Aggregate Index of Systemic Inflammation (AISI), Disease Severity, and Mortality in COVID-19: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
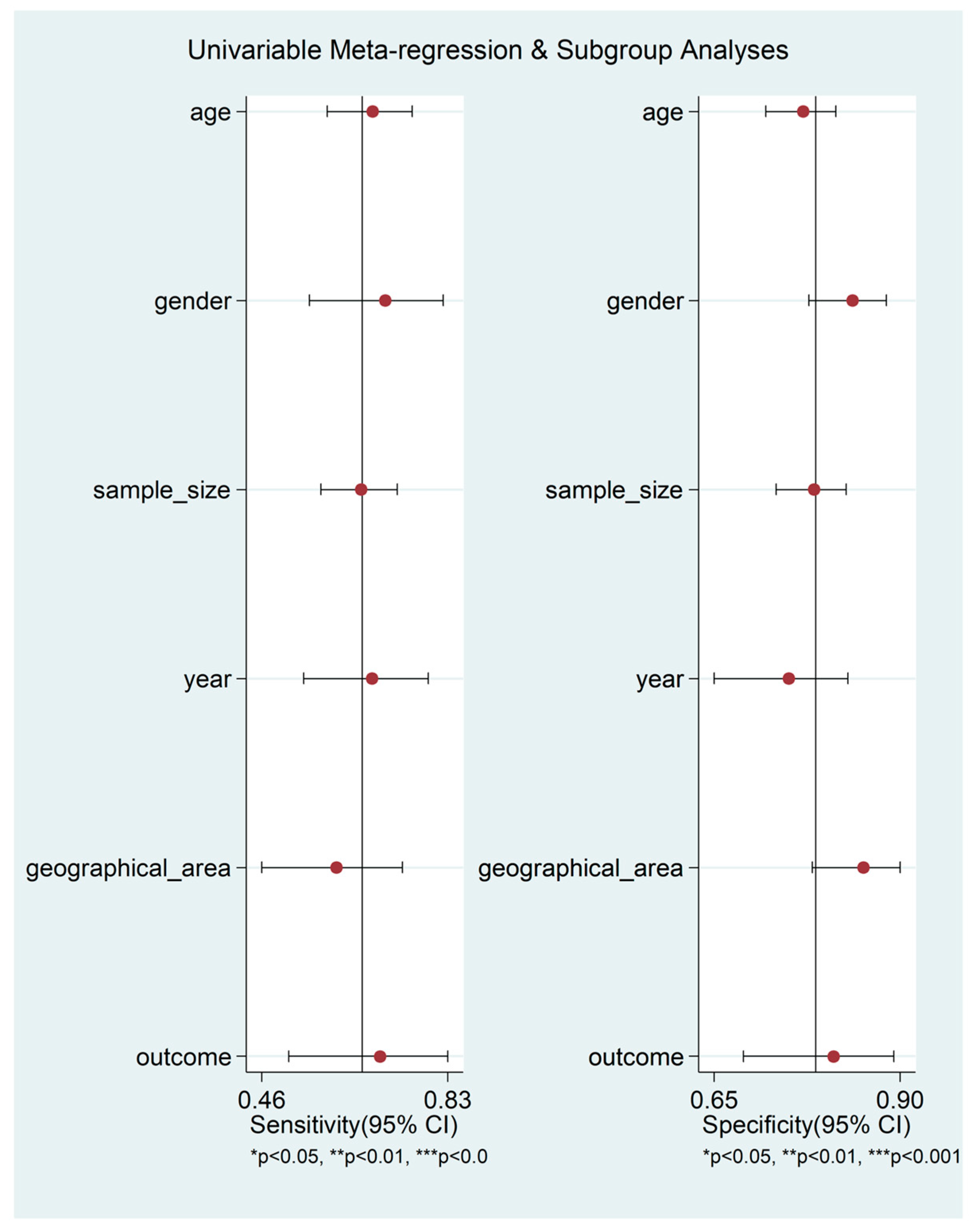
3. Results
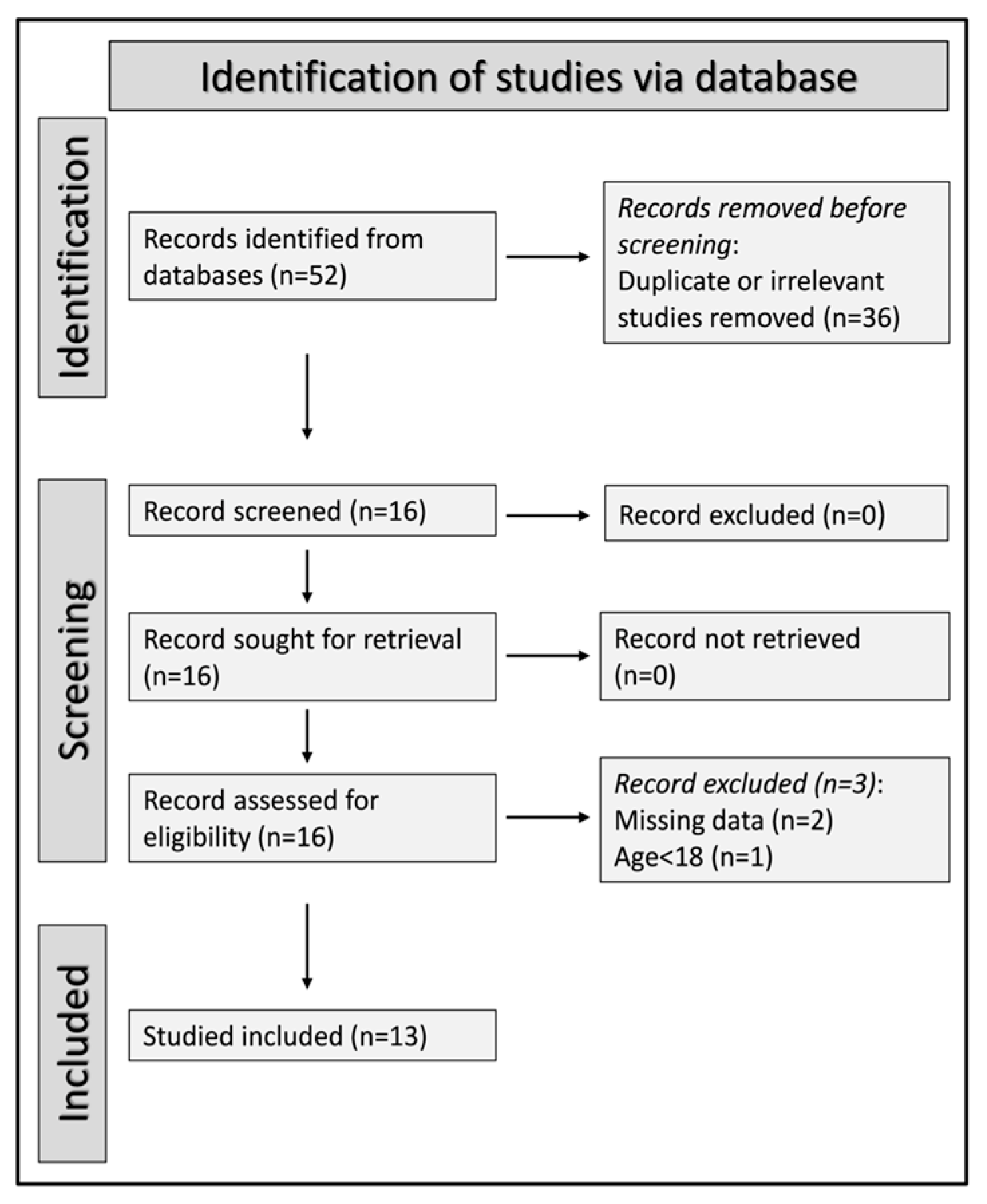
3.1. Study Selection
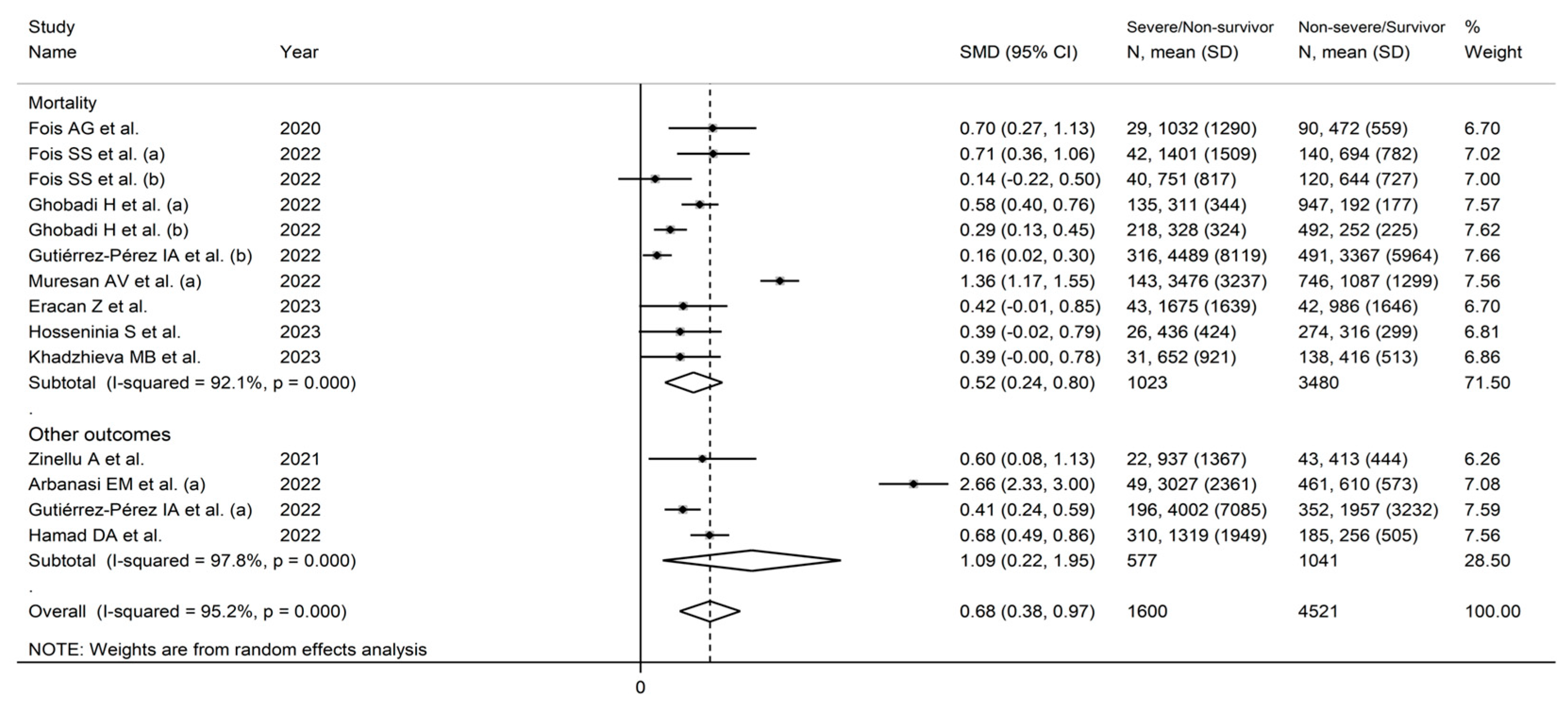
3.2. Standardized Mean Differences
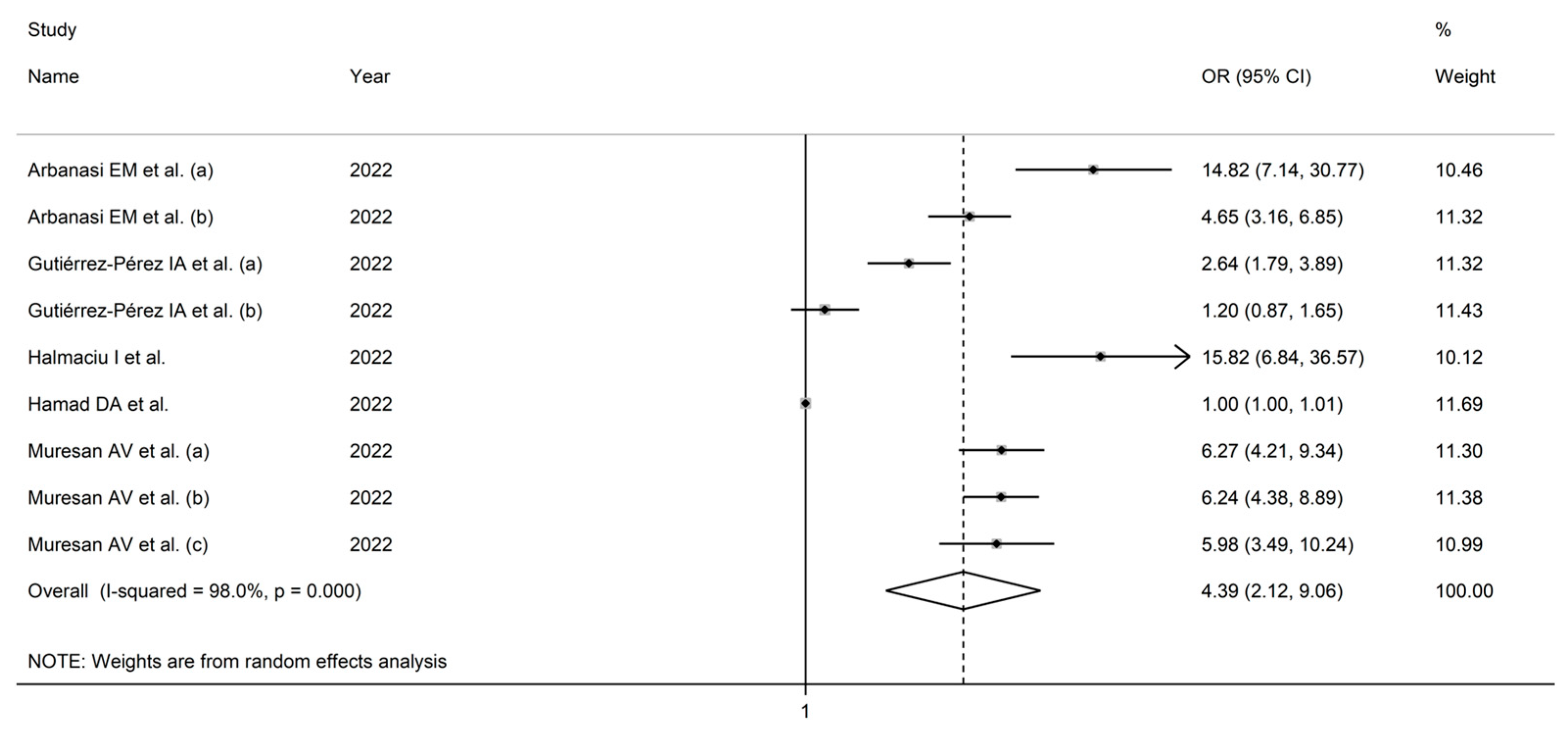
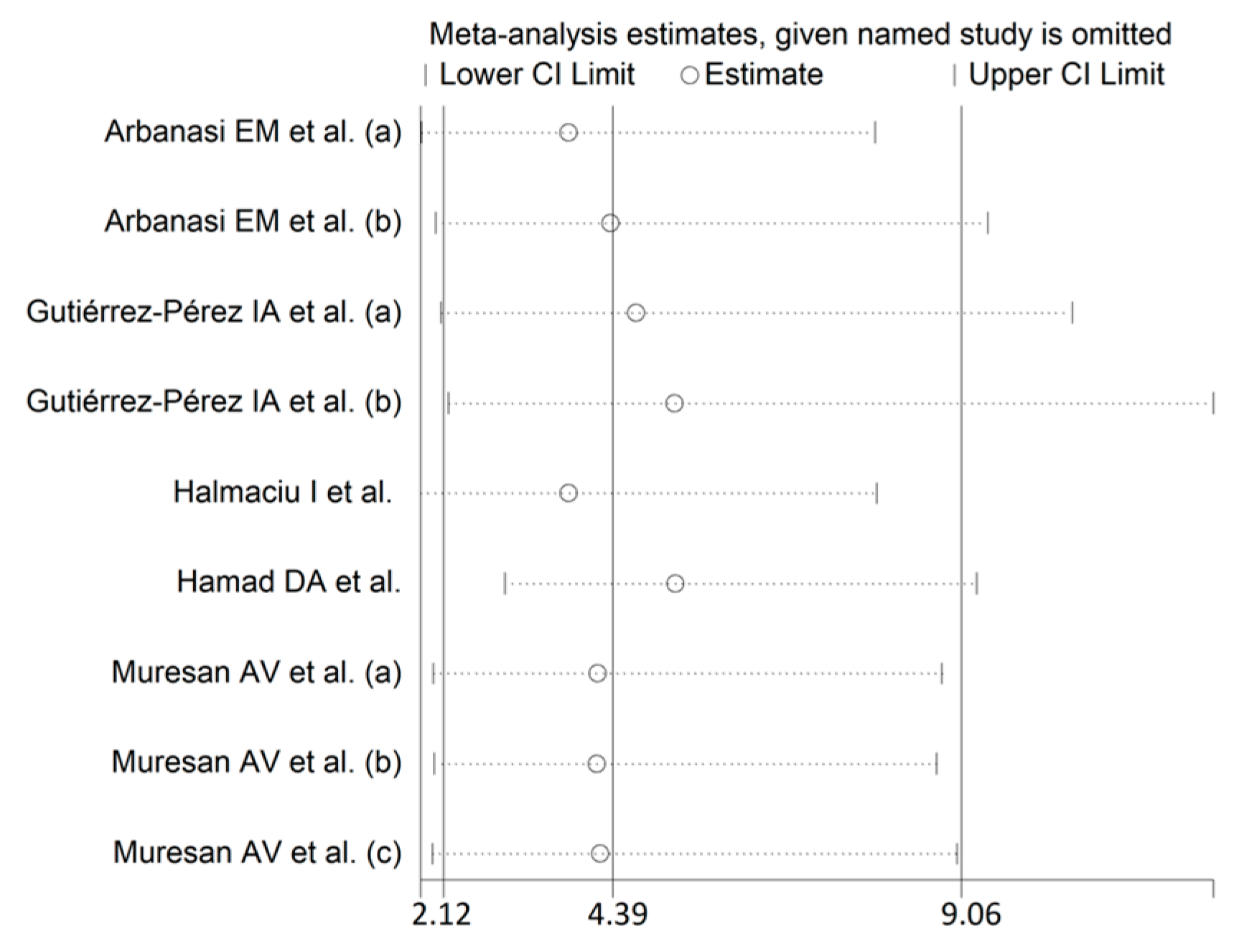
3.3. Odds Ratios
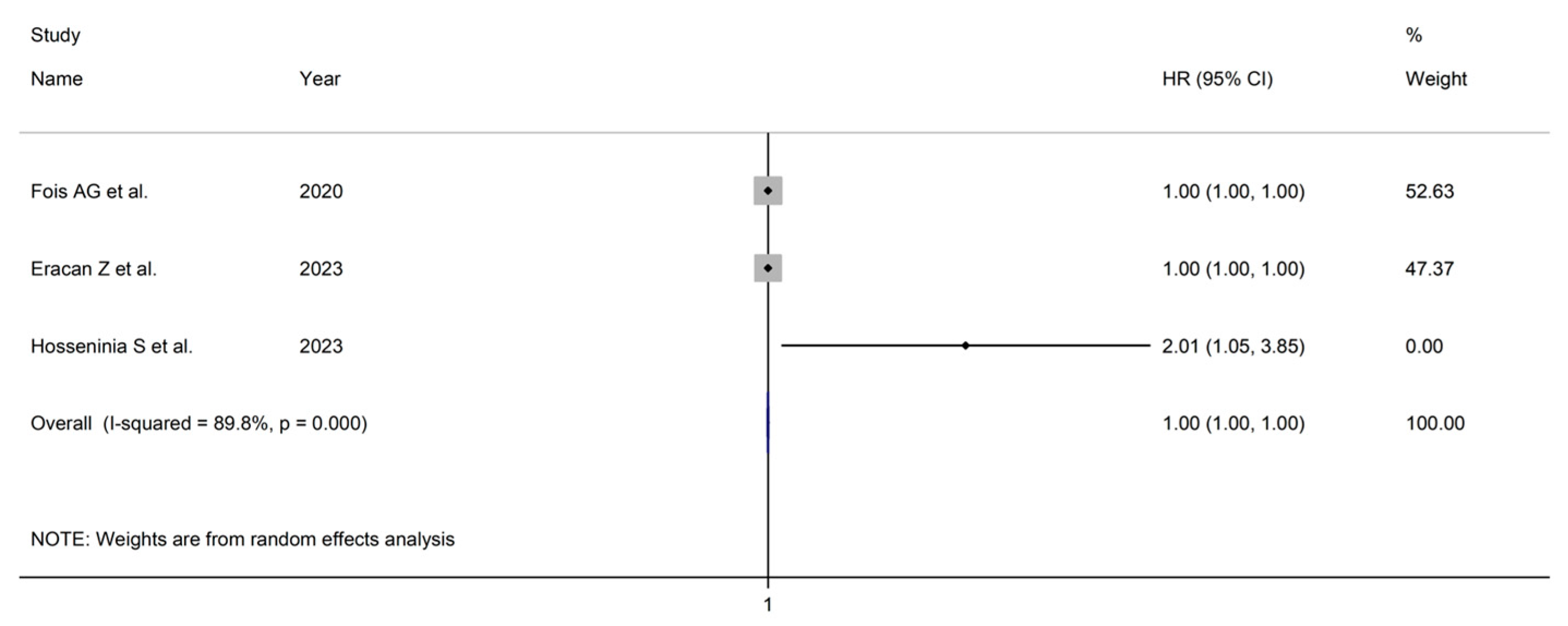
3.4. Hazard Ratios
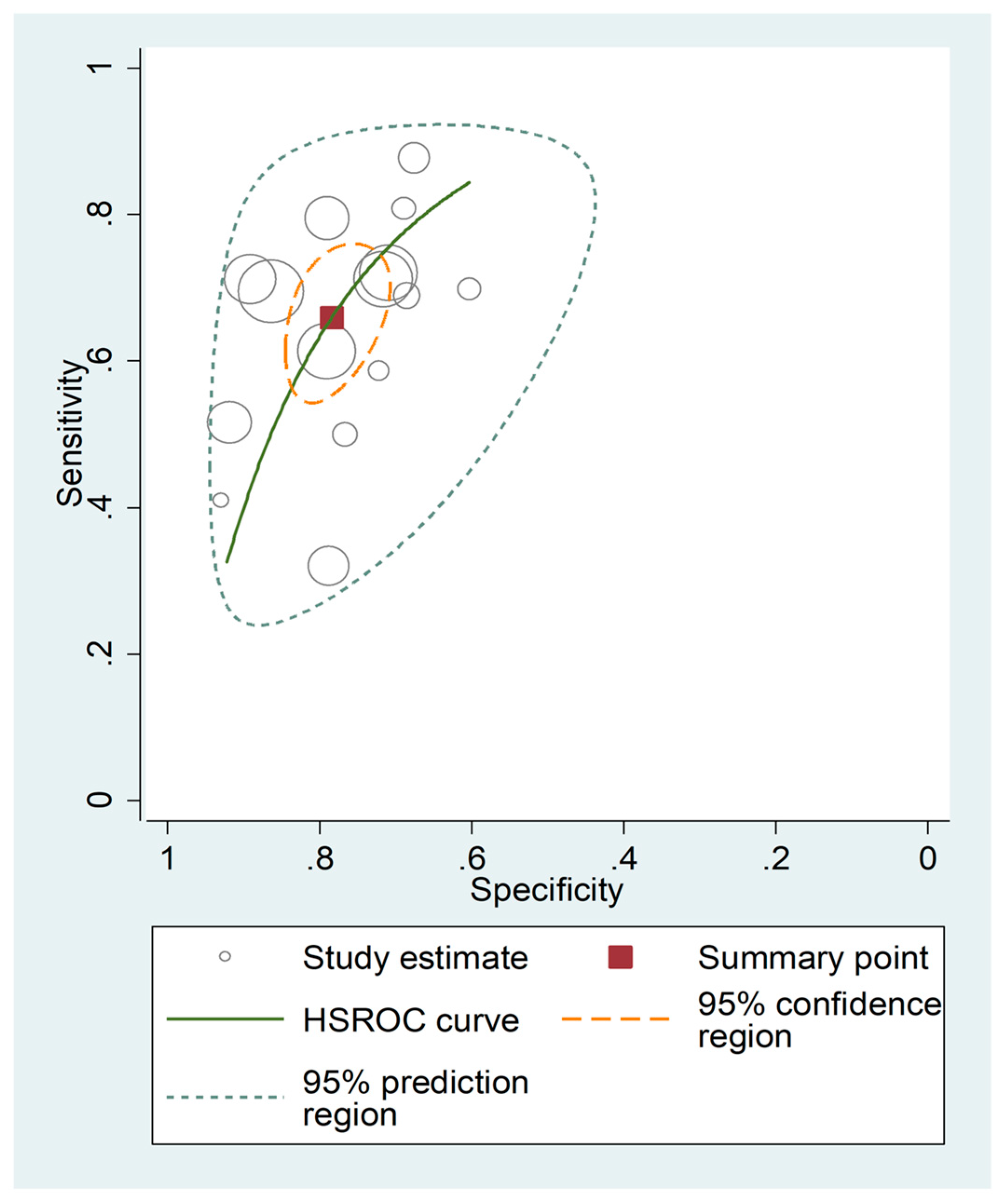
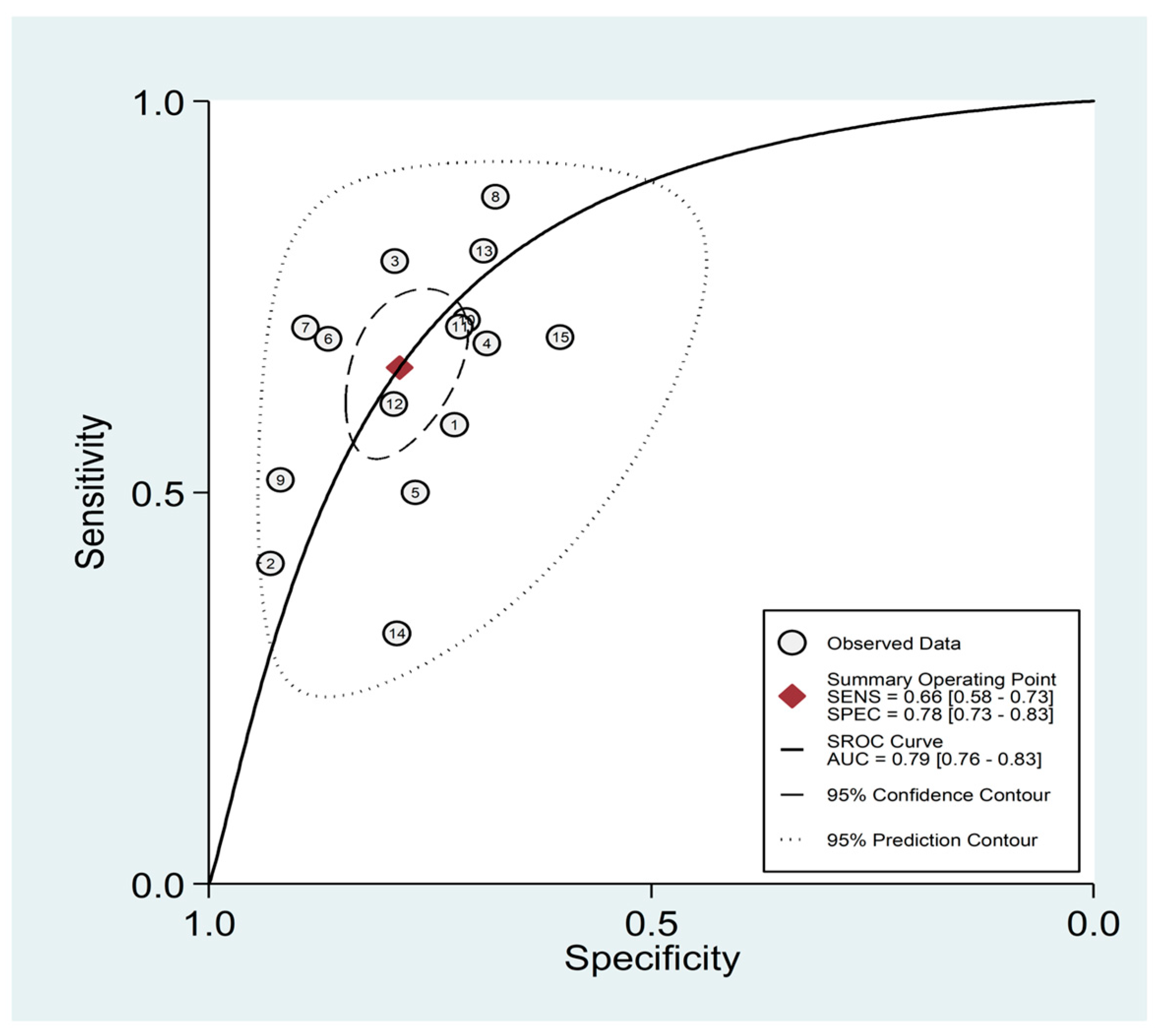
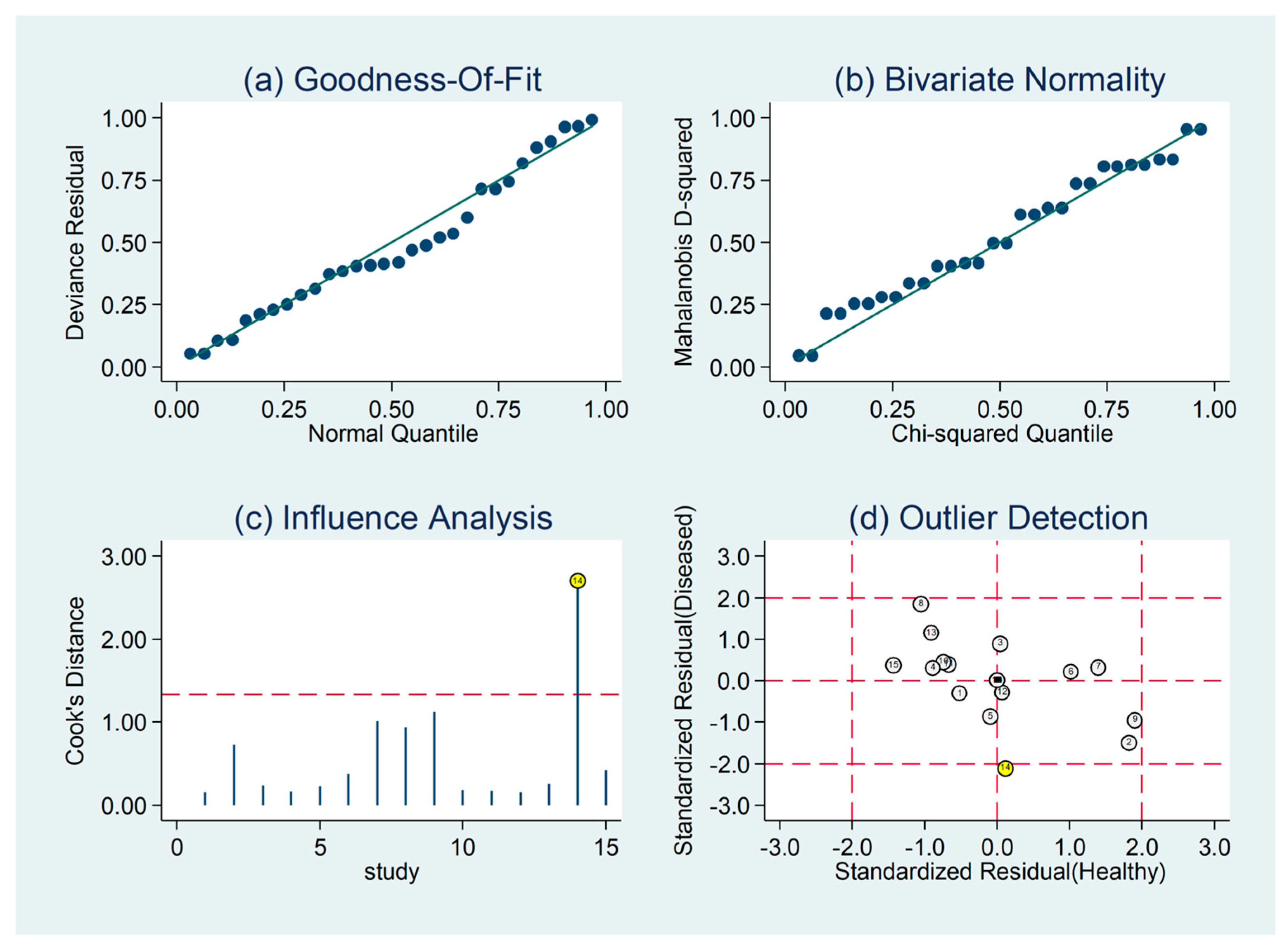
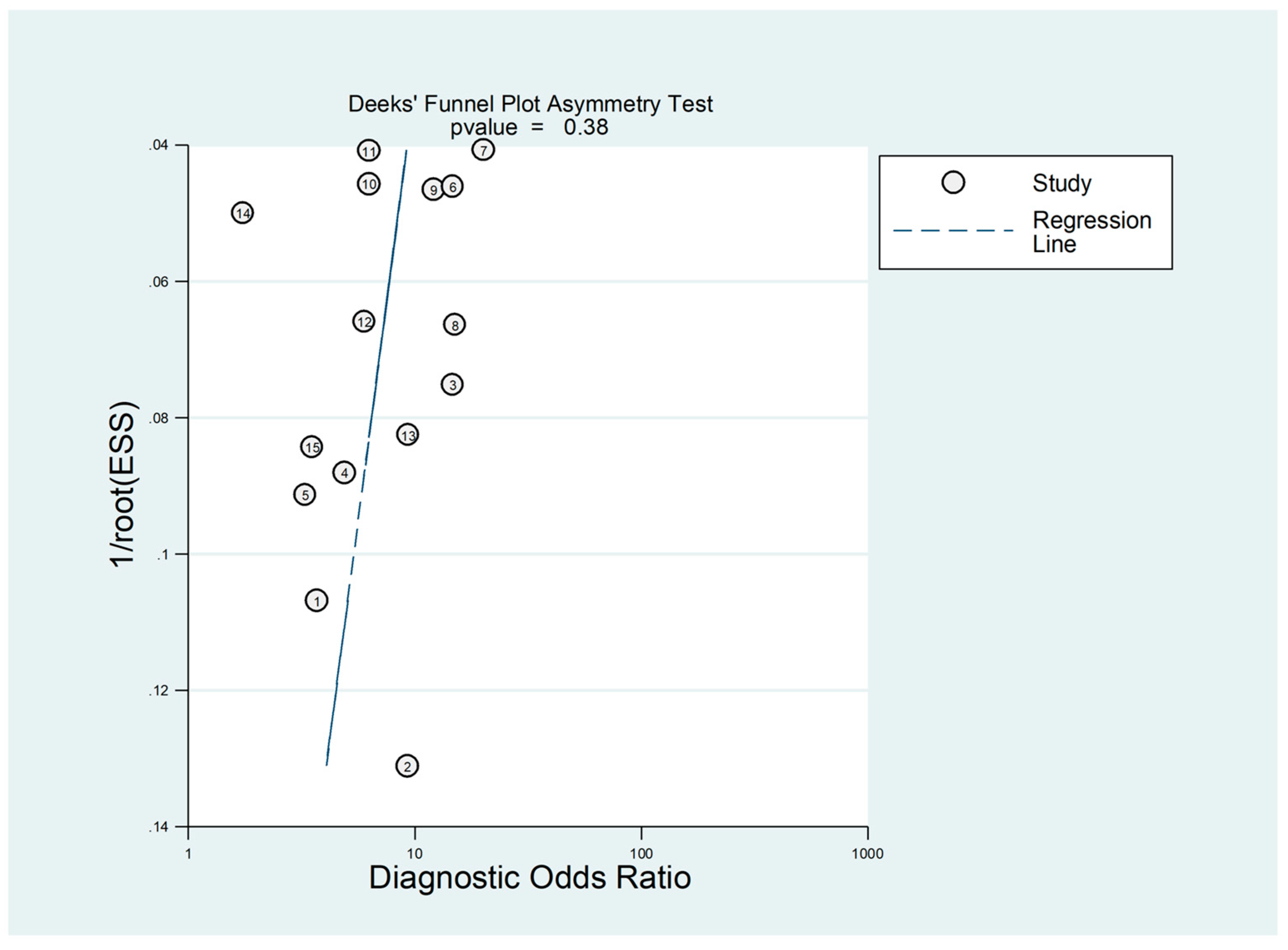
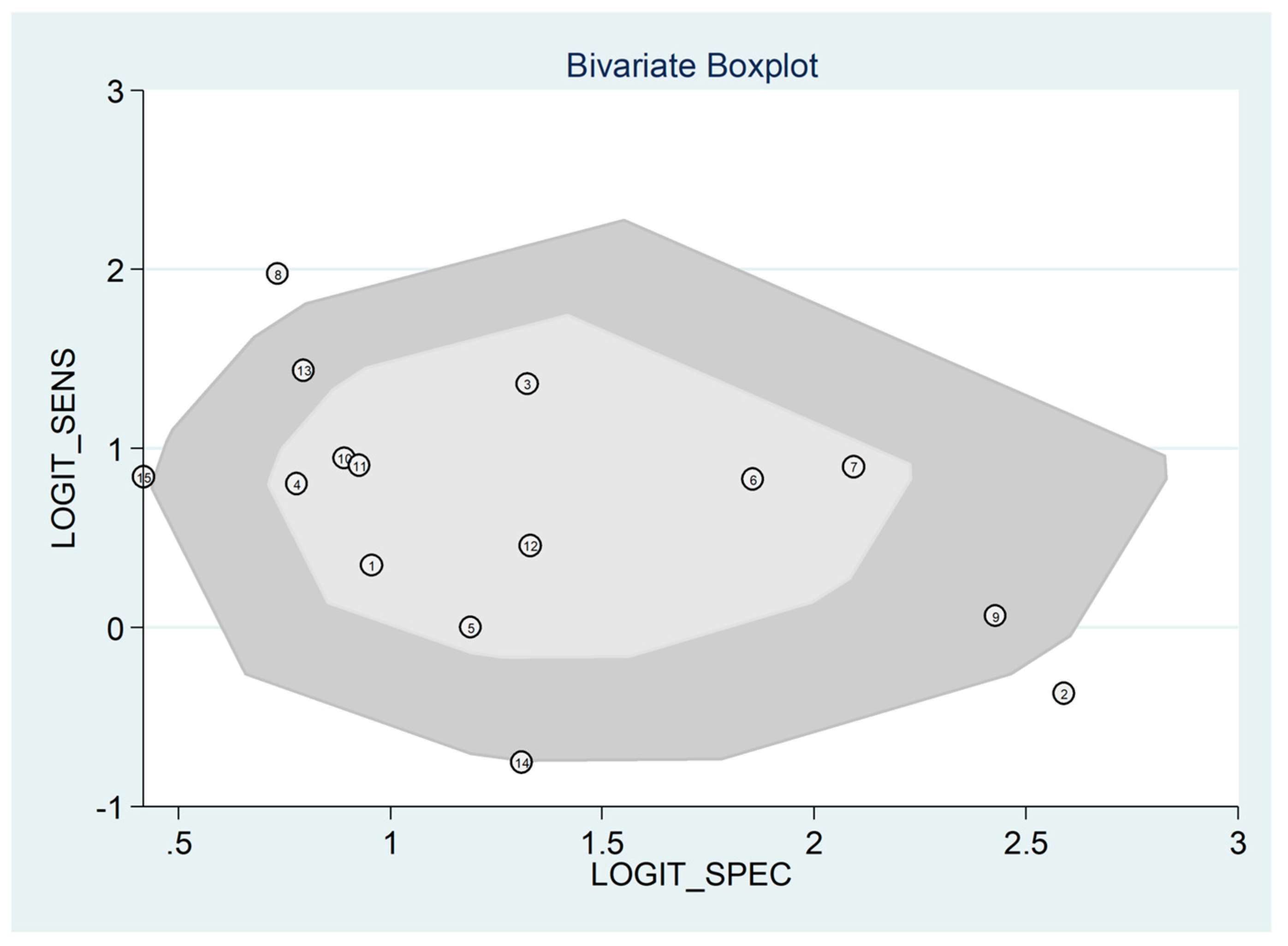
3.5. Diagnostic Accuracy for Prediction of Severe Disease or Death
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Merad, M.; Blish, C.A.; Sallusto, F.; Iwasaki, A. The immunology and immunopathology of COVID-19. Science 2022, 375, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Y.; Sun, Q.; Knopf, J.; Herrmann, M.; Lin, L.; Jiang, J.; Shao, C.; Li, P.; He, X.; et al. Immune response in COVID-19: What is next? Cell Death Differ. 2022, 29, 1107–1122. [Google Scholar] [CrossRef] [PubMed]
- Silberberg, E.; Filep, J.G.; Ariel, A. Weathering the Storm: Harnessing the Resolution of Inflammation to Limit COVID-19 Pathogenesis. Front. Immunol. 2022, 13, 863449. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, S.A.; de Souza, D.A.; Torres, A.L.; de Lima, C.F.G.; Ebram, M.C.; Celano, R.M.G.; Schattner, M.; Chudzinski-Tavassi, A.M. Pathophysiology of COVID-19: Critical Role of Hemostasis. Front. Cell Infect. Microbiol. 2022, 12, 896972. [Google Scholar] [CrossRef]
- Ghofrani Nezhad, M.; Jami, G.; Kooshkaki, O.; Chamani, S.; Naghizadeh, A. The Role of Inflammatory Cytokines (Interleukin-1 and Interleukin-6) as a Potential Biomarker in the Different Stages of COVID-19 (Mild, Severe, and Critical). J. Interferon Cytokine Res. 2023, 43, 147–163. [Google Scholar] [CrossRef]
- Silva, M.J.A.; Ribeiro, L.R.; Gouveia, M.I.M.; Marcelino, B.D.R.; Santos, C.S.D.; Lima, K.V.B.; Lima, L. Hyperinflammatory Response in COVID-19: A Systematic Review. Viruses 2023, 15, 553. [Google Scholar] [CrossRef]
- Nasrollahi, H.; Talepoor, A.G.; Saleh, Z.; Eshkevar Vakili, M.; Heydarinezhad, P.; Karami, N.; Noroozi, M.; Meri, S.; Kalantar, K. Immune responses in mildly versus critically ill COVID-19 patients. Front. Immunol. 2023, 14, 1077236. [Google Scholar] [CrossRef]
