscle Mass in Obese Patients With Type 2 Diabetes: A Prospective Case Series. J. Clin. Med. Res. 2019, 11, 219–224. [Google Scholar] [CrossRef] [Green Version]
- Yaribeygi, H.; Sathyapalan, T.; Sahebkar, A. Molecular mechanisms by which GLP-1 RA and DPP-4i induce insulin sensitivity. Life Sci. 2019, 234, 116776. [Google Scholar] [CrossRef]
- Challa, T.D.; Beaton, N.; Arnold, M.; Rudofsky, G.; Langhans, W.; Wolfrum, C. Regulation of adipocyte formation by GLP-1/GLP-1R signaling. J. Biol. Chem. 2012, 287, 6421–6430. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zhao, H.; Ma, X.; Zhang, Y.; Lu, S.; Wang, Y.; Zong, C.; Qin, D.; Wang, Y.; Yingfeng Yang, Y.; et al. GLP-1/GLP-1R Signaling in Regulation of Adipocyte Differentiation and Lipogenesis. Cell. Physiol. Biochem. 2017, 42, 1165–1176. [Google Scholar] [CrossRef]
- Li, H.; Donelan, W.; Wang, F.; Zhang, P.; Yang, L.; Ding, Y.; Tang, D.; Li, S. GLP-1 Induces the Expression of FNDC5 Derivatives That Execute Lipolytic Actions. Front. Cell Dev. Biol. 2021, 9, 777026. [Google Scholar] [CrossRef]
- Sulaiman, M.A.; Al-Farsi, Y.M.; Al-Khaduri, M.M.; Saleh, J.; Waly, M.I. Polycystic ovarian syndrome is linked to increased oxidative stress in Omani women. Int. J. Womens Health 2018, 10, 763–771. [Google Scholar] [CrossRef] [Green Version]
- Betteridge, D.J. What is oxidative stress? Metabolism 2000, 49, 3–8. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus. Oxid. Med. Cell. Longev. 2020, 2020, 8609213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michos, E.D. Polycystic Ovarian Syndrome: How Your Ovaries Can Affect Your Heart. Available online: https://www.hopkinsmedicine.org/health/conditions-and-diseases/polycystic-ovarian-syndrome-how-your-ovaries-can-affect-your-heart (accessed on 10 August 2022).
- Antipolis, S. Young Women with Polycystic Ovary Syndrome Have Raised Risk of Heart Disease. Available online: https://www.escardio.org/The-ESC/Press-Office/Press-releases/Young-women-with-polycystic-ovary-syndrome-have-raised-risk-of-heart-disease (accessed on 6 August 2022).
- Rizzo, M.; Abate, N.; Chandalia, M.; Rizvi, A.A.; Giglio, R.V.; Nikolic, D.; Marino Gammazza, A.; Barbagallo, I.; Isenovic, E.R.; Banach, M.; et al. Liraglutide reduces oxidative stress and restores heme oxygenase-1 and ghrelin levels in patients with type 2 diabetes: A prospective pilot study. J. Clin. Endocrinol. Metab. 2015, 100, 603–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Millán, E.; Martín, M.A.; Goya, L.; Lizárraga-Mollinedo, E.; Escrivá, F.; Ramos, S.; Álvarez, C. Glucagon-like peptide-1 improves beta-cell antioxidant capacity via extracellular regulated kinases pathway and Nrf2 translocation. Free. Radic. Biol. Med. 2016, 95, 16–26. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; She, M.; Xu, M.; Chen, H.; Li, J.; Chen, X.; Zheng, D.; Liu, J.; Chen, S.; Zhu, J.; et al. GLP-1 treatment protects endothelial cells from oxidative stress-induced autophagy and endothelial dysfunction. Int. J. Biol. Sci. 2018, 14, 1696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frostegård, J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013, 11, 117. [Google Scholar] [CrossRef] [Green Version]
- Sathyapalan, T.; Atkin, S.L. Mediators of inflammation in polycystic ovary syndrome in relation to adiposity. Mediat. Inflamm. 2010, 2010, 758656. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.S.; Jun, H.S. Anti-Inflammatory Effects of GLP-1-Based Therapies beyond Glucose Control. Mediat. Inflamm. 2016, 2016, 3094642. [Google Scholar] [CrossRef] [Green Version]
