24-h Urinary Calcium Excretion and Renal Outcomes in Hospitalized Patients with and without Chronic Kidney Disease
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Exposure and Outcomes
2.3. Covariates
2.4. Statistical Analysis
3. Results
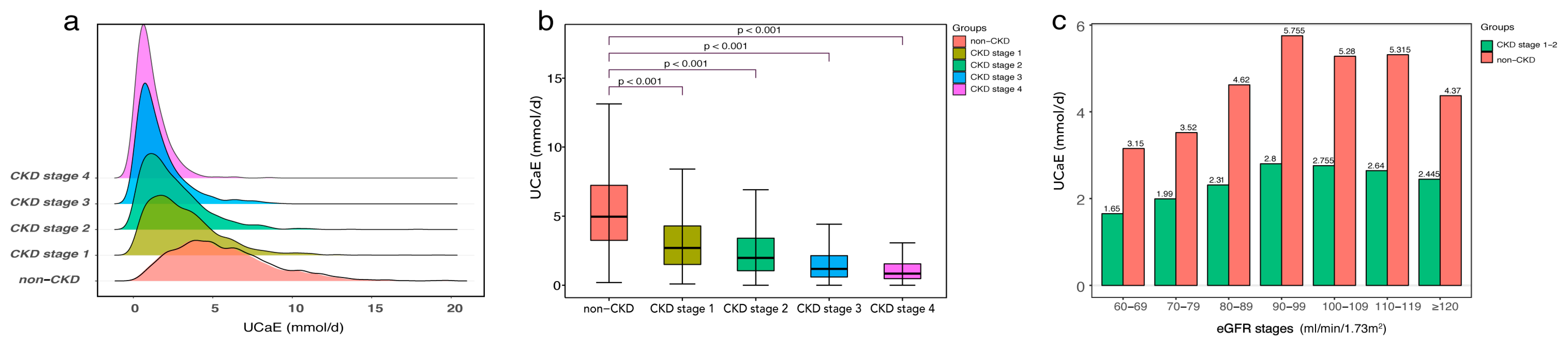
3.1. Baseline Characteristics
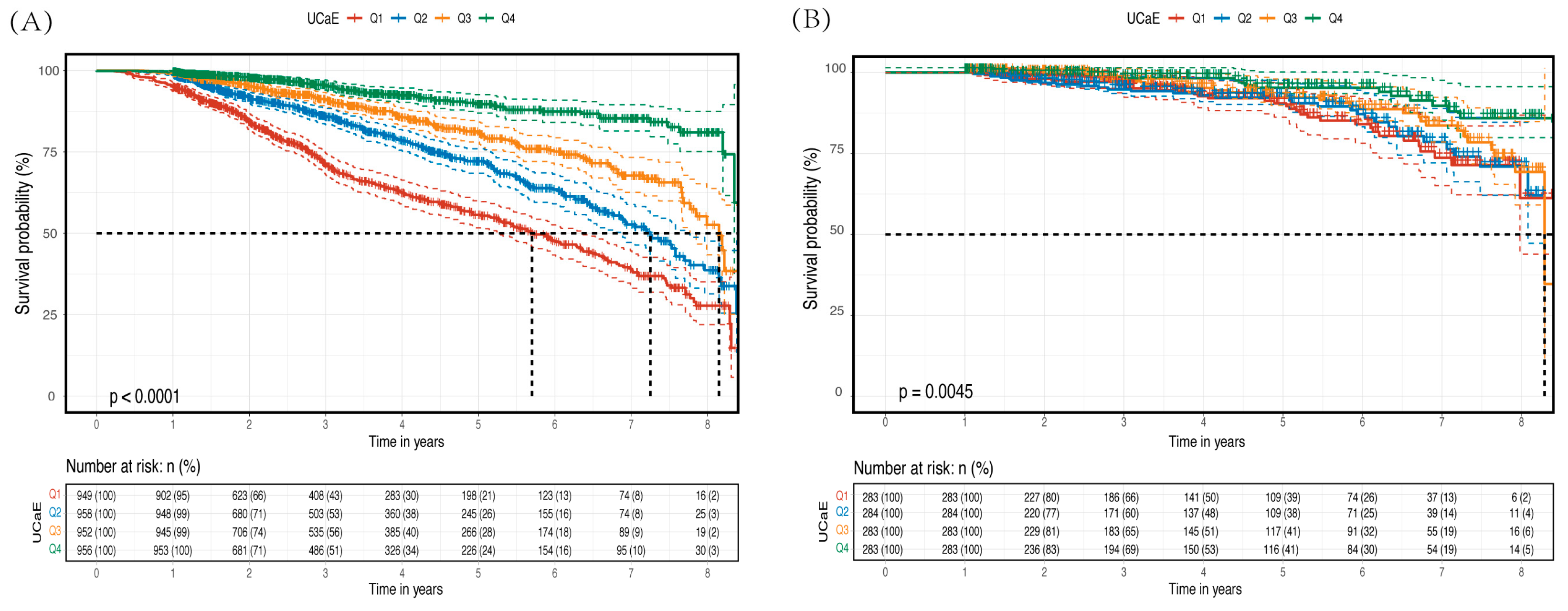
3.2. Primary Outcomes
3.3. Secondary Outcomes
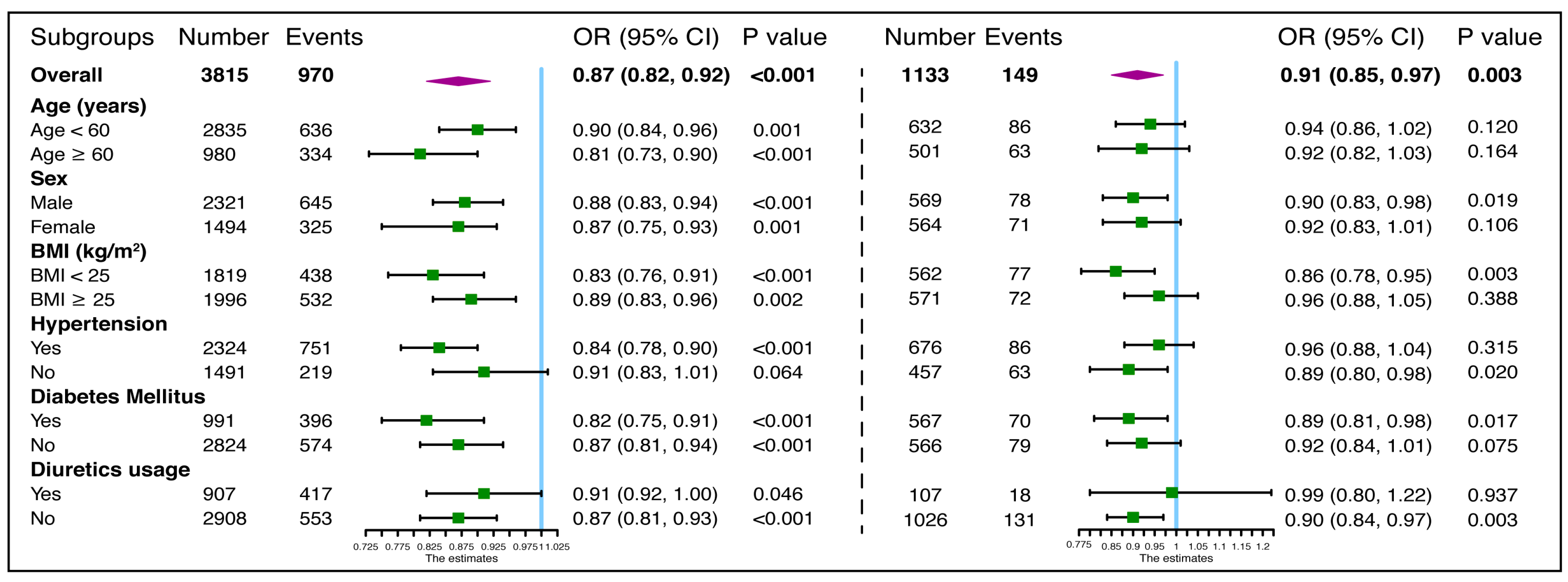
3.4. Further Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robertson, W.G.; Marshall, R.W. Ionized calcium in body fluids. Crit. Rev. Clin. Lab. Sci. 1981, 15, 85–125. [Google Scholar] [CrossRef]
- Shrimanker, I.; Bhattarai, S. Electrolytes. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Weaver, C.M.; Peacock, M. Calcium. Adv. Nutr. 2019, 10, 546–548. [Google Scholar] [CrossRef] [PubMed]
- Moe, S.M. Calcium Homeostasis in Health and in Kidney Disease. Compr. Physiol. 2016, 6, 1781–1800. [Google Scholar] [CrossRef]
- Sutton, R.A. Disorders of renal calcium excretion. Kidney Int. 1983, 23, 665–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaine, J.; Chonchol, M.; Levi, M. Renal control of calcium, phosphate, and magnesium homeostasis. Clin. J. Am. Soc. Nephrol. 2015, 10, 1257–1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Downie, M.L.; Alexander, R.T. Molecular mechanisms altering tubular calcium reabsorption. Pediatr. Nephrol. 2022, 37, 707–718. [Google Scholar] [CrossRef]
- Ogata, S.; Akashi, Y.; Sakusabe, T.; Yoshizaki, S.; Maeda, Y.; Nishimura, K.; Maeda, K.; Nakai, S. A multiple 24-hour urine collection study indicates that kidney function decline is related to urinary sodium and potassium excretion in patients with chronic kidney disease. Kidney Int. 2022, 101, 164–173. [Google Scholar] [CrossRef]
- Ma, Y.; He, F.J.; Sun, Q.; Yuan, C.; Kieneker, L.M.; Curhan, G.C.; MacGregor, G.A.; Bakker, S.J.L.; Campbell, N.R.C.; Wang, M.; et al. 24-Hour Urinary Sodium and Potassium Excretion and Cardiovascular Risk. N. Engl. J. Med. 2022, 386, 252–263. [Google Scholar] [CrossRef]
- Cirillo, M.; Stellato, D.; Panarelli, P.; Laurenzi, M.; De Santo, N. Cross-sectional and prospective data on urinary calcium and urinary stone disease. Kidney Int. 2003, 63, 2200–2206. [Google Scholar] [CrossRef]
- Prochaska, M.; Taylor, E.; Vaidya, A.; Curhan, G. Low Bone Density and Bisphosphonate Use and the Risk of Kidney Stones. Clin. J. Am. Soc. Nephrol. 2017, 12, 1284–1290. [Google Scholar] [CrossRef] [Green Version]
