Memory Deficits in Parkinson’s Disease Are Associated with Impaired Attentional Filtering and Memory Consolidation Processes
Abstract
:1. Introduction
1.1. Working Memory Deficits in PD
1.2. Episodic Memory Deficits in PD
1.3. Introduction to Current Hypotheses
2. Materials and Methods
2.1. Participants
2.2. Sample Size Justification
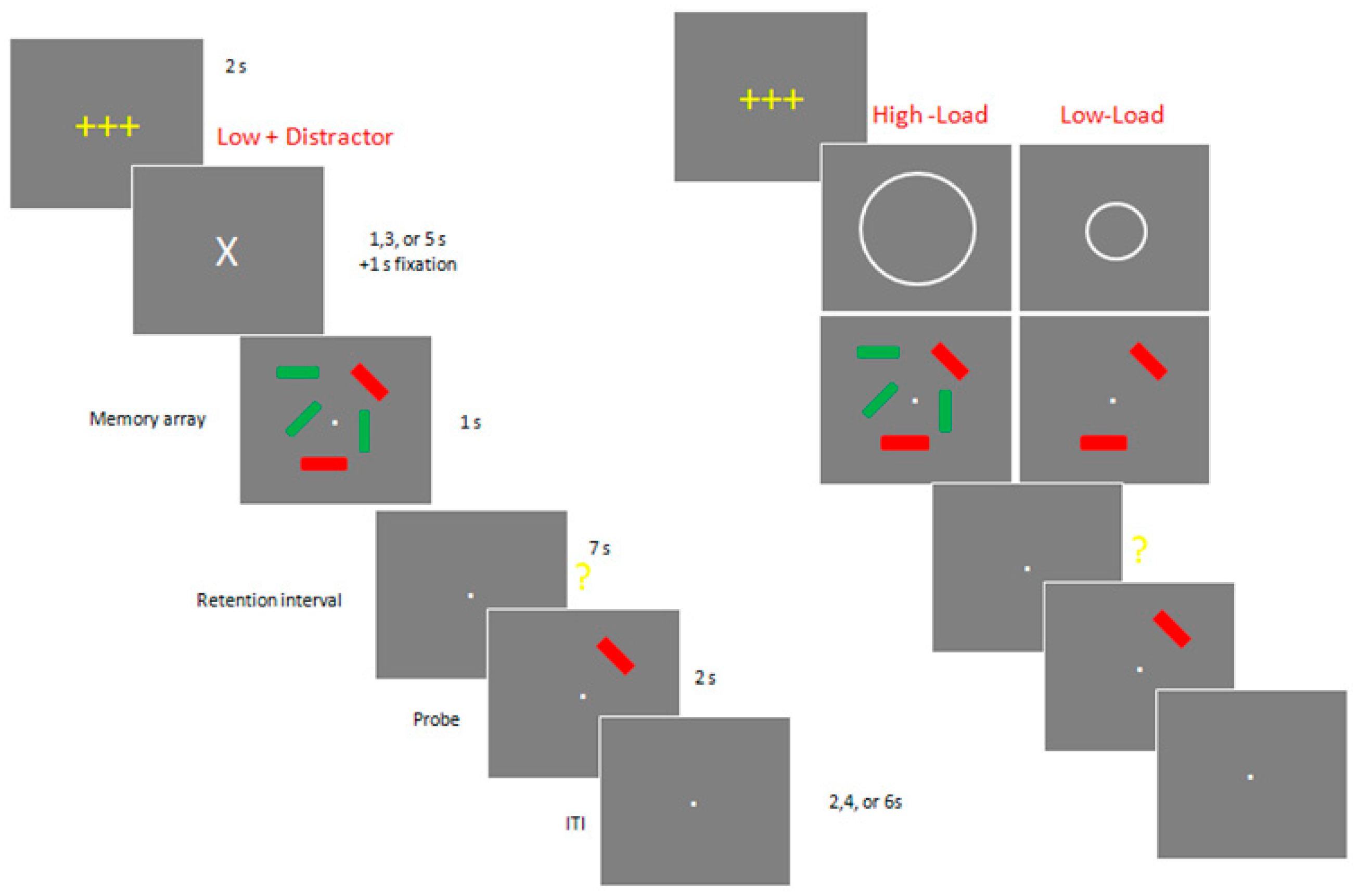
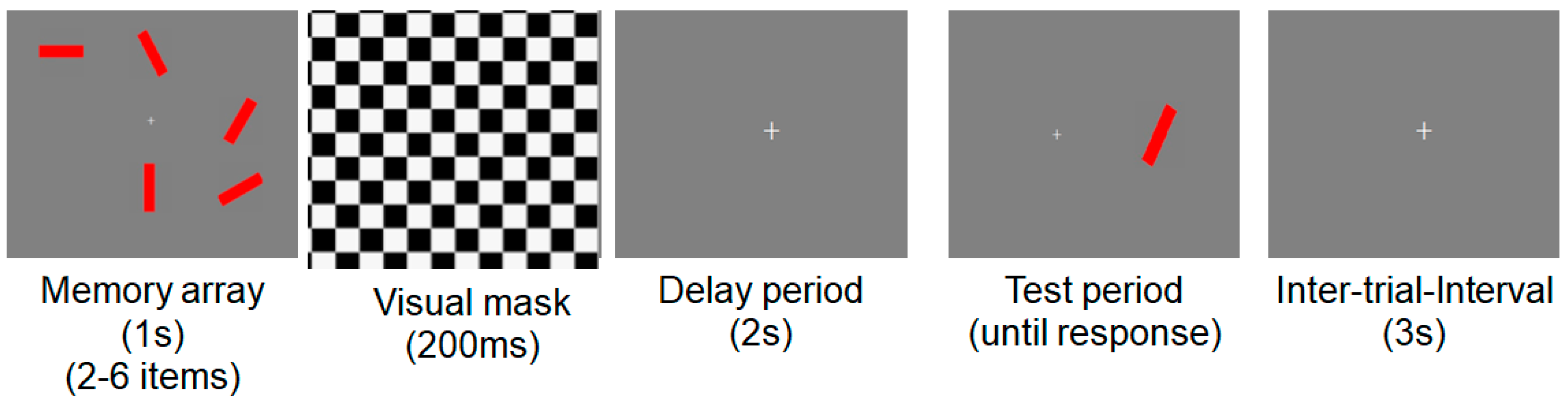
2.3. Stimuli and Procedures for Neuropsychological Experiments
2.4. MRI Image Acquisition and Image Processing
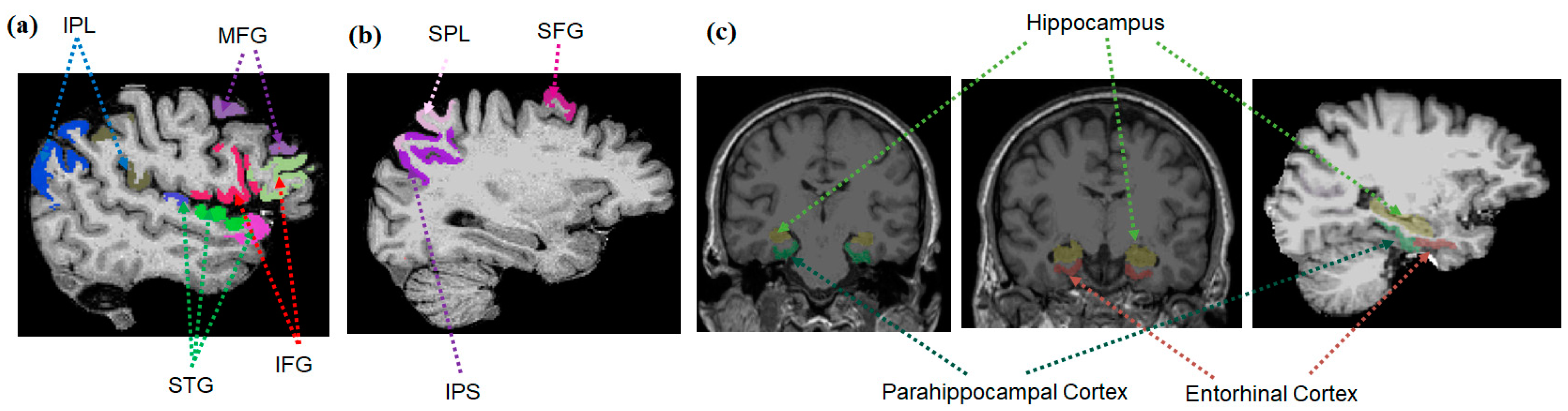
2.4.1. Brain Regions of Interest
2.4.2. Hippocampal Volumes and Cortical Thickness
2.5. Statistical Analysis
3. Results
3.1. Demographics
3.2. Group Comparison of Memory Metrics
3.3. Group Comparison of MRI Structural Metrics
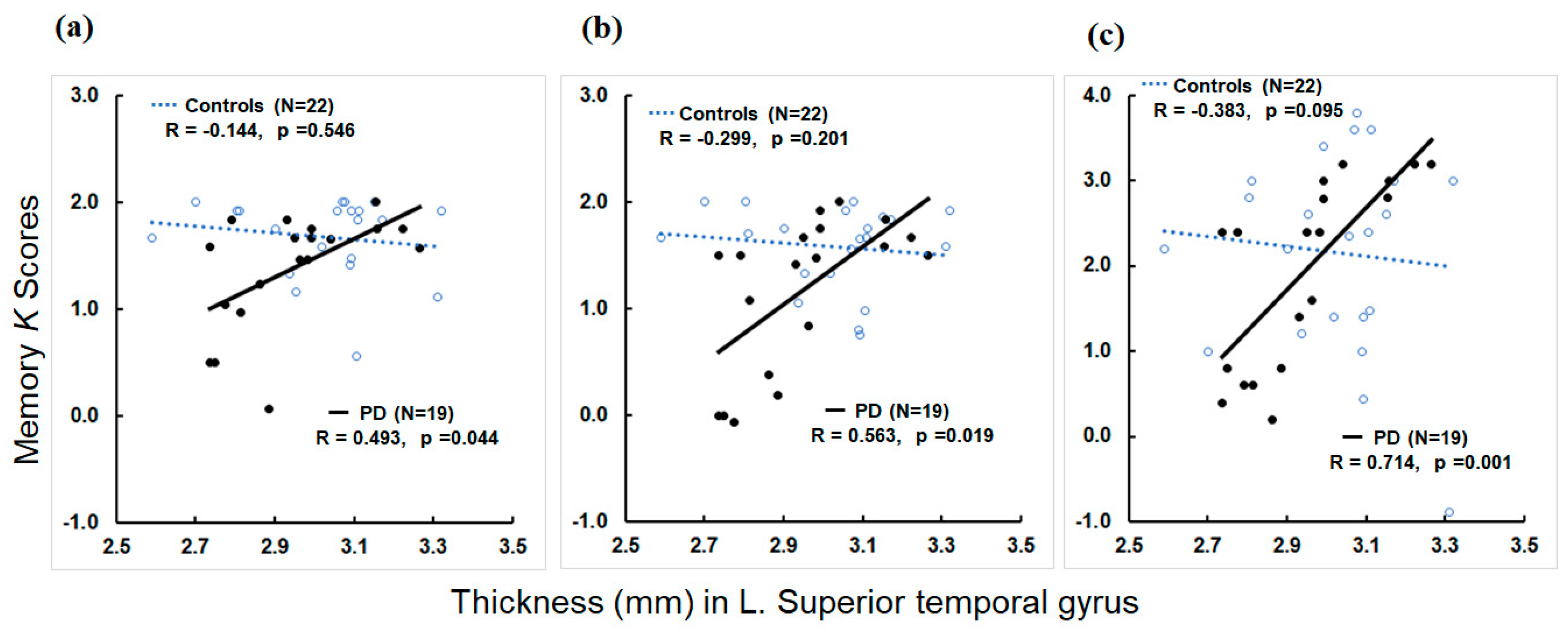
3.4. Associations of MRI Structural Metrics with Memory Metrics
3.5. Stepwise Regression Analysis to Determine Factors Predicting Memory Metrics
4. Discussion
4.1. Memory Deficits in PD Due to Impaired Memory Consolidation Process
4.2. Memory Deficits in PD Due to Impaired Attentional Filtering
4.3. Neural Correlates of Memory Deficits in PD
5. Limitations and Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gabrieli, J.D.; Singh, J.; Stebbins, G.T.; Goetz, C.G. Reduced working memory span in Parkinson’s disease: Evidence for the role of frontostriatal system in working and strategic memory. Neuropsychology 1996, 10, 322. [Google Scholar] [CrossRef]
- Zgaljardic, D.J.; Borod, J.C.; Foldi, N.S.; Mattis, P. A review of the cognitive and behavioral sequelae of Parkinson’s disease: Relationship to frontostriatal circuitry. Cogn. Behav. Neurol. 2003, 16, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Owen, A.M. Cognitive dysfunction in Parkinson’s disease: The role of frontostriatal circuitry. Neuroscientist 2004, 10, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yu, C.; Zhang, N.; Liu, J.; Liu, W. Cognitive impairment in patients with Parkinson’s disease: A 30-month follow-up study. Clin. Neurol. Neurosurg. 2016, 151, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.J.; Dove, A.; Robbins, T.W.; Barker, R.A.; Owen, A.M. Cognitive impairments in early Parkinson’s disease are accompanied by reductions in activity in frontostriatal neural circuitry. J. Neurosci. 2003, 23, 6351–6356. [Google Scholar] [CrossRef]
- Cacciola, A.; Calamuneri, A.; Milardi, D.; Mormina, E.; Chillemi, G.; Marino, S.; Naro, A.; Rizzo, G.; Anastasi, G.; Quartarone, A. A connectomic analysis of the human basal ganglia network. Front. Neuroanat. 2017, 11, 85. [Google Scholar] [CrossRef]
- Morris, R.G.; Downes, J.J.; Sahakian, B.J.; Evenden, J.L.; Heald, A.; Robbins, T.W. Planning and spatial working memory in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1988, 51, 757–766. [Google Scholar] [CrossRef]