- Kanda, H.; Tateya, S.; Tamori, Y.; Kotani, K.; Hiasa, K.; Kitazawa, R.; Kitazawa, S.; Miyachi, H.; Maeda, S.; Egashira, K.; et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Investig. 2006, 116, 1494–1505. [Google Scholar] [CrossRef]
- Lee, Y.S.; Park, M.S.; Choung, J.S.; Kim, S.S.; Oh, H.H.; Choi, C.S.; Ha, S.Y.; Kang, Y.; Kim, Y.; Jun, H.S. Glucagon-like peptide-1 inhibits adipose tissue macrophage infiltration and inflammation in an obese mouse model of diabetes. Diabetologia 2012, 55, 2456–2468. [Google Scholar] [CrossRef] [Green Version]
- Giuliani, C.; Bucci, I.; Napolitano, G. The Role of the Transcription Factor Nuclear Factor-kappa B in Thyroid Autoimmunity and Cancer. Front. Endocrinol. 2018, 9, 471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, C.; Huang, T.; Chen, A.; Chen, X.; Wang, L.; Shen, F.; Gu, X. Glucagon-like peptide 1 improves insulin resistance in vitro through anti-inflammation of macrophages. Braz. J. Med. Biol. Res. 2016, 49, e5826. [Google Scholar] [CrossRef] [PubMed]
- Kim Chung le, T.; Hosaka, T.; Yoshida, M.; Harada, N.; Sakaue, H.; Sakai, T.; Nakaya, Y. Exendin-4, a GLP-1 receptor agonist, directly induces adiponectin expression through protein kinase A pathway and prevents inflammatory adipokine expression. Biochem. Biophys. Res. Commun. 2009, 390, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Walsh, K. Adiponectin as an anti-inflammatory factor. Clin. Chim. Acta 2007, 380, 24–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawson, A.J.; Sathyapalan, T.; Vince, R.; Coady, A.M.; Ajjan, R.A.; Kilpatrick, E.S.; Atkin, S.L. The Effect of Exenatide on Cardiovascular Risk Markers in Women With Polycystic Ovary Syndrome. Front. Endocrinol. 2019, 10, 189. [Google Scholar] [CrossRef] [PubMed]
- Foltyn, W.; Strzelczyk, J.; Marek, B.; Kajdaniuk, D.; Siemińska, L.; Zemczak, A.; Blicharz-Dorniak, J.; Kos-Kudła, B. Selected markers of endothelial dysfunction in women with polycystic ovary syndrome. Endokrynol. Pol. 2011, 62, 243–248. [Google Scholar]
- Kahal, H.; Aburima, A.; Ungvari, T.; Rigby, A.S.; Coady, A.M.; Vince, R.V.; Ajjan, R.A.; Kilpatrick, E.S.; Naseem, K.M.; Atkin, S.L. The effects of treatment with liraglutide on atherothrombotic risk in obese young women with polycystic ovary syndrome and controls. BMC Endocr. Disord. 2015, 15, 14. [Google Scholar] [CrossRef] [Green Version]
- Krasner, N.M.; Ido, Y.; Ruderman, N.B.; Cacicedo, J.M. Glucagon-like peptide-1 (GLP-1) analog liraglutide inhibits endothelial cell inflammation through a calcium and AMPK dependent mechanism. PLoS ONE 2014, 9, e97554. [Google Scholar] [CrossRef] [Green Version]
- Vassilatou, E. Nonalcoholic fatty liver disease and polycystic ovary syndrome. World J. Gastroenterol. 2014, 20, 8351–8363. [Google Scholar] [CrossRef]
- Somm, E.; Montandon, S.A.; Loizides-Mangold, U.; Gaïa, N.; Lazarevic, V.; De Vito, C.; Perroud, E.; Bochaton-Piallat, M.-L.; Dibner, C.; Schrenzel, J.; et al. The GLP-1R agonist liraglutide limits hepatic lipotoxicity and inflammatory response in mice fed a methionine-choline deficient diet. Transl. Res. 2021, 227, 75–88. [Google Scholar] [CrossRef]