- Shang, N.; Khan, A.; Polubriaginof, F.; Zanoni, F.; Mehl, K.; Fasel, D.; Drawz, P.E.; Carrol, R.J.; Denny, J.C.; Hathcock, M.A.; et al. Medical records-based chronic kidney disease phenotype for clinical care and “big data” observational and genetic studies. NPJ Digit. Med. 2021, 4, 70. [Google Scholar] [CrossRef]
- Okada, R.; Tsushita, K.; Wakai, K.; Kato, K.; Wada, T.; Shinohara, Y. Healthy lifestyle reduces incidence of trace/positive proteinuria and rapid kidney function decline after 2 years: From the Japan Ningen Dock study. Nephrol. Dial. Transplant. 2021, 36, 1039–1048. [Google Scholar] [CrossRef]
- Chapter 2: Definition, identification, and prediction of CKD progression. Kidney Int. Suppl. (2011) 2013, 3, 63–72. [CrossRef] [PubMed] [Green Version]
- Jorgensen, F.S. Effect of thiazide diuretics upon calcium metabolism. Dan. Med. Bull. 1976, 23, 223–230. [Google Scholar] [PubMed]
- Courbebaisse, M.; Thervet, E.; Souberbielle, J.C.; Zuber, J.; Eladari, D.; Martinez, F.; Mamzer-Bruneel, M.F.; Urena, P.; Legendre, C.; Friedlander, G.; et al. Effects of vitamin D supplementation on the calcium-phosphate balance in renal transplant patients. Kidney Int. 2009, 75, 646–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piemonte, S.; Romagnoli, E.; Cipriani, C.; Fassino, V.; Del Fiacco, R.; Carnevale, V.; Scillitani, A.; D’Erasmo, E.; Tancredi, A.; Minisola, S. The effect of recombinant PTH(1-34) and PTH(1-84) on serum ionized calcium, 1,25-dihydroxyvitamin D, and urinary calcium excretion: A pilot study. Calcif. Tissue Int. 2009, 85, 287–292. [Google Scholar] [CrossRef]
- Leaf, D.E.; Korets, R.; Taylor, E.N.; Tang, J.; Asplin, J.R.; Goldfarb, D.S.; Gupta, M.; Curhan, G.C. Effect of vitamin D repletion on urinary calcium excretion among kidney stone formers. Clin. J. Am. Soc. Nephrol. 2012, 7, 829–834. [Google Scholar] [CrossRef] [Green Version]
- Isakova, T.; Gutierrez, O.; Shah, A.; Castaldo, L.; Holmes, J.; Lee, H.; Wolf, M. Postprandial mineral metabolism and secondary hyperparathyroidism in early CKD. J. Am. Soc. Nephrol. 2008, 19, 615–623. [Google Scholar] [CrossRef] [Green Version]
- Ince, B.; Anderson, E.; Neer, R. Lowering dietary protein to U.S. Recommended dietary allowance levels reduces urinary calcium excretion and bone resorption in young women. J. Clin. Endocrinol. Metab. 2004, 89, 3801–3807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, J.J.; Nielsen, F.H. Acid diet (high-meat protein) effects on calcium metabolism and bone health. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 698–702. [Google Scholar] [CrossRef]
- Zhu, K.; Meng, X.; Kerr, D.A.; Devine, A.; Solah, V.; Binns, C.W.; Prince, R.L. The effects of a two-year randomized, controlled trial of whey protein supplementation on bone structure, IGF-1, and urinary calcium excretion in older postmenopausal women. J. Bone Miner. Res. 2011, 26, 2298–2306. [Google Scholar] [CrossRef] [PubMed]
