Safety Issues in the Development of an Innovative Medical Parallel Robot Used in Renal Single-Incision Laparoscopic Surgery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Identification and Prioritization of Technical Characteristics for a Parallel Robotic Structure Used in SILS
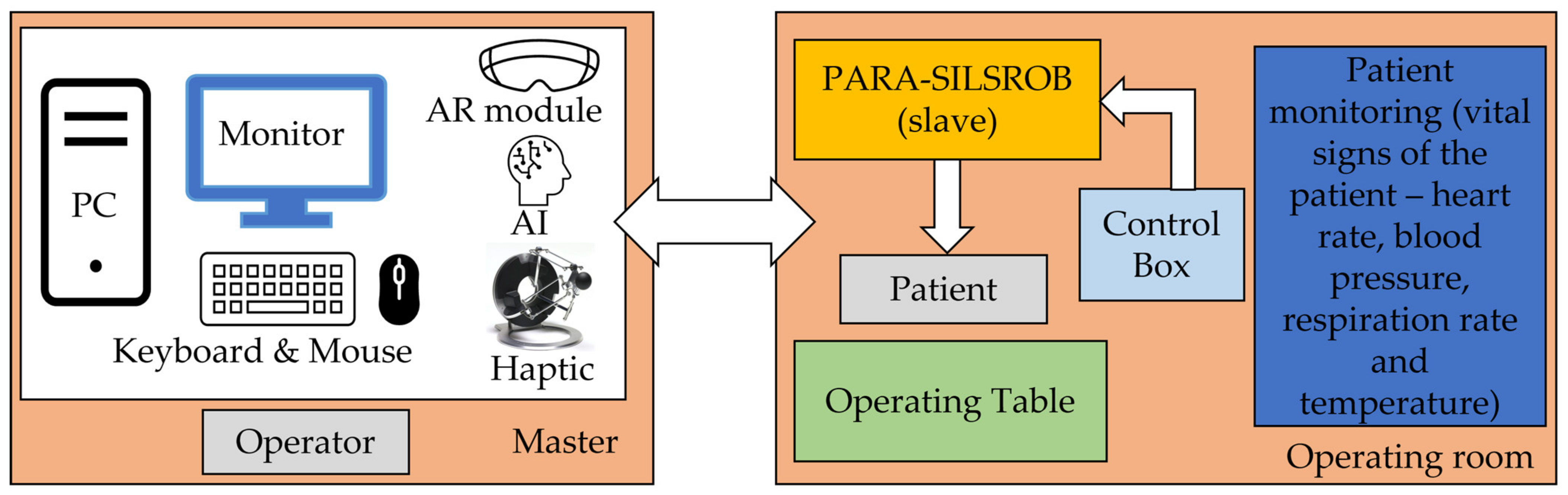
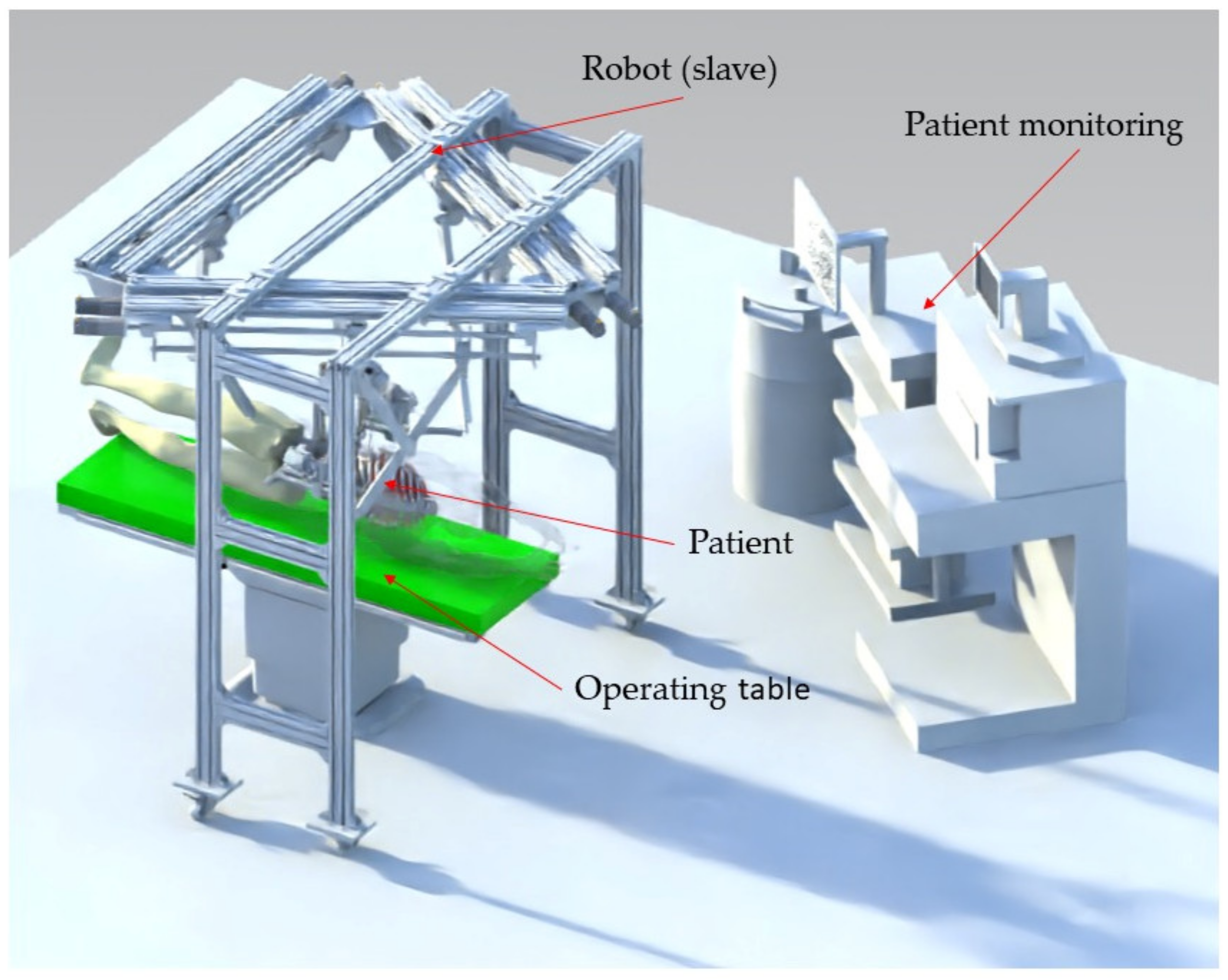