- Astrup, A.; Rössner, S.; Van Gaal, L.; Rissanen, A.; Niskanen, L.; Al Hakim, M.; Madsen, J.; Rasmussen, M.F.; Lean, M.E. Effects of liraglutide in the treatment of obesity: A randomised, double-blind, placebo-controlled study. Lancet 2009, 374, 1606–1616. [Google Scholar] [CrossRef]
- Lyu, X.; Lyu, T.; Wang, X.; Zhu, H.; Pan, H.; Wang, L.; Yang, H.; Gong, F. The Antiobesity Effect of GLP-1 Receptor Agonists Alone or in Combination with Metformin in Overweight/Obese Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Int. J. Endocrinol. 2021, 2021, 6616693. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Ding, X.; Wang, Y.; Deng, Y.; Sun, A. The therapeutic effects of glucagon-like peptide-1 receptor agonists and metformin on polycystic ovary syndrome: A protocol for systematic review and meta-analysis. Medicine 2021, 100, e26295. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.A.; Deshmukh, H.; Atkin, S.; Sathyapalan, T. The potential role of incretin-based therapies for polycystic ovary syndrome: A narrative review of the current evidence. Ther. Adv. Endocrinol. Metab. 2021, 12, 2042018821989238. [Google Scholar] [CrossRef]
- Sun, F.; Chai, S.; Yu, K.; Quan, X.; Yang, Z.; Wu, S.; Zhang, Y.; Ji, L.; Wang, J.; Shi, L. Gastrointestinal adverse events of glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: A systematic review and network meta-analysis. Diabetes Technol. 2015, 17, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Ratner, R.; Han, J.; Nicewarner, D.; Yushmanova, I.; Hoogwerf, B.J.; Shen, L. Cardiovascular safety of exenatide BID: An integrated analysis from controlled clinical trials in participants with type 2 diabetes. Cardiovasc. Diabetol. 2011, 10, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filippatos, T.D.; Panagiotopoulou, T.V.; Elisaf, M.S. Adverse Effects of GLP-1 Receptor Agonists. Rev. Diabet. Stud. 2014, 11, 202–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, L.; Costello, R.A. Glucagon-Like Peptide-1 Receptor Agonists; BTI—StatPearls: Tampa, FL, USA, 2022. [Google Scholar]
- Garber, A.J. Long-acting glucagon-like peptide 1 receptor agonists: A review of their efficacy and tolerability. Diabetes Care 2011, 34 (Suppl. S2), S279–S284. [Google Scholar] [CrossRef] [Green Version]
- Anderson, S.L.; Trujillo, J.M. Association of pancreatitis with glucagon-like peptide-1 agonist use. Ann. Pharm. 2010, 44, 904–909. [Google Scholar] [CrossRef]
- Bjerre Knudsen, L.; Madsen, L.W.; Andersen, S.; Almholt, K.; de Boer, A.S.; Drucker, D.J.; Gotfredsen, C.; Egerod, F.L.; Hegelund, A.C.; Jacobsen, H.; et al. Glucagon-like Peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology 2010, 151, 1473–1486. [Google Scholar] [CrossRef] [Green Version]
- Boess, F.; Bertinetti-Lapatki, C.; Zoffmann, S.; George, C.; Pfister, T.; Roth, A.; Lee, S.M.; Thasler, W.E.; Singer, T.; Suter, L. Effect of GLP1R agonists taspoglutide and liraglutide on primary thyroid C-cells from rodent and man. J. Mol. Endocrinol. 2013, 50, 325–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, W.Y.; Shih, S.-R.; Tseng, C.-H. A review on the association between glucagon-like peptide-1 receptor agonists and thyroid cancer. Exp. Diabetes Res. 2012, 2012, 924168. [Google Scholar] [CrossRef] [Green Version]
- FDA. FDA Approves Novel, Dual-Targeted Treatment for Type 2 Diabetes. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-novel-dual-targeted-treatment-type-2-diabetes (accessed on 8 June 2022).