- Dawson-Hughes, B.; Harris, S.S.; Palermo, N.J.; Gilhooly, C.H.; Shea, M.K.; Fielding, R.A.; Ceglia, L. Potassium Bicarbonate Supplementation Lowers Bone Turnover and Calcium Excretion in Older Men and Women: A Randomized Dose-Finding Trial. J. Bone Miner. Res. 2015, 30, 2103–2111. [Google Scholar] [CrossRef] [PubMed]
- Bayomy, O.; Zaheer, S.; Williams, J.; Curhan, G.; Vaidya, A. Disentangling the Relationships Between the Renin-Angiotensin-Aldosterone System, Calcium Physiology, and Risk for Kidney Stones. J. Clin. Endocrinol. Metab. 2020, 105, 1937–1946. [Google Scholar] [CrossRef] [PubMed]
- Nouvenne, A.; Meschi, T.; Prati, B.; Guerra, A.; Allegri, F.; Vezzoli, G.; Soldati, L.; Gambaro, G.; Maggiore, U.; Borghi, L. Effects of a low-salt diet on idiopathic hypercalciuria in calcium-oxalate stone formers: A 3-mo randomized controlled trial. Am. J. Clin. Nutr. 2010, 91, 565–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostroverkhova, D.S.; Hu, J.; Tarasov, V.V.; Melnikova, T.I.; Porozov, Y.B.; Mutig, K. Calcium-Sensing Receptor and Regulation of WNK Kinases in the Kidney. Cells 2020, 9, 1644. [Google Scholar] [CrossRef]
- Toka, H.R.; Al-Romaih, K.; Koshy, J.M.; DiBartolo, S., 3rd; Kos, C.H.; Quinn, S.J.; Curhan, G.C.; Mount, D.B.; Brown, E.M.; Pollak, M.R. Deficiency of the calcium-sensing receptor in the kidney causes parathyroid hormone-independent hypocalciuria. J. Am. Soc. Nephrol. 2012, 23, 1879–1890. [Google Scholar] [CrossRef] [Green Version]
- Toka, H.; Genovese, G.; Mount, D.; Pollak, M.; Curhan, G. Frequency of rare allelic variation in candidate genes among individuals with low and high urinary calcium excretion. PLoS ONE 2013, 8, e71885. [Google Scholar] [CrossRef] [Green Version]
- de Groot, T.; Lee, K.; Langeslag, M.; Xi, Q.; Jalink, K.; Bindels, R.J.; Hoenderop, J.G. Parathyroid hormone activates TRPV5 via PKA-dependent phosphorylation. J. Am. Soc. Nephrol. 2009, 20, 1693–1704. [Google Scholar] [CrossRef] [Green Version]
- Cha, S.K.; Wu, T.; Huang, C.L. Protein kinase C inhibits caveolae-mediated endocytosis of TRPV5. Am. J. Physiol. Renal Physiol. 2008, 294, F1212–F1221. [Google Scholar] [CrossRef]
- Hoenderop, J.G.J.; Müller, D.; van der Kemp, A.W.C.M.; Hartog, A.; Suzuki, M.; Ishibashi, K.; Imai, M.; Sweep, F.; Willems, P.; Os, C.H.V.; et al. Calcitriol controls the epithelial calcium channel in kidney. J. Am. Soc. Nephrol. 2001, 12, 1342–1349. [Google Scholar] [CrossRef]