- Arish, M.; Qian, W.; Narasimhan, H.; Sun, J. COVID-19 immunopathology: From acute diseases to chronic sequelae. J. Med. Virol. 2023, 95, e28122. [Google Scholar] [CrossRef]
- Montazersaheb, S.; Hosseiniyan Khatibi, S.M.; Hejazi, M.S.; Tarhriz, V.; Farjami, A.; Ghasemian Sorbeni, F.; Farahzadi, R.; Ghasemnejad, T. COVID-19 infection: An overview on cytokine storm and related interventions. Virol. J. 2022, 19, 92. [Google Scholar] [CrossRef]
- Tan, L.Y.; Komarasamy, T.V.; Rmt Balasubramaniam, V. Hyperinflammatory Immune Response and COVID-19: A Double Edged Sword. Front. Immunol. 2021, 12, 742941. [Google Scholar] [CrossRef]
- Selvarajan, S.; Anandaradje, A.; Shivabasappa, S.; Melepurakkal Sadanandan, D.; Nair, N.S.; George, M. Efficacy of pharmacological interventions in COVID-19: A network meta-analysis. Br. J. Clin. Pharmacol. 2022, 88, 4080–4091. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Jiao, B.; Qu, L.; Yang, D.; Liu, R. The development of COVID-19 treatment. Front. Immunol. 2023, 14, 1125246. [Google Scholar] [CrossRef] [PubMed]
- Qin, R.; He, L.; Yang, Z.; Jia, N.; Chen, R.; Xie, J.; Fu, W.; Chen, H.; Lin, X.; Huang, R.; et al. Identification of Parameters Representative of Immune Dysfunction in Patients with Severe and Fatal COVID-19 Infection: A Systematic Review and Meta-analysis. Clin. Rev. Allergy Immunol. 2023, 64, 33–65. [Google Scholar] [CrossRef] [PubMed]
- Zinellu, A.; Mangoni, A.A. Serum Prealbumin Concentrations, COVID-19 Severity, and Mortality: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 638529. [Google Scholar] [CrossRef]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Elalamy, I.; Kastritis, E.; Sergentanis, T.N.; Politou, M.; Psaltopoulou, T.; Gerotziafas, G.; Dimopoulos, M.A. Hematological findings and complications of COVID-19. Am. J. Hematol. 2020, 95, 834–847. [Google Scholar] [CrossRef]
- Rahman, A.; Niloofa, R.; Jayarajah, U.; De Mel, S.; Abeysuriya, V.; Seneviratne, S.L. Hematological Abnormalities in COVID-19: A Narrative Review. Am. J. Trop. Med. Hyg. 2021, 104, 1188–1201. [Google Scholar] [CrossRef]
- Zinellu, A.; Mangoni, A.A. A systematic review and meta-analysis of the association between the neutrophil, lymphocyte, and platelet count, neutrophil-to-lymphocyte ratio, and platelet-to-lymphocyte ratio and COVID-19 progression and mortality. Expert Rev. Clin. Immunol. 2022, 18, 1187–1202. [Google Scholar] [CrossRef]