- Coskun, T.; Sloop, K.W.; Loghin, C.; Alsina-Fernandez, J.; Urva, S.; Bokvist, K.B.; Cui, X.; Briere, D.A.; Cabrera, O.; Roell, W.C.; et al. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: From discovery to clinical proof of concept. Mol. Metab. 2018, 18, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Willard, F.S.; Douros, J.D.; Gabe, M.B.; Showalter, A.D.; Wainscott, D.B.; Suter, T.M.; Capozzi, M.E.; van der Velden, W.J.; Stutsman, C.; Cardona, G.R.; et al. Tirzepatide is an imbalanced and biased dual GIP and GLP-1 receptor agonist. JCI Insight 2020, 5, e140532. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, S.; Paulsson, J.F.; Kofoed, J.; Zosel, F.; Olsen, J.; Jeppesen, C.B.; Spetzler, J.; Ynddal, L.; Schleiss, L.G.; Christoffersen, B.Ø.; et al. The effect of fatty diacid acylation of human PYY3-36 on Y2 receptor potency and half-life in minipigs. Sci. Rep. 2021, 11, 21179. [Google Scholar] [CrossRef] [PubMed]
- Min, T.; Bain, S.C. The Role of Tirzepatide, Dual GIP and GLP-1 Receptor Agonist, in the Management of Type 2 Diabetes: The SURPASS Clinical Trials. Diabetes 2021, 12, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A.; Lund, A.; Knop, F.K.; Vilsbøll, T. Glucagon-like peptide 1 in health and disease. Nat. Rev. Endocrinol. 2018, 14, 390–403. [Google Scholar] [CrossRef]
- Roose, S.P. Compliance: The impact of adverse events and tolerability on the physician’s treatment decisions. Eur. Neuropsychopharmacol. 2003, 13 (Suppl. S3), S85–S92. [Google Scholar] [CrossRef]
- Usdin, T.B.; Mezey, E.; Button, D.C.; Brownstein, M.J.; Bonner, T.I. Gastric inhibitory polypeptide receptor, a member of the secretin-vasoactive intestinal peptide receptor family, is widely distributed in peripheral organs and the brain. Endocrinology 1993, 133, 2861–2870. [Google Scholar] [CrossRef]
- Kaneko, S. Tirzepatide: A Novel, Once-weekly Dual GIP and GLP-1 Receptor Agonist for the Treatment of Type 2 Diabetes. Touchrev. Endocrinol. 2022, 18, 10–19. [Google Scholar] [CrossRef]
- Nauck, M.A.; Meier, J.J. GIP and GLP-1: Stepsiblings Rather Than Monozygotic Twins Within the Incretin Family. Diabetes 2019, 68, 897–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, R.; Virendra, S.A.; Chawla, P.A. Bumps and humps in the success of Tirzepatide as the first GLP1 and GIP receptor agonist. Health Sci. Rev. 2022, 4, 100032. [Google Scholar] [CrossRef]
- Boer, G.A.-O.X.; Holst, J.A.-O. Incretin Hormones and Type 2 Diabetes-Mechanistic Insights and Therapeutic Approaches. Biology 2020, 9, 473. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Winter, K.; Nian, C.; Tsuneoka, M.; Koda, Y.; McIntosh, C.H. Glucose-dependent insulinotropic polypeptide (GIP) stimulation of pancreatic beta-cell survival is dependent upon phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB) signaling, inactivation of the forkhead transcription factor Foxo1, and down-regulation of bax expression. J. Biol. Chem. 2005, 280, 22297–22307. [Google Scholar]
- Christensen, M.B.; Calanna, S.; Holst, J.J.; Vilsbøll, T.; Knop, F.K. Glucose-dependent insulinotropic polypeptide: Blood glucose stabilizing effects in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2014, 99, E418–E426. [Google Scholar] [CrossRef] [Green Version]
- Harada, N.; Hamasaki, A.; Yamane, S.; Muraoka, A.; Joo, E.; Fujita, K.; Inagaki, N. Plasma gastric inhibitory polypeptide and glucagon-like peptide-1 levels after glucose loading are associated with different factors in Japanese subjects. J. Diabetes Investig. 2011, 2, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Seino, Y.; Fukushima, M.; Yabe, D. GIP and GLP-1, the two incretin hormones: Similarities and differences. J. Diabetes Investig. 2010, 1, 8–23. [Google Scholar] [CrossRef] [Green Version]
- Gastaldelli, A.; Stefan, N.; Häring, H.U. Liver-targeting drugs and their effect on blood glucose and hepatic lipids. Diabetologia 2021, 64, 1461–1479. [Google Scholar] [CrossRef]
- FDA. Highlights of Prescribing Information; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2022.