- Halliday, G.M.; Leverenz, J.B.; Schneider, J.S.; Adler, C.H. The neurobiological basis of cognitive impairment in Parkinson’s disease. Mov. Disord. 2014, 29, 634–650. [Google Scholar] [CrossRef]
- Biundo, R.; Weis, L.; Antonini, A. Cognitive decline in Parkinson’s disease: The complex picture. NPJ Park. Dis. 2016, 2, 1–7. [Google Scholar] [CrossRef]
- Siquier, A.; Andrés, P. Episodic memory impairment in Parkinson’s disease: Disentangling the role of encoding and retrieval. J. Int. Neuropsychol. Soc. 2021, 27, 261–269. [Google Scholar] [CrossRef]
- Das, T.; Hwang, J.J.; Poston, K.L. Episodic recognition memory and the hippocampus in Parkinson’s disease: A review. Cortex 2019, 113, 191–209. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.T. Measures of short-term memory: A historical review. Cortex 2007, 43, 635–650. [Google Scholar] [CrossRef] [PubMed]
- Miller, G. Human memory and the storage of information. IRE Trans. Inf. Theory 1956, 2, 129–137. [Google Scholar] [CrossRef]
- Baddeley, A.D.; Hitch, G. Working memory. In Psychology of Learning and Motivation; Elsevier: Amsterdam, The Netherlands, 1974; Volume 8, pp. 47–89. [Google Scholar]
- Baddeley, A.D.; Hitch, G.J. Development of working memory: Should the Pascual-Leone and the Baddeley and Hitch models be merged? J. Exp. Child Psychol. 2000, 77, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Baddeley, A.; Hitch, G.; Allen, R. A multicomponent model of working memory. In Working Memory: The State of the Science; Oxford Academic: Oxford, UK, 2021; pp. 10–43. [Google Scholar] [CrossRef]
- D’Esposito, M.; Postle, B.R. The cognitive neuroscience of working memory. Annu. Rev. Psychol. 2015, 66, 115–142. [Google Scholar] [CrossRef]
- Cowan, N.; Elliott, E.M.; Saults, J.S.; Morey, C.C.; Mattox, S.; Hismjatullina, A.; Conway, A.R. On the capacity of attention: Its estimation and its role in working memory and cognitive aptitudes. Cogn. Psychol. 2005, 51, 42–100. [Google Scholar] [CrossRef]
- Baddeley, A. Working memory: Looking back and looking forward. Nat. Rev. Neurosci. 2003, 4, 829–839. [Google Scholar] [CrossRef]
- Eriksson, J.; Vogel, E.K.; Lansner, A.; Bergström, F.; Nyberg, L. Neurocognitive architecture of working memory. Neuron 2015, 88, 33–46. [Google Scholar] [CrossRef]
- Feldmann-Wüstefeld, T.; Vogel, E.K. Neural evidence for the contribution of active suppression during working memory filtering. Cereb. Cortex 2019, 29, 529–543. [Google Scholar] [CrossRef]
- Nani, A.; Manuello, J.; Mancuso, L.; Liloia, D.; Costa, T.; Cauda, F. The neural correlates of consciousness and attention: Two sister processes of the brain. Front. Neurosci. 2019, 13, 1169. [Google Scholar] [CrossRef]