- Wei, T.; Li, J.; Cheng, Z.; Jiang, L.; Zhang, J.; Wang, H.; Zhou, L. Hematological characteristics of COVID-19 patients with fever infected by the Omicron variant in Shanghai: A retrospective cohort study in China. J. Clin. Lab. Anal. 2023, 37, e24808. [Google Scholar] [CrossRef]
- Paliogiannis, P.; Ginesu, G.C.; Tanda, C.; Feo, C.F.; Fancellu, A.; Fois, A.G.; Mangoni, A.A.; Sotgia, S.; Carru, C.; Porcu, A.; et al. Inflammatory cell indexes as preoperative predictors of hospital stay in open elective thoracic surgery. ANZ J. Surg. 2018, 88, 616–620. [Google Scholar] [CrossRef]
- Pinna, A.; Porcu, T.; D’Amico-Ricci, G.; Dore, S.; Boscia, F.; Paliogiannis, P.; Carru, C.; Zinellu, A. Complete Blood Cell Count-Derived Inflammation Biomarkers in Men with Age-Related Macular Degeneration. Ocul. Immunol. Inflamm. 2019, 27, 932–936. [Google Scholar] [CrossRef]
- Zinellu, A.; Paliogiannis, P.; Sotgiu, E.; Mellino, S.; Mangoni, A.A.; Zinellu, E.; Negri, S.; Collu, C.; Pintus, G.; Serra, A.; et al. Blood Cell Count Derived Inflammation Indexes in Patients with Idiopathic Pulmonary Fibrosis. Lung 2020, 198, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Campesi, I.; Montella, A.; Sotgiu, G.; Dore, S.; Carru, C.; Zinellu, A.; Palermo, M.; Franconi, F. Combined oral contraceptives modify the effect of smoking on inflammatory cellular indexes and endothelial function in healthy subjects. Eur. J. Pharmacol. 2021, 891, 173762. [Google Scholar] [CrossRef] [PubMed]
- Zinellu, A.; Collu, C.; Nasser, M.; Paliogiannis, P.; Mellino, S.; Zinellu, E.; Traclet, J.; Ahmad, K.; Mangoni, A.A.; Carru, C.; et al. The Aggregate Index of Systemic Inflammation (AISI): A Novel Prognostic Biomarker in Idiopathic Pulmonary Fibrosis. J. Clin. Med. 2021, 10, 4134. [Google Scholar] [CrossRef] [PubMed]
- Ginesu, G.C.; Paliogiannis, P.; Feo, C.F.; Cossu, M.L.; Scanu, A.M.; Fancellu, A.; Fois, A.G.; Zinellu, A.; Perra, T.; Veneroni, S.; et al. Inflammatory Indexes as Predictive Biomarkers of Postoperative Complications in Oncological Thoracic Surgery. Curr. Oncol. 2022, 29, 3425–3432. [Google Scholar] [CrossRef]
- Fois, S.S.; Zinellu, E.; Zinellu, A.; Merella, M.; Pau, M.C.; Carru, C.; Fois, A.G.; Pirina, P. Comparison of Clinical Features, Complete Blood Count Parameters, and Outcomes between Two Distinct Waves of COVID-19: A Monocentric Report from Italy. Healthcare 2022, 10, 2427. [Google Scholar] [CrossRef]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K.; et al. Systematic reviews of etiology and risk. Joanna Briggs Inst. Rev. Man. 2017, 1–7. [Google Scholar]