- Dahl, D.; Onishi, Y.; Norwood, P.; Huh, R.; Bray, R.; Patel, H.; Rodríguez, Á. Effect of Subcutaneous Tirzepatide vs. Placebo Added to Titrated Insulin Glargine on Glycemic Control in Patients With Type 2 Diabetes: The SURPASS-5 Randomized Clinical Trial. JAMA 2022, 327, 534–545. [Google Scholar] [CrossRef]
- Del Prato, S.; Kahn, S.E.; Pavo, I.; Weerakkody, G.J.; Yang, Z.; Doupis, J.; Aizenberg, D.; Wynne, A.G.; Riesmeyer, J.S.; Heine, R.J.; et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): A randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet 2021, 398, 1811–1824. [Google Scholar] [CrossRef]
- Frías, J.P.; Davies, M.J.; Rosenstock, J.; Pérez Manghi, F.C.; Fernández Landó, L.; Bergman, B.K.; Liu, B.; Cui, X.; Brown, K. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 503–515. [Google Scholar] [CrossRef]
- Ludvik, B.; Giorgino, F.; Jódar, E.; Frias, J.P.; Fernández Landó, L.; Brown, K.; Bray, R.; Rodríguez, Á. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): A randomised, open-label, parallel-group, phase 3 trial. Lancet 2021, 398, 583–598. [Google Scholar] [CrossRef]
- Rosenstock, J.; Wysham, C.; Frías, J.P.; Kaneko, S.; Lee, C.J.; Fernández Landó, L.; Mao, H.; Cui, X.; Karanikas, C.A.; Thieu, V.T. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): A double-blind, randomised, phase 3 trial. Lancet 2021, 398, 143–155. [Google Scholar] [CrossRef]
- Zoler, M.L. Tirzepatide Powers ‘Unprecedented’ Weight Loss in Obesity Trial. Available online: https://www.medscape.com/viewarticle/975061 (accessed on 17 August 2022).
- ADA. SURMOUNT-1 Study Finds Individuals with Obesity Lost Up to 22.5% of Their Body Weight when Taking Tirzepatide. Available online: https://diabetes.org/newsroom/press-releases/2022/surmount-1-study-finds-individuals-%20with-obesity-lost-up-to-22.5-percent-body-weight-taking-tirzepatide (accessed on 19 July 2022).
- Jastreboff, A.M.; Aronne, L.J.; Ahmad, N.N.; Wharton, S.; Connery, L.; Alves, B.; Kiyosue, A.; Zhang, S.; Liu, B.; Bunck, M.C.; et al. Tirzepatide Once Weekly for the Treatment of Obesity. N. Engl. J. Med. 2022, 387, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Mazin, A. Drug Leads to Drastic Weight Loss with Diet and Exercise. Available online: https://www.lifespan.io/news/drug-leads-to-drastic-weight-loss-with-diet-and-exercise/ (accessed on 16 July 2022).