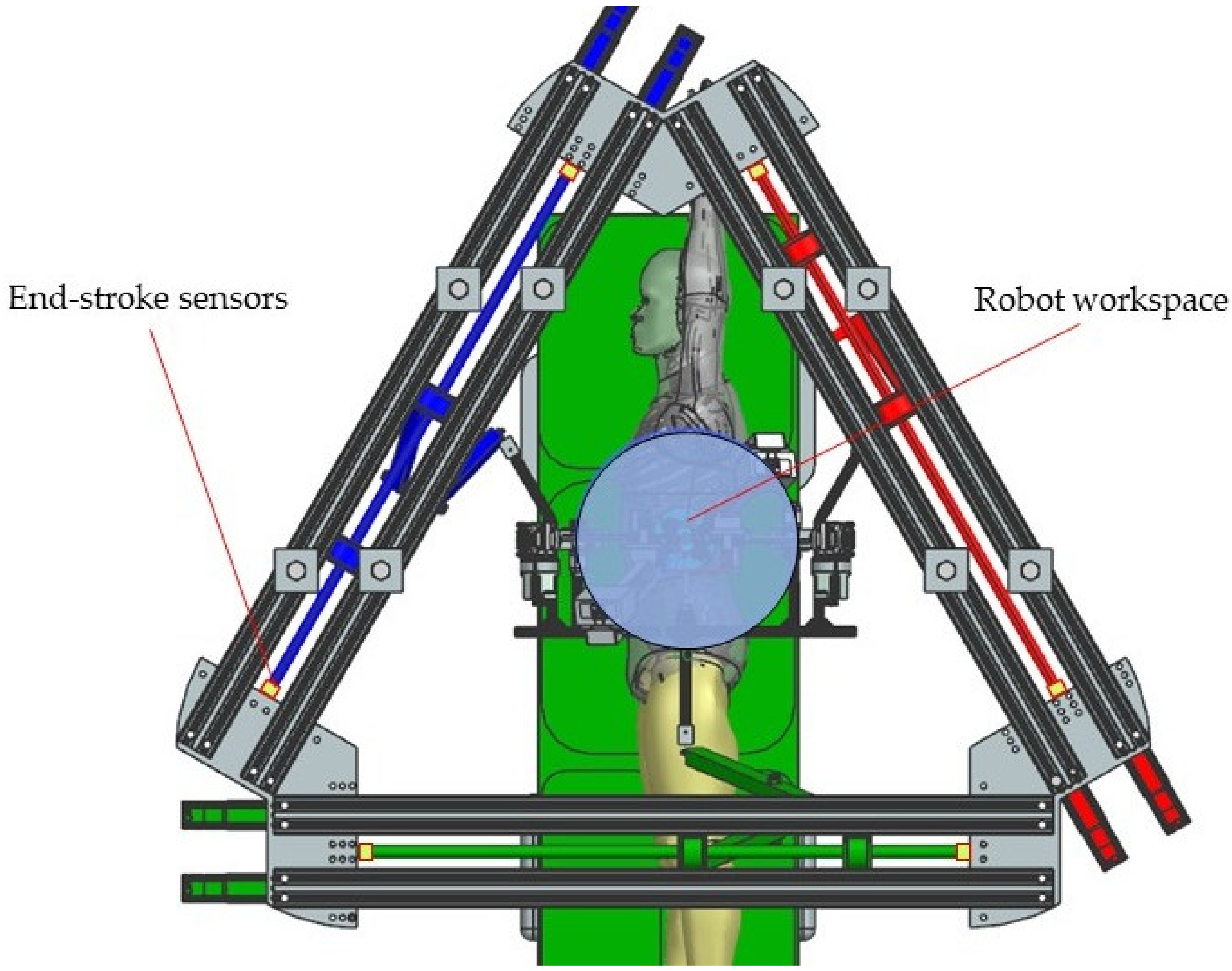
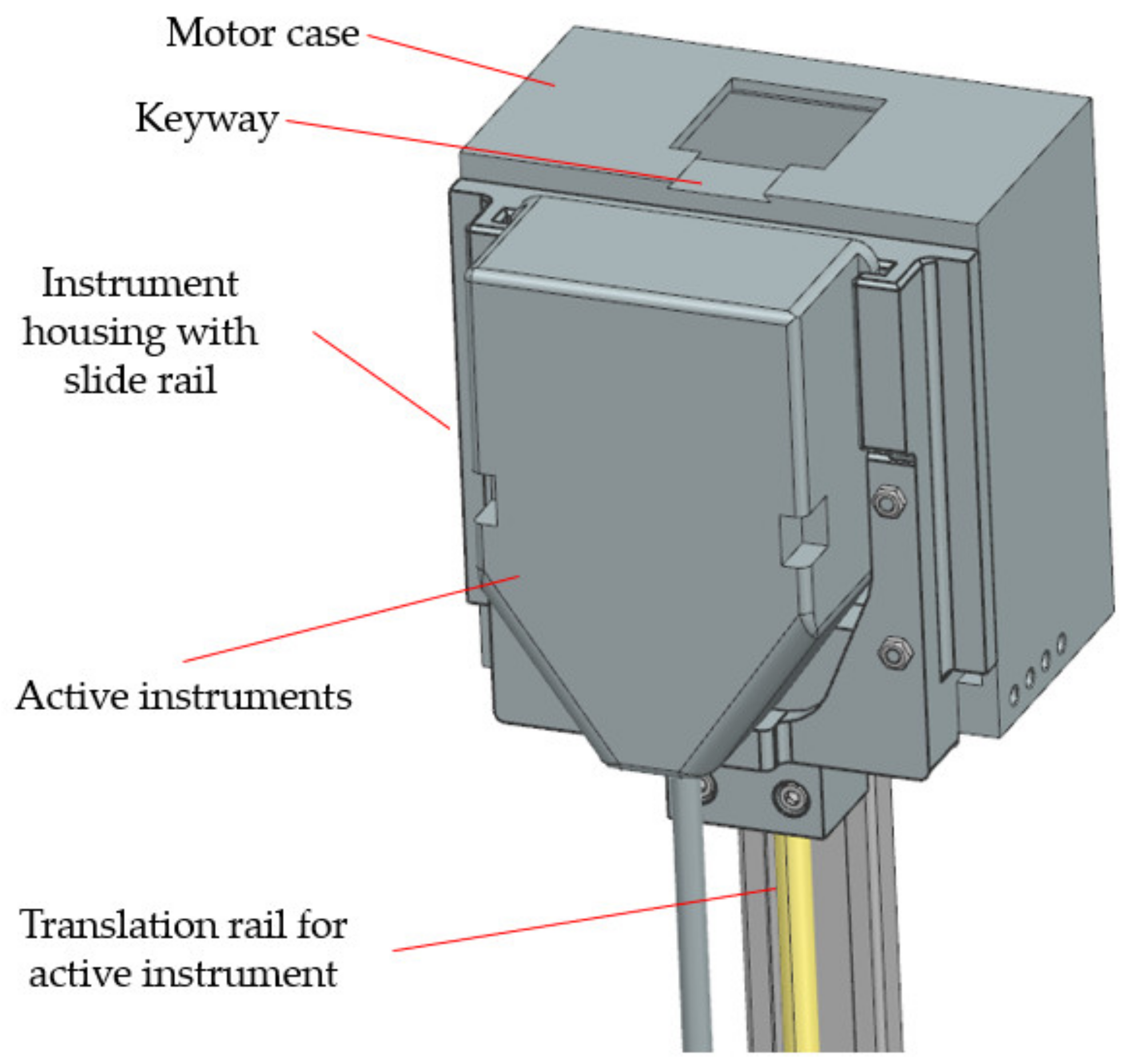
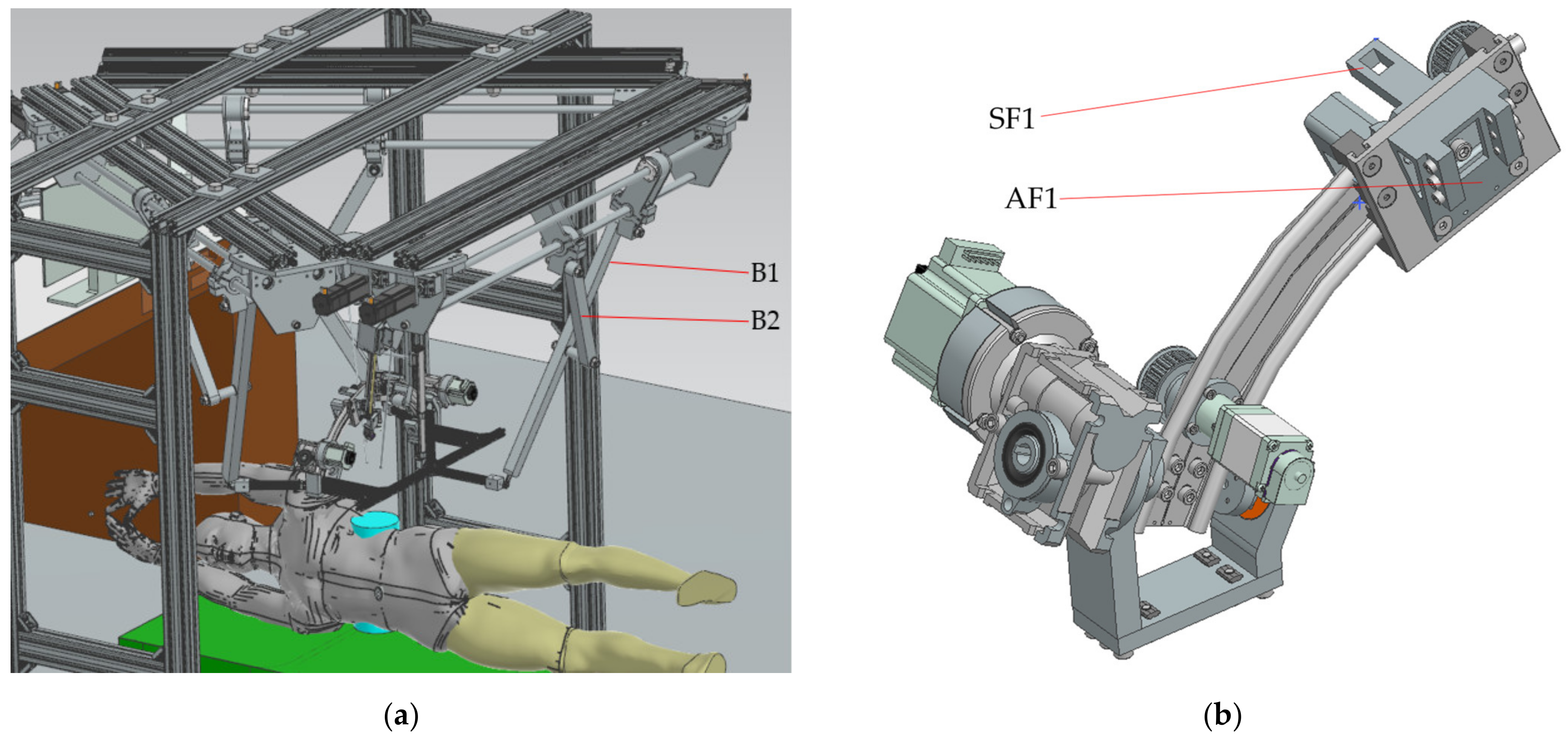
2.2. The PARA-SILSROB Robotic System
- -
- Preparation: patient anesthesia, operating room preparation, position the robot to the SILS port trocars (where the Artificial Intelligence module developed in [35] can be used), save the laparoscope