- Balshem, H.; Helfand, M.; Schunemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Deeks, J.J.; Higgins, J.P.T.; Altman, D.G. Analysing data and undertaking meta-analyses. Cochrane Handb. Syst. Rev. Interv. 2022, 241–284. [Google Scholar]
- Tobias, A. Assessing the influence of a single study in the meta-analysis estimate. Stata Tech. Bull. 1999, 47, 15–17. [Google Scholar]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Egger, M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J. Clin. Epidemiol. 2001, 54, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Rutter, C.M.; Gatsonis, C.A. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat. Med. 2001, 20, 2865–2884. [Google Scholar] [CrossRef]
- Reitsma, J.B.; Glas, A.S.; Rutjes, A.W.; Scholten, R.J.; Bossuyt, P.M.; Zwinderman, A.H. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J. Clin. Epidemiol. 2005, 58, 982–990. [Google Scholar] [CrossRef]
- Harbord, R.M.; Whiting, P. Metandi: Meta-analysis of diagnostic accuracy using hierarchical logistic regression. Stata J. 2009, 9, 211–229. [Google Scholar] [CrossRef] [Green Version]
- Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2009, 36, 1–48. [Google Scholar]
- Deeks, J.J.; Macaskill, P.; Irwig, L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J. Clin. Epidemiol. 2005, 58, 882–893. [Google Scholar] [CrossRef]
- Fagan, T.J. Letter: Nomogram for Bayes theorem. N. Engl. J. Med. 1975, 293, 257. [Google Scholar] [CrossRef]
- Fois, A.G.; Paliogiannis, P.; Scano, V.; Cau, S.; Babudieri, S.; Perra, R.; Ruzzittu, G.; Zinellu, E.; Pirina, P.; Carru, C.; et al. The Systemic Inflammation Index on Admission Predicts In-Hospital Mortality in COVID-19 Patients. Molecules 2020, 25, 5725. [Google Scholar] [CrossRef]
- Zinellu, A.; Scano, V.; Masotto, E.; De Riu, G.; Vaira, L.A.; Carru, C.; Pirina, P.; Babudieri, S.; Mangoni, A.A.; Fois, A.G. The Systemic Inflammation Index on admission is independently associated with length of stay in hospitalized COVID-19 patients. Minerva Respir. Med. 2021, 60, 65–72. [Google Scholar] [CrossRef]
- Arbanasi, E.M.; Halmaciu, I.; Kaller, R.; Muresan, A.V.; Arbanasi, E.M.; Suciu, B.A.; Cosarca, C.M.; Cojocaru, I.I.; Melinte, R.M.; Russu, E. Systemic Inflammatory Biomarkers and Chest CT Findings as Predictors of Acute Limb Ischemia Risk, Intensive Care Unit Admission, and Mortality in COVID-19 Patients. Diagnostics 2022, 12, 2379. [Google Scholar] [CrossRef] [PubMed]