- Rottschy, C.; Langner, R.; Dogan, I.; Reetz, K.; Laird, A.R.; Schulz, J.B.; Fox, P.T.; Eickhoff, S.B. Modelling neural correlates of working memory: A coordinate-based meta-analysis. Neuroimage 2012, 60, 830–846. [Google Scholar] [CrossRef] [PubMed]
- Christophel, T.B.; Klink, P.C.; Spitzer, B.; Roelfsema, P.R.; Haynes, J.-D. The distributed nature of working memory. Trends Cogn. Sci. 2017, 21, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Haber, S.N. Corticostriatal circuitry. Dialogues Clin. Neurosci. 2022, 18, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.A.; Machado, L. A comprehensive meta-analysis on short-term and working memory dysfunction in Parkinson’s disease. Neuropsychol. Rev. 2021, 31, 288–311. [Google Scholar] [CrossRef]
- Siegert, R.J.; Weatherall, M.; Taylor, K.D.; Abernethy, D.A. A meta-analysis of performance on simple span and more complex working memory tasks in Parkinson’s disease. Neuropsychology 2008, 22, 450. [Google Scholar] [CrossRef]
- Lee, E.-Y.; Cowan, N.; Vogel, E.K.; Rolan, T.; Valle-Inclan, F.; Hackley, S.A. Visual working memory deficits in patients with Parkinson’s disease are due to both reduced storage capacity and impaired ability to filter out irrelevant information. Brain J. Neurol. 2010, 133, 2677–2689. [Google Scholar] [CrossRef]
- Kensinger, E.A.; Shearer, D.K.; Locascio, J.J.; Growdon, J.H.; Corkin, S. Working memory in mild Alzheimer’s disease and early Parkinson’s disease. Neuropsychology 2003, 17, 230. [Google Scholar] [CrossRef]
- Salmi, J.; Ritakallio, L.; Fellman, D.; Ellfolk, U.; Rinne, J.O.; Laine, M. Disentangling the role of working memory in Parkinson’s disease. Front. Aging Neurosci. 2020, 12, 572037. [Google Scholar] [CrossRef]
- Zhao, G.; Chen, F.; Zhang, Q.; Shen, M.; Gao, Z. Feature-based information filtering in visual working memory is impaired in Parkinson’s disease. Neuropsychologia 2018, 111, 317–323. [Google Scholar] [CrossRef]
- Tulving, E. Precis of elements of episodic memory. Behav. Brain Sci. 1984, 7, 223–238. [Google Scholar] [CrossRef]
- Sugar, J.; Moser, M.B. Episodic memory: Neuronal codes for what, where, and when. Hippocampus 2019, 29, 1190–1205. [Google Scholar] [CrossRef] [PubMed]
- Eichenbaum, H.; Lipton, P.A. Towards a functional organization of the medial temporal lobe memory system: Role of the parahippocampal and medial entorhinal cortical areas. Hippocampus 2008, 18, 1314–1324. [Google Scholar] [CrossRef] [PubMed]
- Umbach, G.; Kantak, P.; Jacobs, J.; Kahana, M.; Pfeiffer, B.E.; Sperling, M.; Lega, B. Time cells in the human hippocampus and entorhinal cortex support episodic memory. Proc. Natl. Acad. Sci. USA 2020, 117, 28463–28474. [Google Scholar] [CrossRef] [PubMed]
- Bäckman, L.; Small, B.J.; Fratiglioni, L. Stability of the preclinical episodic memory deficit in Alzheimer’s disease. Brain J. Neurol. 2001, 124, 96–102. [Google Scholar] [CrossRef]