- Lee, J.; Ju, K.; Kim, H.; Tsogbadrakh, B.; Ryu, H.; Kang, E.; Kang, M.; Yang, J.; Kang, H.; Ahn, C.; et al. Soluble α-klotho anchors TRPV5 to the distal tubular cell membrane independent of FGFR1 by binding TRPV5 and galectin-1 simultaneously. Am. J. Physiol. Renal Physiol. 2021, 320, F559–F568. [Google Scholar] [CrossRef] [PubMed]
- Andrukhova, O.; Smorodchenko, A.; Egerbacher, M.; Streicher, C.; Zeitz, U.; Goetz, R.; Shalhoub, V.; Mohammadi, M.; Pohl, E.E.; Lanske, B.; et al. FGF23 promotes renal calcium reabsorption through the TRPV5 channel. EMBO J. 2014, 33, 229–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.; Vallon, V. Reduced renal calcium excretion in the absence of sclerostin expression: Evidence for a novel calcium-regulating bone kidney axis. J. Am. Soc. Nephrol. 2014, 25, 2159–2168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, T.; Lin, T.; Ma, J.; Guo, L.; Zhang, L.; Zhou, X.; Ye, T. Regulation of TRPV5 transcription and expression by E2/ERα signalling contributes to inhibition of osteoclastogenesis. J. Cell. Mol. Med. 2018, 22, 4738–4750. [Google Scholar] [CrossRef]
- Hsu, Y.; Dimke, H.; Schoeber, J.; Hsu, S.; Lin, S.; Chu, P.; Hoenderop, J.; Bindels, R. Testosterone increases urinary calcium excretion and inhibits expression of renal calcium transport proteins. Kidney Int. 2010, 77, 601–608. [Google Scholar] [CrossRef] [Green Version]
- Meurer, M.; Höcherl, K. Endotoxaemia differentially regulates the expression of renal Ca2+ transport proteins in mice. Acta Physiol. 2019, 225, e13175. [Google Scholar] [CrossRef]
- Fluck, E.; Yazici, A.; Rohacs, T.; Moiseenkova-Bell, V. Structural basis of TRPV5 regulation by physiological and pathophysiological modulators. Cell Rep. 2022, 39, 110737. [Google Scholar] [CrossRef]
- Nie, M.; Bal, M.; Yang, Z.; Liu, J.; Rivera, C.; Wenzel, A.; Beck, B.; Sakhaee, K.; Marciano, D.; Wolf, M. Mucin-1 Increases Renal TRPV5 Activity In Vitro, and Urinary Level Associates with Calcium Nephrolithiasis in Patients. J. Am. Soc. Nephrol. 2016, 27, 3447–3458. [Google Scholar] [CrossRef] [Green Version]
- Wolf, M.; Wu, X.; Huang, C. Uromodulin upregulates TRPV5 by impairing caveolin-mediated endocytosis. Kidney Int. 2013, 84, 130–137. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.; Renigunta, V.; Nie, M.; Sunq, A.; Himmerkus, N.; Quintanova, C.; Bleich, M.; Renigunta, A.; Wolf, M. Phosphorylated claudin-16 interacts with Trpv5 and regulates transcellular calcium transport in the kidney. Proc. Natl. Acad. Sci. USA 2019, 116, 19176–19186. [Google Scholar] [CrossRef] [Green Version]