RCM and find the RCML and RCMR positions using the 6-DOF parallel robot;
- -
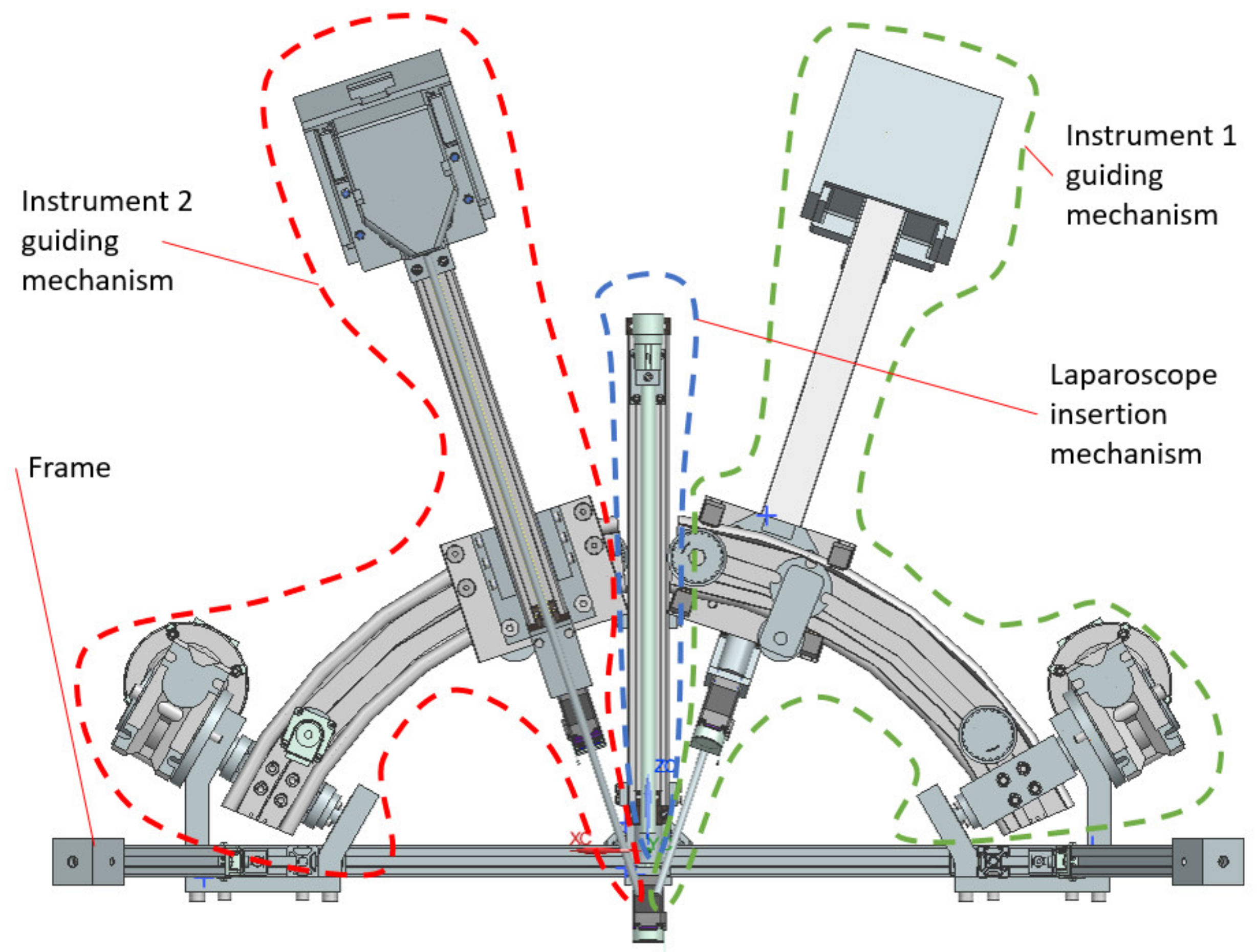
- Instruments insertion: the three instruments are placed in the surgical field. Usually, for the vast majority of SILS procedures (including liver cancer—hepatocellular carcinoma—or stomach cancer), the incision and insertion of the SILS port are performed at the level of the umbilicus, with the patient positioned on his back. However, there may be situations where the incision is made retroperitoneally below the 12th rib, with the patient positioned on one side (in the case of kidney surgeries). The maximum orientation of the mobile platform after inserting the endoscopic camera and active instruments into the patient’s body is limited to ±30° [22]. The endoscopic camera is inserted into the patient’s body approximately 150 mm, and the maximum insertion depth of the active instruments is 270 mm [28,36].
- -
- MP positioning towards the targeted lesion: the amplitude of the movements of the modules attached to the mobile platform is limited to ±45° [29].
- -
- Surgical task: the MP is locked in position, and the two modules guiding the active instruments perform individual tasks, remotely guided by the surgeon. It becomes obvious that if the laparoscope requires further orientation, which is performed with the MP, the RCML and RCMR positions will be altered, and a compensation motion will be from the two modules is required.