- Kakoly, N.S.; Khomami, M.B.; Joham, A.E.; Cooray, S.D.; Misso, M.L.; Norman, R.J.; Harrison, C.L.; Ranasinha, S.; Teede, H.J.; Moran, L.J. Ethnicity, obesity and the prevalence of impaired glucose tolerance and type 2 diabetes in PCOS: A systematic review and meta-regression. Hum. Reprod. Update 2018, 24, 455–467. [Google Scholar] [CrossRef]
- Sacerdote, A. Rare and Underappreciated Causes of Polycystic Ovarian Syndrome. In Polycystic Ovary Syndrome; Zhengchao, W., Ed.; IntechOpen: Rijeka, Croatia, 2022; p. Ch. 4. [Google Scholar]
- Guerciolini, R. Mode of action of orlistat. Int. J. Obes. Relat. Metab. Disord. 1997, 21 (Suppl. S3), S12–S23. [Google Scholar] [PubMed]
- Jayagopal, V.; Kilpatrick, E.S.; Holding, S.; Jennings, P.E.; Atkin, S.L. Orlistat is as beneficial as metformin in the treatment of polycystic ovarian syndrome. J. Clin. Endocrinol. Metab. 2005, 90, 729–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graff, S.K.; Mario, F.M.; Ziegelmann, P.; Spritzer, P.M. Effects of orlistat vs. metformin on weight loss-related clinical variables in women with PCOS: Systematic review and meta-analysis. Int. J. Clin. Prac. 2016, 70, 450–461. [Google Scholar] [CrossRef] [Green Version]
- Panidis, D.; Tziomalos, K.; Papadakis, E.; Chatzis, P.; Kandaraki, E.A.; Tsourdi, E.A.; Katsikis, I. The role of orlistat combined with lifestyle changes in the management of overweight and obese patients with polycystic ovary syndrome. Clin. Endocrinol. 2014, 80, 432–438. [Google Scholar] [CrossRef]
- Setter, S.M.; Iltz, J.L.; Thams, J.; Campbell, R.K. Metformin hydrochloride in the treatment of type 2 diabetes mellitus: A clinical review with a focus on dual therapy. Clin. Ther. 2003, 25, 2991–3026. [Google Scholar] [CrossRef]
- Lord, J.M.; Flight, I.H.; Norman, R.J. Metformin in polycystic ovary syndrome: Systematic review and meta-analysis. BMJ 2003, 327, 951–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trolle, B.; Flyvbjerg, A.; Kesmodel, U.; Lauszus, F.F. Efficacy of metformin in obese and non-obese women with polycystic ovary syndrome: A randomized, double-blinded, placebo-controlled cross-over trial. Hum. Reprod. 2007, 22, 2967–2973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lashen, H. Review: Role of metformin in the management of polycystic ovary syndrome. Ther. Adv. Endocrinol. Metab. 2010, 1, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Aroda, V.R.; Edelstein, S.L.; Goldberg, R.B.; Knowler, W.C.; Marcovina, S.M.; Orchard, T.J.; Bray, G.A.; Schade, D.S.; Temprosa, M.G.; White, N.H.; et al. Long-term Metformin Use and Vitamin B12 Deficiency in the Diabetes Prevention Program Outcomes Study. J. Clin. Endocrinol. Metab. 2016, 101, 1754–1761. [Google Scholar] [CrossRef]
- Rives-Lange, C.; Rassy, N.; Carette, C.; Phan, A.; Barsamian, C.; Thereaux, J.; Moszkowicz, D.; Poghosyan, T.; Czernichow, S. Seventy years of bariatric surgery: A systematic mapping review of randomized controlled trials. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2022, 23, e13420. [Google Scholar] [CrossRef]
- Lee, R.; Joy Mathew, C.; Jose, M.T.; Elshaikh, A.O.; Shah, L.; Cancarevic, I. A Review of the Impact of Bariatric Surgery in Women With Polycystic Ovary Syndrome. Cureus 2020, 12, e10811. [Google Scholar] [CrossRef]
- SIGN. Management of Obesity: A National Clinical Guideline. Available online: https://www.sign.ac.uk/assets/sign115.pdf (accessed on 29 June 2022).