- Rajan, K.B.; Weuve, J.; Barnes, L.L.; McAninch, E.A.; Wilson, R.S.; Evans, D.A. Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020–2060). Alzheimer’s Dement. 2021, 17, 1966–1975. [Google Scholar] [CrossRef]
- Li, H.; Habes, M.; Wolk, D.A.; Fan, Y.; Initiative, A.S.D.N. A deep learning model for early prediction of Alzheimer’s disease dementia based on hippocampal magnetic resonance imaging data. Alzheimer’s Dement. 2019, 15, 1059–1070. [Google Scholar] [CrossRef]
- Grueso, S.; Viejo-Sobera, R. Machine learning methods for predicting progression from mild cognitive impairment to Alzheimer’s disease dementia: A systematic review. Alzheimer’s Res. Ther. 2021, 13, 1–29. [Google Scholar] [CrossRef]
- El-Sappagh, S.; Abuhmed, T.; Islam, S.R.; Kwak, K.S. Multimodal multitask deep learning model for Alzheimer’s disease progression detection based on time series data. Neurocomputing 2020, 412, 197–215. [Google Scholar] [CrossRef]
- Weintraub, D.; Simuni, T.; Caspell-Garcia, C.; Coffey, C.; Lasch, S.; Siderowf, A.; Aarsland, D.; Barone, P.; Burn, D.; Chahine, L.M. Cognitive performance and neuropsychiatric symptoms in early, untreated Parkinson’s disease. Mov. Disord. 2015, 30, 919–927. [Google Scholar] [CrossRef]
- Yarnall, A.J.; Breen, D.P.; Duncan, G.W.; Khoo, T.K.; Coleman, S.Y.; Firbank, M.J.; Nombela, C.; Winder-Rhodes, S.; Evans, J.R.; Rowe, J.B. Characterizing mild cognitive impairment in incident Parkinson disease: The ICICLE-PD study. Neurology 2014, 82, 308–316. [Google Scholar] [CrossRef]
- Wallace, E.R.; Segerstrom, S.C.; van Horne, C.G.; Schmitt, F.A.; Koehl, L.M. Meta-analysis of cognition in Parkinson’s disease mild cognitive impairment and dementia progression. Neuropsychol. Rev. 2021, 32, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Breen, K.C.; Drutyte, G. Non-motor symptoms of Parkinson’s disease: The patient’s perspective. J. Neural Transm. 2013, 120, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Baiano, C.; Barone, P.; Trojano, L.; Santangelo, G. Prevalence and clinical aspects of mild cognitive impairment in Parkinson’s disease: A meta-analysis. Mov. Disord. 2020, 35, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Brønnick, K.; Alves, G.; Aarsland, D.; Tysnes, O.-B.; Larsen, J.P. Verbal memory in drug-naive, newly diagnosed Parkinson’s disease. The retrieval deficit hypothesis revisited. Neuropsychology 2011, 25, 114. [Google Scholar] [CrossRef]
- Scimeca, J.M.; Badre, D. Striatal contributions to declarative memory retrieval. Neuron 2012, 75, 380–392. [Google Scholar] [CrossRef]
- Bezdicek, O.; Ballarini, T.; Buschke, H.; Růžička, F.; Roth, J.; Albrecht, F.; Růžička, E.; Mueller, K.; Schroeter, M.L.; Jech, R. Memory impairment in Parkinson’s disease: The retrieval versus associative deficit hypothesis revisited and reconciled. Neuropsychology 2019, 33, 391. [Google Scholar] [CrossRef]