- Ghobadi, H.; Mohammadshahi, J.; Javaheri, N.; Fouladi, N.; Mirzazadeh, Y.; Aslani, M.R. Role of leukocytes and systemic inflammation indexes (NLR, PLR, MLP, dNLR, NLPR, AISI, SIR-I, and SII) on admission predicts in-hospital mortality in non-elderly and elderly COVID-19 patients. Front. Med. 2022, 9, 916453. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Perez, I.A.; Buendia-Roldan, I.; Perez-Rubio, G.; Chavez-Galan, L.; Hernandez-Zenteno, R.J.; Aguilar-Duran, H.; Fricke-Galindo, I.; Zaragoza-Garcia, O.; Falfan-Valencia, R.; Guzman-Guzman, I.P. Outcome predictors in COVID-19: An analysis of emergent systemic inflammation indices in Mexican population. Front. Med. 2022, 9, 1000147. [Google Scholar] [CrossRef]
- Halmaciu, I.; Arbanasi, E.M.; Kaller, R.; Muresan, A.V.; Arbanasi, E.M.; Bacalbasa, N.; Suciu, B.A.; Cojocaru, I.I.; Runcan, A.I.; Grosu, F.; et al. Chest CT Severity Score and Systemic Inflammatory Biomarkers as Predictors of the Need for Invasive Mechanical Ventilation and of COVID-19 Patients’ Mortality. Diagnostics 2022, 12, 2089. [Google Scholar] [CrossRef] [PubMed]
- Hamad, D.A.; Aly, M.M.; Abdelhameid, M.A.; Ahmed, S.A.; Shaltout, A.S.; Abdel-Moniem, A.E.; Ragheb, A.M.R.; Attia, M.N.; Meshref, T.S. Combined Blood Indexes of Systemic Inflammation as a Mirror to Admission to Intensive Care Unit in COVID-19 Patients: A Multicentric Study. J. Epidemiol. Glob. Health 2022, 12, 64–73. [Google Scholar] [CrossRef]
- Muresan, A.V.; Halmaciu, I.; Arbanasi, E.M.; Kaller, R.; Arbanasi, E.M.; Budisca, O.A.; Melinte, R.M.; Vunvulea, V.; Filep, R.C.; Marginean, L.; et al. Prognostic Nutritional Index, Controlling Nutritional Status (CONUT) Score, and Inflammatory Biomarkers as Predictors of Deep Vein Thrombosis, Acute Pulmonary Embolism, and Mortality in COVID-19 Patients. Diagnostics 2022, 12, 2757. [Google Scholar] [CrossRef]
- Ercan, Z.; Evren Oztop, K.; Pinar, M.; Varim, C.; Dheir, H.; Karacaer, C.; Yaylaci, S.; Bilal Genc, A.; Cekic, D.; Nalbant, A.; et al. The aggregate index of systemic inflammation may predict mortality in COVID-19 patients with chronic renal failure. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 3747–3752. [Google Scholar] [CrossRef]
- Haryati, H.; Wicaksono, B.; Syahadatina, M. Complete blood count derived inflammation indexes predict outcome in COVID-19 patients: A study in Indonesia. J. Infect. Dev. Ctries 2023, 17, 319–326. [Google Scholar] [CrossRef]