- Reilly, R.F.; Huang, C.-L. The mechanism of hypocalciuria with NaCl cotransporter inhibition. Nat. Rev. Nephrol. 2011, 7, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Pulskens, W.P.; Verkaik, M.; Sheedfar, F.; van Loon, E.P.; van de Sluis, B.; Vervloet, M.G.; Hoenderop, J.G.; Bindels, R.J. Deregulated Renal Calcium and Phosphate Transport during Experimental Kidney Failure. PLoS ONE 2015, 10, e0142510. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Zheng, D.; Guo, X.; Zhao, M.; Gao, L.; Bai, L. Transient Receptor Potential Channel, Vanilloid 5, Induces Chondrocyte Apoptosis in a Rat Osteoarthritis Model Through the Mediation of Ca2+ Influx. Cell. Physiol. Biochem. 2018, 46, 687–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, J.; Kieneker, L.; de Borst, M.; Visser, S.; Kema, I.; Bakker, S.; Gansevoort, R. Urinary Calcium Excretion and Risk of Chronic Kidney Disease in the General Population. Kidney Int. Rep. 2017, 2, 366–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| CKD Patients (n = 3815) | non-CKD Patients (n = 1133) | |||||||
|---|---|---|---|---|---|---|---|---|
| Quartiles | Q 1 | Q 2 | Q 3 | Q 4 | Q 1 | Q 2 | Q 3 | Q 4 |
| 24-h UCaE (mmol/d) | ~0.84 | 0.85–1.71 | 1.72–3.21 | 3.22~ | ~3.25 | 3.26–4.95 | 4.96–7.23 | 7.24~ |
| Number | 949 | 958 | 952 | 956 | 283 | 284 | 283 | 283 |
| Basic information | ||||||||
| Age (years) | 49 (35, 62) | 46 (34, 60) | 47 (35, 59) | 46 (35, 58) | 59 (48, 70) | 57 (47, 66) | 58 (50, 66) | 57 (48, 65) |
| Male, n (%) | 560 (59.0) | 573 (59.8) | 594 (62.4) | 594 (62.1) | 138 (48.8) | 135 (47.5) | 137 (48.4) | 159 (56.2) |
| BMI (kg/m2) | 24.5 (22.0, 27.0) | 25.0 (22.8, 27.7) | 25.2 (22.7, 28.1) | 25.8 (23.4, 28.4) | 24.7 (22.5, 27.3) | 25.3 (22.7, 27.6) | 24.8 (22.3, 27.2) | 25.4 (23.7, 27.8) |
| MAP (mmHg) | 99 (90, 107) | 98 (89, 108) | 97 (89, 107) | 97 (89, 107) | 93 (86, 102) | 97 (88, 107) | 96 (89, 106) | 99 (91, 107) |
| Current smoker, n (%) | 99 (10.4) | 106 (11.1) | 98 (10.3) | 125 (13.1) | 20 (7.1) | 14 (4.9) | 18 (6.4) | 19 (6.7) |
| Laboratory test | ||||||||
| eGFR (mL/min/1.73 m2) | 46.1 (29.6, 72.9) | 59.9 (37.9, 89.2) | 79.5 (53.4, 100.9) | 92.5 (72.0, 107.9) | 90.0 (78.3, 103.4) | 95.7 (85.7, 105.9) | 95.0 (87.0, 103.3) | 96.0 (90.1, 106.0) |
| Hb (g/L) | 115 (99, 131) | 125 (111, 139) | 131 (118, 147) | 137 (124, 149) | 131.2 ± 16.7 | 133.9 ± 16.2 | 134.2 ± 16.8 | 138.9 ± 15.0 |
| Alb (g/L) | 34.3 (26.0, 39.0) | 36.6 (30.3, 40.5) | 38.0 (31.8, 41.6) | 38.6 (32.6, 42.3) | 41.2 (38.9, 43.5) | 41.6 (39.7, 43.6) | 41.9 (39.8, 43.5) | 42.1 (40.1, 44.1) |
| ALP (U/L) | 62.0 (50.4, 77.8) | 59.6 (49.3, 73.7) | 59.4 (48.6, 71.2) | 57.0 (46.4, 71.5) | 63.9 (51.7, 77.3) | 65.2 (52.3, 78.8) | 64.5 (54.5, 79.0) | 65.9 (55.2, 78.9) |