- Chiaravalloti, N.D.; Ibarretxe-Bilbao, N.; DeLuca, J.; Rusu, O.; Pena, J.; García-Gorostiaga, I.; Ojeda, N. The source of the memory impairment in Parkinson’s disease: Acquisition versus retrieval. Mov. Disord. 2014, 29, 765–771. [Google Scholar] [CrossRef]
- Yesavage, J.A.; Sheikh, J.I. 9/Geriatric depression scale (GDS) recent evidence and development of a shorter version. Clin. Gerontol. 1986, 5, 165–173. [Google Scholar] [CrossRef]
- Rabey, J.; Korczyn, A. The Hoehn and Yahr rating scale for Parkinson’s disease. In Instrumental Methods and Scoring in Extrapyramidal Disorders; Springer: Berlin/Heidelberg, Germany, 1995; pp. 7–17. [Google Scholar]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical power analyses using G* Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Ségonne, F.; Dale, A.M.; Busa, E.; Glessner, M.; Salat, D.; Hahn, H.K.; Fischl, B. A hybrid approach to the skull stripping problem in MRI. Neuroimage 2004, 22, 1060–1075. [Google Scholar] [CrossRef]
- Fischl, B.; Salat, D.H.; Busa, E.; Albert, M.; Dieterich, M.; Haselgrove, C.; Van Der Kouwe, A.; Killiany, R.; Kennedy, D.; Klaveness, S. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron 2002, 33, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Fischl, B.; Salat, D.H.; Van Der Kouwe, A.J.; Makris, N.; Ségonne, F.; Quinn, B.T.; Dale, A.M. Sequence-independent segmentation of magnetic resonance images. Neuroimage 2004, 23, S69–S84. [Google Scholar] [CrossRef] [PubMed]
- Cowan, N. The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behav. Brain Sci. 2001, 24, 87–114. [Google Scholar] [CrossRef] [PubMed]
- Holm, S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar]
- Squire, L.R.; Genzel, L.; Wixted, J.T.; Morris, R.G. Memory consolidation. Cold Spring Harb. Perspect. Biol. 2015, 7, a021766. [Google Scholar] [CrossRef]
- Hanoğlu, L.; Ercan, F.B.; Mantar, N.; Yılmaz, N.H.; Sitrava, S.; Özer, F.; Yuluğ, B. Accelerated forgetting and verbal memory consolidation process in idiopathic nondement Parkinson’s disease. J. Clin. Neurosci. 2019, 70, 208–213. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Maruff, P.; Pietrzak, R.H.; Ames, D.; Ellis, K.A.; Harrington, K.; Lautenschlager, N.T.; Szoeke, C.; Martins, R.N.; Masters, C.L. Effect of amyloid on memory and non-memory decline from preclinical to clinical Alzheimer’s disease. Brain J. Neurol. 2014, 137, 221–231. [Google Scholar] [CrossRef]
- McNab, F.; Klingberg, T. Prefrontal cortex and basal ganglia control access to working memory. Nat. Neurosci. 2008, 11, 103–107. [Google Scholar] [CrossRef]
- Ott, T.; Nieder, A. Dopamine and cognitive control in prefrontal cortex. Trends Cogn. Sci. 2019, 23, 213–234. [Google Scholar] [CrossRef]
- Praamstra, P.; Plat, F. Failed suppression of direct visuomotor activation in Parkinson’s disease. J. Cogn. Neurosci. 2001, 13, 31–43. [Google Scholar] [CrossRef]