- Hosseninia, S.; Ghobadi, H.; Garjani, K.; Hosseini, S.A.H.; Aslani, M.R. Aggregate index of systemic inflammation (AISI) in admission as a reliable predictor of mortality in COPD patients with COVID-19. BMC Pulm. Med. 2023, 23, 107. [Google Scholar] [CrossRef]
- Khadzhieva, M.B.; Gracheva, A.S.; Belopolskaya, O.B.; Chursinova, Y.V.; Redkin, I.V.; Pisarev, M.V.; Kuzovlev, A.N. Serial Changes in Blood-Cell-Count-Derived and CRP-Derived Inflammatory Indices of COVID-19 Patients. Diagnostics 2023, 13, 746. [Google Scholar] [CrossRef] [PubMed]
- Caraguel, C.G.; Vanderstichel, R. The two-step Fagan’s nomogram: Ad hoc interpretation of a diagnostic test result without calculation. Evid. Based Med. 2013, 18, 125–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiu, J.; Lin, X.; Chen, Q.; Yu, P.; Lu, J.; Yang, Y.; Chen, W.; Bao, K.; Wang, J.; Zhu, J.; et al. The aggregate index of systemic inflammation (AISI): A novel predictor for hypertension. Front. Cardiovasc. Med. 2023, 10, 1163900. [Google Scholar] [CrossRef] [PubMed]






| Non-Severe Disease or Survivor (NSDS) | Severe Disease or Non-Survivor (SDNS) | Outcome | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | n | Age (Years) | M/F | AISI (Mean ± SD) | n | Age (Years) | M/F | AISI (Mean ± SD) | |
| Fois AG et al., 2020, Italy [40] | 90 | 68 | 56/34 | 472 ± 559 | 29 | 80 | 21/8 | 1032 ± 1290 | Mortality |
| Zinellu A et al., 2021, Italy [41] | 43 | 66 | 27/16 | 413 ± 444 | 22 | 69 | 16/6 | 937 ± 1367 | Length of stay |
| Arbanasi EM et al. (a) 2022, Romania [42] | 461 | 70 | 284/177 | 610 ± 573 | 49 | 74 | 21/28 | 3027 ± 2361 | ALI |
| Fois SS et al. (a), 2022, Italy [25] | 140 | 68 | 91/49 | 694 ± 782 | 42 | 82 | 32/10 | 1401 ± 1509 | Mortality |
| Fois SS et al. (b), 2022, Italy [25] | 120 | 73 | 66/54 | 644 ± 727 | 40 | 82 | 20/20 | 751 ± 817 | Mortality |
| Ghobadi H et al. (a) 2022, Iran [43] | 947 | 48 | 548/399 | 192 ± 177 | 135 | 54 | 88/47 | 311 ± 344 | Mortality |
| Ghobadi H et al. (b) 2022, Iran [43] | 492 | 76 | 238/254 | 252 ± 225 | 218 | 78 | 114/104 | 328 ± 324 | Mortality |
| Gutiérrez-Pérez IA et al. (a) 2022, Mexico [44] | 352 | NR | NR | 1957 ± 3232 | 196 | NR | NR | 4002 ± 7085 | IMV |
| Gutiérrez-Pérez IA et al. (b) 2022, Mexico [44] | 491 | NR | NR | 3367 ± 5964 | 316 | NR | NR | 4489 ± 8119 | Mortality |