| TC (mmol/L) | 4.6 (3.8, 5.9) | 4.6 (3.9, 5.6) | 4.5 (3.8, 5.6) | 4.6 (3.8, 5.8) | 4.0 (3.4, 4.7) | 4.2 (3.6, 4.8) | 4.1 (3.5, 4.8) | 4.2 (3.5, 4.9) |
| TG (mmol/L) | 1.7 (1.2, 2.6) | 1.7 (1.2, 2.5) | 1.7 (1.2, 2.5) | 1.8 (1.2, 2.6) | 1.2 (0.9, 1.8) | 1.3 (0.9, 1.9) | 1.2 (0.9, 1.7) | 1.4 (1.0, 1.9) |
| 24-h proteinuria (g/d) | 2.5 (0.9, 4.4) | 1.9 (0.8, 4.0) | 1.3 (0.5, 3.5) | 1.1 (0.4, 2.8) | 0.1 (0.0, 0.1) | 0.1 (0.0, 0.1) | 0.1 (0.0, 0.1) | 0.1 (0.0, 0.1) |
| Serum Ca (mmol/L) | 2.1 (2.0, 2.2) | 2.2 (2.1, 2.3) | 2.2 (2.1, 2.3) | 2.2 (2.1, 2.3) | 2.2 (2.2, 2.3) | 2.3 (2.2, 2.3) | 2.3 (2.2, 2.3) | 2.3 (2.2, 2.4) |
| Serum P (mmol/L) | 1.2 (1.1, 1.4) | 1.2 (1.1, 1.3) | 1.2 (1.0, 1.3) | 1.2 (1.0, 1.3) | 1.1 (1.0, 1.3) | 1.1 (1.0, 1.3) | 1.1(1.0, 1.3) | 1.2 (1.0, 1.3) |
| 24-h UNaE (mmol/d) | 94 (58, 138) | 118 (79, 163) | 130 (89, 184) | 166 (116, 224) | 114 (78, 160) | 134 (94, 179) | 156 (120, 208) | 195 (142, 247) |
| 24-h UKE (mmol/d) | 33.3 (23.9, 43.9) | 35.4 (26.7, 46.6) | 38.2 (28.8, 49.6) | 40.8 (31.3, 54.3) | 38.7 (28.8, 51.9) | 38.7 (30.4, 50.1) | 42.5 (33.3, 52.9) | 48.1 (37.5, 62.4) |
| Comorbidities | ||||||||
| HTN, n (%) | 618 (65.1) | 618 (64.5) | 554 (58.2) | 534 (55.9) | 160 (56.5) | 179 (63.0) | 164 (58.0) | 173 (61.1) |
| DM, n (%) | 257 (27.1) | 214 (22.3) | 229 (24.1) | 291 (30.4) | 120 (42.4) | 133 (46.8) | 149 (52.7) | 166 (58.7) |
| CVD, n (%) | 60 (6.3) | 62 (6.5) | 57 (6.0) | 46 (4.8) | 41 (14.5) | 24 (8.5) | 24 (8.5) | 21 (7.4) |
| Medications | ||||||||
| ACEI/ARB, n (%) | 650 (68.5) | 683 (71.3) | 686 (72.1) | 667 (69.8) | 104 (36.7) | 101 (35.6) | 105 (37.1) | 108 (38.2) |
| Diuretics, n (%) | 342 (36.0) | 223 (23.3) | 181 (19.0) | 161 (16.8) | 39 (13.8) | 29 (10.2) | 21 (7.4) | 17 (6.0) |
| Glucocorticoids, n (%) | 201 (21.2) | 195 (20.4) | 183 (19.2) | 212 (22.2) | 10 (3.5) | 10 (3.5) | 9 (3.2) | 5 (1.8) |
| Statins, n (%) | 455 (47.9) | 427 (44.6) | 428 (45.0) | 434 (45.4) | 148 (52.3) | 126 (44.4) | 138 (48.8) | 147 (51.9) |
| CaCO3 supplement, n (%) | 378 (39.8) | 340 (35.5) | 330 (34.7) | 386 (40.4) | 73 (25.8) | 82 (28.9) | 94 (33.2) | 89 (31.4) |
| Vitamin D3 supplement, n (%) | 103 (10.9) | 84 (8.8) | 79 (8.3) | 91 (9.5) | 40 (14.1) | 51 (18.0) | 38 (13.4) | 53 (18.7) |
| Calcitriol, n (%) | 262 (27.6) | 244 (25.5) | 244 (25.6) | 295 (30.9) | 64 (22.6) | 62 (21.8) | 77 (27.2) | 73 (25.8) |
| P-binders, n (%) | 9 (0.9) | 2 (0.2) | 4 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| CKD Patients (CKD Progression) | non-CKD Patients (Kidney Function Decline) | ||||
|---|---|---|---|---|---|