- Gatto, E.M.; Aldinio, V. Impulse control disorders in Parkinson’s disease. A brief and comprehensive review. Front. Neurol. 2019, 10, 351. [Google Scholar] [CrossRef] [PubMed]
- Siquier, A.; Andrés, P. Cognitive and behavioral inhibition deficits in Parkinson’s disease: The hayling test as a reliable marker. Front. Aging Neurosci. 2021, 12, 621603. [Google Scholar] [CrossRef] [PubMed]
- Walton, L.; Domellöf, M.E.; Boraxbekk, C.-J.; Domellöf, E.; Rönnqvist, L.; Bäckström, D.; Forsgren, L.; Stigsdotter Neely, A. The Effects of Working Memory Updating Training in Parkinson’s Disease: A Feasibility and Single-Subject Study on Cognition, Movement and Functional Brain Response. Front. Psychol. 2021, 11, 587925. [Google Scholar] [CrossRef] [PubMed]
- Giguère-Rancourt, A.; Plourde, M.; Racine, E.; Couture, M.; Langlois, M.; Dupré, N.; Simard, M. Goal management training and psychoeducation/mindfulness for treatment of executive dysfunction in Parkinson’s disease: A feasibility pilot trial. PLoS ONE 2022, 17, e0263108. [Google Scholar] [CrossRef] [PubMed]
- Hemphill, L.; Valenzuela, Y.; Luna, K.; Szymkowicz, S.M.; Jones, J.D. Synergistic associations of depressive symptoms and aging on cognitive decline in early Parkinson’s disease. Clin. Park. Relat. Disord. 2023, 8, 100192. [Google Scholar] [CrossRef]
- Austgen, G.; Marsh, L. Cognitive dysfunction and neuropsychiatric aspects of Parkinson’s disease. Prog. Brain Res. 2022, 269, 59–90. [Google Scholar]
- Randver, R. Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex to alleviate depression and cognitive impairment associated with Parkinson’s disease: A review and clinical implications. J. Neurol. Sci. 2018, 393, 88–99. [Google Scholar] [CrossRef]
- Postle, B.R.; Ferrarelli, F.; Hamidi, M.; Feredoes, E.; Massimini, M.; Peterson, M.; Alexander, A.; Tononi, G. Repetitive transcranial magnetic stimulation dissociates working memory manipulation from retention functions in the prefrontal, but not posterior parietal, cortex. J. Cogn. Neurosci. 2006, 18, 1712–1722. [Google Scholar] [CrossRef]
- Velenosi, L.A.; Wu, Y.-H.; Schmidt, T.T.; Blankenburg, F. Intraparietal sulcus maintains working memory representations of somatosensory categories in an adaptive, context-dependent manner. NeuroImage 2020, 221, 117146. [Google Scholar] [CrossRef]
- Zacharopoulos, G.; Cohen, R.K. Predicting working memory capacity based on glutamatergic concentration and its modulation of functional connectivity. Neuroscience 2021, 457, 12–19. [Google Scholar] [CrossRef]