| Hamad DA et al., 2022, Egypt [46] | 185 | 33 | 91/94 | 256 ± 505 | 310 | 58 | 181/129 | 1319 ± 1949 | ICU admission |
| Muresan AV et al. (a), 2022 Romania [47] | 746 | 70 | 397/349 | 1087 ± 1299 | 143 | 72 | 77/66 | 3476 ± 3237 | Mortality |
| Ercan Z et al., 2023, Turkey [48] | 42 | 74 | 23/19 | 986 ± 1646 | 43 | 73 | 16/27 | 1675 ± 1639 | Mortality |
| Hosseninia S et al., 2023, Romania [50] | 274 | 68 | 70/53 | 316 ± 299 | 26 | 73 | 28/18 | 436 ± 424 | Mortality |
| Khadzhieva MB et al., 2023, Russia [51] | 138 | 57 | 73/65 | 416 ± 513 | 31 | 62 | 18/13 | 652 ± 921 | Mortality |
| Study | Design | n | Age (Years) | M/F | OR | 95% CI | Outcome |
|---|---|---|---|---|---|---|---|
| Arbanasi EM et al. (a) 2022, Romania [42] | R | 510 | 70 | 305/205 | 14.82 | 7.14–30.77 | Acute limb ischemia |
| Arbanasi EM et al. (b) 2022, Romania [42] | R | 510 | 70 | 305/205 | 4.65 | 3.16–6.85 | Admission to intensive care unit |
| Gutiérrez-Pérez IA et al. (a) 2022, Mexico [44] | R | 548 | NR | 352/196 | 2.64 | 1.79–3.89 | Invasive mechanical ventilation |
| Gutiérrez-Pérez IA et al. (b) 2022, Mexico [44] | R | 807 | NR | 491/316 | 1.2 | 0.87–1.65 | Mortality |
| Halmaciu I et al., 2022, Romania [45] | R | 267 | 71 | 159/108 | 15.82 | 6.86–36.65 | Invasive mechanical ventilation |
| Hamad DA et al., 2022, Egypt [46] | R | 485 | 49 | 272/213 | 1.003 | 1.001–1.005 | Admission to intensive care unit |
| Muresan AV et al. (a) 2022, Romania [47] | R | 889 | 71 | 474/415 | 6.27 | 4.21–9.34 | Mortality |
| Muresan AV et al. (b) 2022, Romania [47] | R | 889 | 71 | 474/415 | 6.24 | 4.38–8.89 | Deep vein thrombosis |
| Muresan AV et al. (c) 2022, Romania [47] | R | 889 | 71 | 474/415 | 5.98 | 3.49–10.24 | Acute pulmonary embolism |
| Study | Design | n | Age (Years) | M/F | HR | 95% CI | Outcome |
| Fois AG et al., 2020, Italy [40] | R | 119 | 72 | 77/42 | 1 | 1–1.0001 | Mortality |
| Ercan Z et al., 2023, Turkey [48] | R | 85 | 72 | 39/46 | 1.001 | 1–1.001 | Mortality |
| Hosseninia S et al., 2023, Iran [50] | R | 300 | 69 | 98/71 | 2.01 | 1.048–3.855 | Mortality |
| Study | Design | n | AUC (95% CI) | Cut-Off | Sensitivity (%) | Specificity (%) | Outcome |
|---|---|---|---|---|---|---|---|
| Fois AG et al., 2020, Italy [40] | R | 119 | 0.640 | 798 | 0.59 | 0.72 | Mortality |
| 0.546–0.726 | |||||||
| Zinellu A et al., 2021, Italy [41] | R | 65 | 0.674 | 1153 | 0.4 | 0.93 | Length of stay |
| 0.547–0.786 | |||||||