| Models | HR (95% CI) | p | Models | HR (95% CI) | p |
| Crude | 0.64 (0.56, 0.66) | <0.001 | Crude | 0.89 (0.83, 0.96) | 0.001 |
| Model 1 a | 0.61 (0.56, 0.66) | <0.001 | Model 1 d | 0.89 (0.83, 0.95) | <0.001 |
| Model 2 b | 0.79 (0.73, 0.86) | <0.001 | Model 2 e | 0.90 (0.84, 0.96) | 0.002 |
| Model 3 c | 0.85 (0.77, 0.93) | <0.001 | Model 3 f | 0.89 (0.82, 0.95) | 0.001 |
| No. of events | 813 | No. of events | 109 | ||
| UCaE | Q 1 | Q 2 | Q 3 | Q 4 | |||
| CKD Patients (n = 3815) | |||||||
| UCaE and annual eGFR decline | |||||||
| Models | β (95% CI) | p | β (95% CI) | β (95% CI) | β (95% CI) | p # | |
| Crude | 0.168 (0.116, 0.221) | <0.001 | Reference | 0.639 (0.338, 0.940) | 0.878 (0.577, 1.179) | 1.110 (0.809, 1.411) | <0.001 |
| Model 1 a | 0.165 (0.113, 0.217) | <0.001 | Reference | 0.596 (0.298, 0.895) | 0.854 (0.555, 1.152) | 1.062 (0.763, 1.361) | <0.001 |
| Model 2 b | 0.136 (0.077, 0.194) | <0.001 | Reference | 0.564 (0.263, 0.865) | 0.765 (0.449, 1.081) | 0.944 (0.606, 1.282) | <0.001 |
| Model 3 c | 0.122 (0.057, 0.188) | <0.001 | Reference | 0.420 (0.121, 0.719) | 0.583 (0.259, 0.908) | 0.810 (0.435, 1.184) | <0.001 |
| UCaE and RKFD | |||||||
| Models | OR (95% CI) | p | OR (95% CI) | OR (95% CI) | OR (95% CI) | p # | |
| Crude | 0.784 (0.741, 0.830) | <0.001 | Reference | 0.568 (0.456, 0.708) | 0.449 (0.356, 0.565) | 0.294 (0.227, 0.380) | <0.001 |
| Model 1 a | 0.786 (0.743, 0.832) | <0.001 | Reference | 0.578 (0.463, 0.722) | 0.450 (0.357, 0.569) | 0.298 (0.230, 0.385) | <0.001 |
| Model 2 b | 0.860 (0.809, 0.914) | <0.001 | Reference | 0.636 (0.505, 0.801) | 0.578 (0.449, 0.744) | 0.436 (0.325, 0.584) | <0.001 |
| Model 3 c | 0.903 (0.843, 0.969) | 0.004 | Reference | 0.742 (0.578, 0.953) | 0.712 (0.538, 0.942) | 0.545 (0.387, 0.767) | 0.005 |
| Events | 654 | 257 | 167 | 136 | 94 | ||
| non-CKD patients (n = 1133) | |||||||
| UCaE and annual eGFR decline | |||||||
| Models | β (95% CI) | p | β (95% CI) | β (95% CI) | β (95% CI) | p # | |
| Crude | 0.043 (0.016, 0.070) | 0.002 | Reference | 0.018 (−0.213, 0.248) | 0.149 (−0.081, 0.379) | 0.352 (0.121, 0.582) | 0.010 |
| Model 1 d | 0.044 (0.017, 0.070) | 0.001 | Reference | 0.041 (−0.187, 0.270) | 0.158 (−0.070, 0.386) | 0.366 (0.138, 0.595) | 0.008 |
| Model 2 e | 0.044 (0.017, 0.072) | 0.001 | Reference | 0.020 (−0.207, 0.246) | 0.134 (−0.094, 0.362) | 0.357 (0.125, 0.590) | 0.009 |
| Model 3 f | 0.046 (0.019, 0.074) | 0.001 | Reference | 0.032 (−0.195, 0.259) | 0.153 (−0.075, 0.382) | 0.376 (0.140, 0.612) | 0.007 |
| UCaE and RKFD | |||||||
| Models | OR (95% CI) | p | OR (95% CI) | OR (95% CI) | OR (95% CI) | p # | |