- Ezzyat, Y.; Wanda, P.A.; Levy, D.F.; Kadel, A.; Aka, A.; Pedisich, I.; Sperling, M.R.; Sharan, A.D.; Lega, B.C.; Burks, A. Closed-loop stimulation of temporal cortex rescues functional networks and improves memory. Nat. Commun. 2018, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Curot, J.; Busigny, T.; Valton, L.; Denuelle, M.; Vignal, J.-P.; Maillard, L.; Chauvel, P.; Pariente, J.; Trebuchon, A.; Bartolomei, F. Memory scrutinized through electrical brain stimulation: A review of 80 years of experiential phenomena. Neurosci. Biobehav. Rev. 2017, 78, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Suthana, N.; Haneef, Z.; Stern, J.; Mukamel, R.; Behnke, E.; Knowlton, B.; Fried, I. Memory enhancement and deep-brain stimulation of the entorhinal area. N. Engl. J. Med. 2012, 366, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.; Miller, J.; Lee, S.A.; Coffey, T.; Watrous, A.J.; Sperling, M.R.; Sharan, A.; Worrell, G.; Berry, B.; Lega, B. Direct electrical stimulation of the human entorhinal region and hippocampus impairs memory. Neuron 2016, 92, 983–990. [Google Scholar] [CrossRef]
- Das, A.; Menon, V. Concurrent-and after-effects of medial temporal lobe stimulation on directed information flow to and from prefrontal and parietal cortices during memory formation. J. Neurosci. 2023, 43, 3159–3175. [Google Scholar] [CrossRef]
- Mercier, M.R.; Dubarry, A.-S.; Tadel, F.; Avanzini, P.; Axmacher, N.; Cellier, D.; Del Vecchio, M.; Hamilton, L.S.; Hermes, D.; Kahana, M.J. Advances in human intracranial electroencephalography research, guidelines and good practices. Neuroimage 2022, 260, 119438. [Google Scholar] [CrossRef]
- Yang, Y.; Qiao, S.; Sani, O.G.; Sedillo, J.I.; Ferrentino, B.; Pesaran, B.; Shanechi, M.M. Modelling and prediction of the dynamic responses of large-scale brain networks during direct electrical stimulation. Nat. Biomed. Eng. 2021, 5, 324–345. [Google Scholar] [CrossRef]
- Leff, A.P.; Schofield, T.M.; Crinion, J.T.; Seghier, M.L.; Grogan, A.; Green, D.W.; Price, C.J. The left superior temporal gyrus is a shared substrate for auditory short-term memory and speech comprehension: Evidence from 210 patients with stroke. Brain J. Neurol. 2009, 132, 3401–3410. [Google Scholar] [CrossRef]
- Ekert, J.O.; Gajardo-Vidal, A.; Lorca-Puls, D.L.; Hope, T.M.; Dick, F.; Crinion, J.T.; Green, D.W.; Price, C.J. Dissociating the functions of three left posterior superior temporal regions that contribute to speech perception and production. NeuroImage 2021, 245, 118764. [Google Scholar] [CrossRef]
- Sharma, S.; Mandal, P.K. A comprehensive report on machine learning-based early detection of alzheimer’s disease using multi-modal neuroimaging data. ACM Comput. Surv. (CSUR) 2022, 55, 1–44. [Google Scholar] [CrossRef]