| Arbanasi EM et al. (a) 2022, Romania [42] | R | 510 | 0.851 | 1296.62 | 0.8 | 0.79 | Acute limb ischemia |
| 0.789–0.913 | |||||||
| Arbanasi EM et al. (b) 2022, Romania [42] | R | 510 | 0.738 | 650.58 | 0.679 | 0.687 | Admission to intensive care unit |
| 0.692–0.783 | |||||||
| Fois SS et al. (a) 2022, Italy [25] | R | 182 | 0.645 | 1282 | 0.51 | 0.77 | Mortality |
| 0.570–0.715 | |||||||
| Ghobadi H et al. (a) 2022, Iran [43] | R | 1082 | 0.871 | 492 | 0.693 | 0.865 | Mortality |
| 0.849–0.890 | |||||||
| Ghobadi H et al. (b) 2022, Iran [43] | R | 710 | 0.826 | 518 | 0.711 | 0.89 | Mortality |
| 0.796–0.853 | |||||||
| Halmaciu I et al., 2022, Romania [45] | R | 267 | 0.813 | 994.76 | 0.883 | 0.676 | Invasive mechanical ventilation |
| 0.754–0.871 | |||||||
| Hamad DA et al., 2022, Egypt [46] | R | 485 | 0.807 | 729 | 0.517 | 0.919 | Admission to intensive care unit |
| 0.767–0.846 | |||||||
| Muresan AV et al. (a) 2022, Romania [47] | R | 889 | 0.780 | 1696.18 | 0.72 | 0.709 | Mortality |
| 0.740–0.821 | |||||||
| Muresan AV et al. (b) 2022, Romania [47] | R | 889 | 0.784 | 1605.4 | 0.712 | 0.716 | Deep vein thrombosis |
| 0.745–0.823 | |||||||
| Muresan AV et al. (c) 2022, Romania [47] | R | 889 | 0.750 | 2769.85 | 0.613 | 0.791 | Acute pulmonary oedema |
| 0.684–0.816 | |||||||
| Ercan Z et al., 2023, Turkey [48] | R | 85 | 0.820 | 621.1 | 0.81 | 0.691 | Mortality |
| 0.733–0.903 | |||||||
| Haryati H et al., 2023, Indonesia [49] | R | 445 | 0.558 | 1422 | 0.32 | 0.788 | Mortality |
| 0.510–0.614 | |||||||
| Hosseninia S et al., 2023, Iran [50] | R | 300 | 0.630 | 260 | 0.696 | 0.61 | Mortality |
| 0.552–0.703 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zinellu, A.; Paliogiannis, P.; Mangoni, A.A. Aggregate Index of Systemic Inflammation (AISI), Disease Severity, and Mortality in COVID-19: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 4584. https://doi.org/10.3390/jcm12144584
Zinellu A, Paliogiannis P, Mangoni AA. Aggregate Index of Systemic Inflammation (AISI), Disease Severity, and Mortality in COVID-19: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2023; 12(14):4584. https://doi.org/10.3390/jcm12144584
Chicago/Turabian StyleZinellu, Angelo, Panagiotis Paliogiannis, and Arduino A. Mangoni. 2023. "Aggregate Index of Systemic Inflammation (AISI), Disease Severity, and Mortality in COVID-19: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 12, no. 14: 4584. https://doi.org/10.3390/jcm12144584