| Crude | 0.897 (0.831, 0.968) | 0.005 | Reference | 0.738 (0.430, 1.266) | 0.772 (0.453, 1.318) | 0.410 (0.218, 0.771) | 0.053 |
| Model 1 d | 0.896 (0.830, 0.967) | 0.005 | Reference | 0.714 (0.415, 1.230) | 0.773 (0.452, 1.323) | 0.401 (0.212, 0.756) | 0.045 |
| Model 2 e | 0.886 (0.819, 0.958) | 0.002 | Reference | 0.764 (0.439, 1.331) | 0.799 (0.460, 1.391) | 0.377 (0.195, 0.731) | 0.037 |
| Model 3 f | 0.889 (0.820, 0.963) | 0.004 | Reference | 0.778 (0.445, 1.361) | 0.791 (0.451, 1.388) | 0.393 (0.199, 0.775) | 0.060 |
| Events | 102 | 34 | 26 | 27 | 15 | ||
| eGFR Annual Decline in CKD Participants | ||||||
| Follow-up | n | Q 1 (n = 949) | Q 2 (n = 958) | Q 3 (n = 952) | Q 4 (n = 956) | p # |
| Overall | 3815 | −2.94 (−5.35, −1.20) | −2.33 (−4.10, −0.95) | −1.99 (−3.47, −0.89) | −1.99 (−3.13, −0.85) | <0.001 |
| 1–2 years | 1747 | −2.74 (−5.47, −1.41) | −2.32 (−3.55, −1.31) | −2.21 (−3.27, −1.29) | −2.22 (−3.11, −1.41) | <0.001 |
| 3–4 years | 1035 | −3.40 (−5.80, −1.25) | −2.32 (−4.56, −0.74) | −1.93 (−3.85, −0.68) | −1.67 (−3.49, −0.63) | <0.001 |
| 5~ years | 1033 | −2.65 (−4.93, −0.76) | −2.37 (−4.41, −0.92) | −1.67 (−3.50, −0.65) | −1.40 (−2.91, −0.46) | <0.001 |
| eGFR Annual Decline in non-CKD Participants | ||||||
| Follow-up | n | Q 1 (n = 283) | Q 2 (n = 284) | Q 3 (n = 283) | Q 4 (n = 283) | p # |
| Overall | 1133 | −1.68 (−2.37, −0.83) | −1.58 (−2.22, −0.84) | −1.43 (−2.21, −0.72) | −1.34 (−2.01, −0.64) | 0.002 |
| 1–2 years | 399 | −2.16 (−2.65, −1.45) | −2.13 (−2.49, −1.70) | −2.09 (−2.55, −1.58) | −1.97 (−2.41, −1.41) | 0.198 |
| 3–4 years | 283 | −1.37 (−2.18, −0.56) | −1.24 (−1.90, −0.76) | −1.30 (−1.91, −0.73) | −1.55 (−1.95, −1.02) | 0.286 |
| 5~ years | 451 | −1.32 (−1.99, −0.77) | −0.91 (−1.62, −0.43) | −0.94 (−1.57, −0.37) | −0.80 (−1.25, −0.24) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Wang, W.; Ma, Y.; Liang, Y.; Zhou, Y.; Cai, G. 24-h Urinary Calcium Excretion and Renal Outcomes in Hospitalized Patients with and without Chronic Kidney Disease. J. Clin. Med. 2023, 12, 4600. https://doi.org/10.3390/jcm12144600
Guo X, Wang W, Ma Y, Liang Y, Zhou Y, Cai G. 24-h Urinary Calcium Excretion and Renal Outcomes in Hospitalized Patients with and without Chronic Kidney Disease. Journal of Clinical Medicine. 2023; 12(14):4600. https://doi.org/10.3390/jcm12144600
Chicago/Turabian StyleGuo, Xinru, Wanling Wang, Yangyang Ma, Yanjun Liang, Yena Zhou, and Guangyan Cai. 2023. "24-h Urinary Calcium Excretion and Renal Outcomes in Hospitalized Patients with and without Chronic Kidney Disease" Journal of Clinical Medicine 12, no. 14: 4600. https://doi.org/10.3390/jcm12144600