- Le Bihan, D.; Mangin, J.F.; Poupon, C.; Clark, C.A.; Pappata, S.; Molko, N.; Chabriat, H. Diffusion tensor imaging: Concepts and applications. J. Magn. Reson. Imaging 2001, 13, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Douaud, G.; Menke, R.A.; Gass, A.; Monsch, A.U.; Rao, A.; Whitcher, B.; Zamboni, G.; Matthews, P.M.; Sollberger, M.; Smith, S. Brain microstructure reveals early abnormalities more than two years prior to clinical progression from mild cognitive impairment to Alzheimer’s disease. J. Neurosci. 2013, 33, 2147–2155. [Google Scholar] [CrossRef] [PubMed]
- Fellgiebel, A.; Dellani, P.R.; Greverus, D.; Scheurich, A.; Stoeter, P.; Müller, M.J. Predicting conversion to dementia in mild cognitive impairment by volumetric and diffusivity measurements of the hippocampus. Psychiatry Res. Neuroimaging 2006, 146, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.J.; Greverus, D.; Weibrich, C.; Dellani, P.R.; Scheurich, A.; Stoeter, P.; Fellgiebel, A. Diagnostic utility of hippocampal size and mean diffusivity in amnestic MCI. Neurobiol. Aging 2007, 28, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.A.; Seidenberg, M.; Smith, J.C.; Nielson, K.A.; Woodard, J.L.; Durgerian, S.; Rao, S.M. Diffusion tensor imaging predictors of episodic memory decline in healthy elders at genetic risk for Alzheimer’s disease. J. Int. Neuropsychol. Soc. 2016, 22, 1005–1015. [Google Scholar] [CrossRef]
- Vogt, N.M.; Hunt, J.F.; Adluru, N.; Dean, D.C., III; Johnson, S.C.; Asthana, S.; Yu, J.-P.J.; Alexander, A.L.; Bendlin, B.B. Cortical microstructural alterations in mild cognitive impairment and Alzheimer’s disease dementia. Cereb. Cortex 2020, 30, 2948–2960. [Google Scholar] [CrossRef] [PubMed]

| Controls (N = 22) | PD Patients (N = 19) | p-Values | |
|---|---|---|---|
| Age (y) | 69.05 ± 5.58 | 66.16 ± 8.81 | 0.211 |
| Gender (m/f) | 12/10 | 14/5 | 0.205 |
| Education (years) | 14.77 ± 3.19 | 16.63 ± 3.25 | 0.073 |
| MMSE | 29.40 ± 0.99 | 29.00 ± 1.29 | 0.284 |
| Hoehn and Yahr Scale (1/2/3) | 0 | 2.03 ± 0.77 (7/8/4) | |
| Disease duration (years) | 0 | 6.65 ± 4.76 | |
| GDS | 2.78 ± 2.17 | 4.11 ± 3.11 | 0.147 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, E.-Y. Memory Deficits in Parkinson’s Disease Are Associated with Impaired Attentional Filtering and Memory Consolidation Processes. J. Clin. Med. 2023, 12, 4594. https://doi.org/10.3390/jcm12144594
Lee E-Y. Memory Deficits in Parkinson’s Disease Are Associated with Impaired Attentional Filtering and Memory Consolidation Processes. Journal of Clinical Medicine. 2023; 12(14):4594. https://doi.org/10.3390/jcm12144594
Chicago/Turabian StyleLee, Eun-Young. 2023. "Memory Deficits in Parkinson’s Disease Are Associated with Impaired Attentional Filtering and Memory Consolidation Processes" Journal of Clinical Medicine 12, no. 14: 4594. https://doi.org/10.3390/jcm12144594
APA StyleLee, E.-Y. (2023). Memory Deficits in Parkinson’s Disease Are Associated with Impaired Attentional Filtering and Memory Consolidation Processes. Journal of Clinical Medicine, 12(14), 4594. https://doi.org/10.3390/jcm12144594