- Yildiz, B.O.; Knochenhauer, E.S.; Azziz, R. Impact of obesity on the risk for polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2008, 93, 162–168. [Google Scholar] [CrossRef] [Green Version]
- Johansson, K.; Cnattingius, S.; Näslund, I.; Roos, N.; Trolle Lagerros, Y.; Granath, F.; Stephansson, O.; Neovius, M. Outcomes of pregnancy after bariatric surgery. N. Engl. J. Med. 2015, 372, 814–824. [Google Scholar] [CrossRef] [Green Version]
- Meek, C.L.; Lewis, H.B.; Reimann, F.; Gribble, F.M.; Park, A.J. The effect of bariatric surgery on gastrointestinal and pancreatic peptide hormones. Peptides 2016, 77, 28–37. [Google Scholar] [CrossRef]
- Moffett, R.C.; Naughton, V. Emerging role of GIP and related gut hormones in fertility and PCOS. Peptides 2020, 125, 170233. [Google Scholar] [CrossRef]
- Khan, D.; Moffett, R.C. Commentary: Emerging role of GIP and related gut hormones in fertility and PCOS. J. Endocrinol. Sci. 2020, 2, 11–15. [Google Scholar] [CrossRef]
- Skubleny, D.; Switzer, N.J.; Gill, R.S.; Dykstra, M.; Shi, X.; Sagle, M.A.; de Gara, C.; Birch, D.W.; Karmali, S. The Impact of Bariatric Surgery on Polycystic Ovary Syndrome: A Systematic Review and Meta-analysis. Obes. Surg. 2016, 26, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, R.; Stener-Victorin, E.; Yildiz, B.O.; Duleba, A.J.; Hoeger, K.; Mason, H.; Homburg, R.; Hickey, T.; Franks, S.; Tapanainen, J.S.; et al. PCOS Forum: Research in polycystic ovary syndrome today and tomorrow. Clin. Endocrinol. 2011, 74, 424–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Trial | SURPASS 1 | SURPASS 2 | SURPASS 3 | SURPASS 4 | SURPASS 5 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tirzepatide | 5 mg/10 mg/15 mg | 5 mg/10 mg/15 mg | 5 mg/10 mg/15 mg | 5 mg/10 mg/15 mg | 5 mg/10 mg/15 mg | ||||||||||
| Comparator | Placebo | Semaglutide 1.0 mg | Insulin degludec | Insulin glargine | Placebo + Insulin glargine | ||||||||||
| Trial duration | 40 weeks | 40 weeks | 52 weeks | 52 weeks | 40 weeks | ||||||||||
| Design | double-blind placebo-controlled | open-label | open-label | open-label | double-blind placebo-controlled | ||||||||||
| Tirzepatide | 5 mg | 10 mg | 15 mg | 5 mg | 10 mg | 15 mg | 5 mg | 10 mg | 15 mg | 5 mg | 10 mg | 15 mg | 5 mg | 10 mg | 15 mg |
| Patients number | 121 | 121 | 121 | 470 | 469 | 470 | 358 | 360 | 359 | 329 | 328 | 338 | 116 | 119 | 120 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anala, A.D.; Saifudeen, I.S.H.; Ibrahim, M.; Nanda, M.; Naaz, N.; Atkin, S.L. The Potential Utility of Tirzepatide for the Management of Polycystic Ovary Syndrome. J. Clin. Med. 2023, 12, 4575. https://doi.org/10.3390/jcm12144575
Anala AD, Saifudeen ISH, Ibrahim M, Nanda M, Naaz N, Atkin SL. The Potential Utility of Tirzepatide for the Management of Polycystic Ovary Syndrome. Journal of Clinical Medicine. 2023; 12(14):4575. https://doi.org/10.3390/jcm12144575
Chicago/Turabian StyleAnala, Alekya Devi, Insiya Sajjad Hussain Saifudeen, Maryam Ibrahim, Moksha Nanda, Nida Naaz, and Stephen L. Atkin. 2023. "The Potential Utility of Tirzepatide for the Management of Polycystic Ovary Syndrome" Journal of Clinical Medicine 12, no. 14: 4575. https://doi.org/10.3390/jcm12144575
APA StyleAnala, A. D., Saifudeen, I. S. H., Ibrahim, M., Nanda, M., Naaz, N., & Atkin, S. L. (2023). The Potential Utility of Tirzepatide for the Management of Polycystic Ovary Syndrome. Journal of Clinical Medicine, 12(14), 4575. https://doi.org/10.3390/jcm12144575