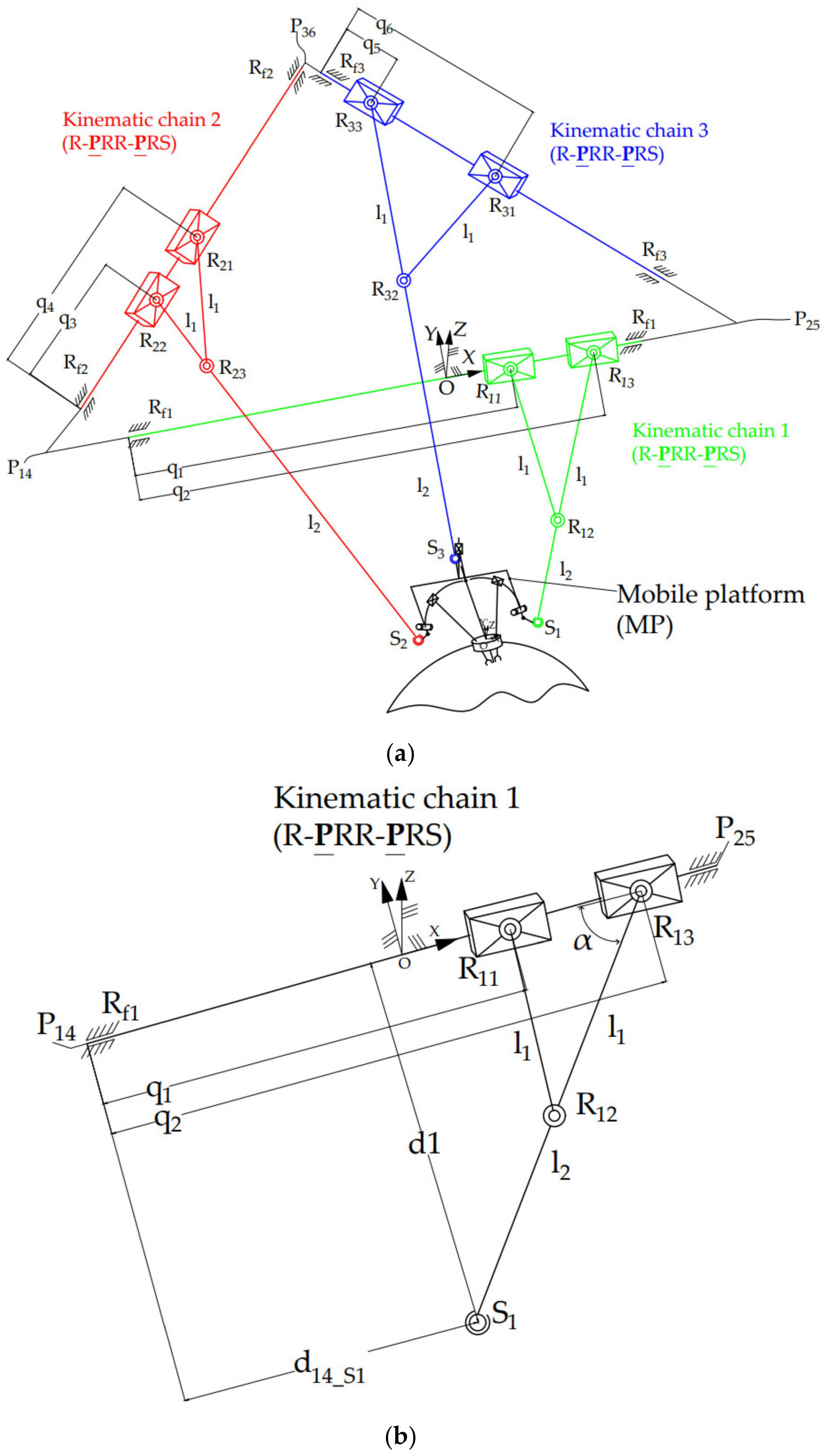
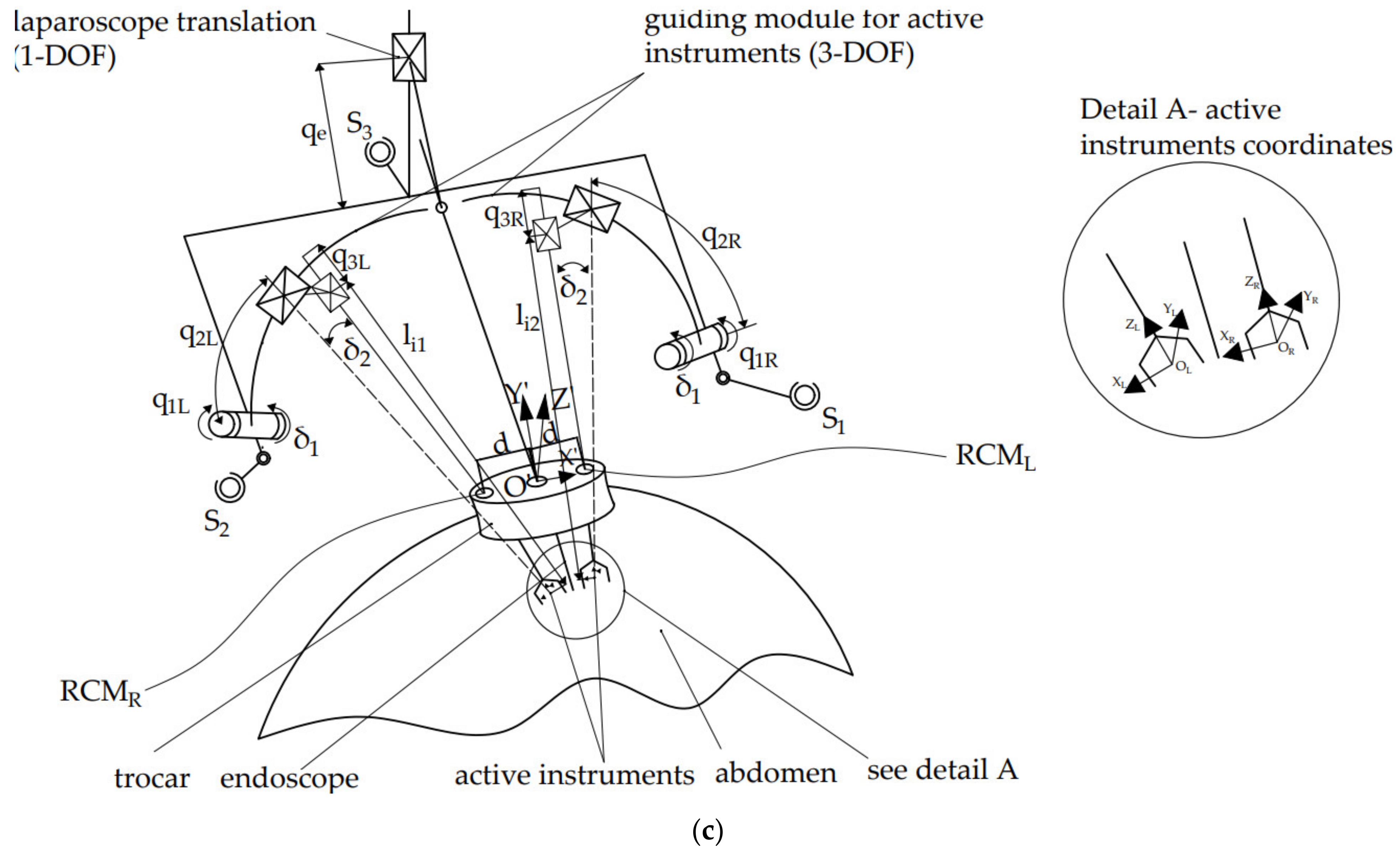
2.3. Inverse Kinematics of the PARA-SILSROB Robotic Structure
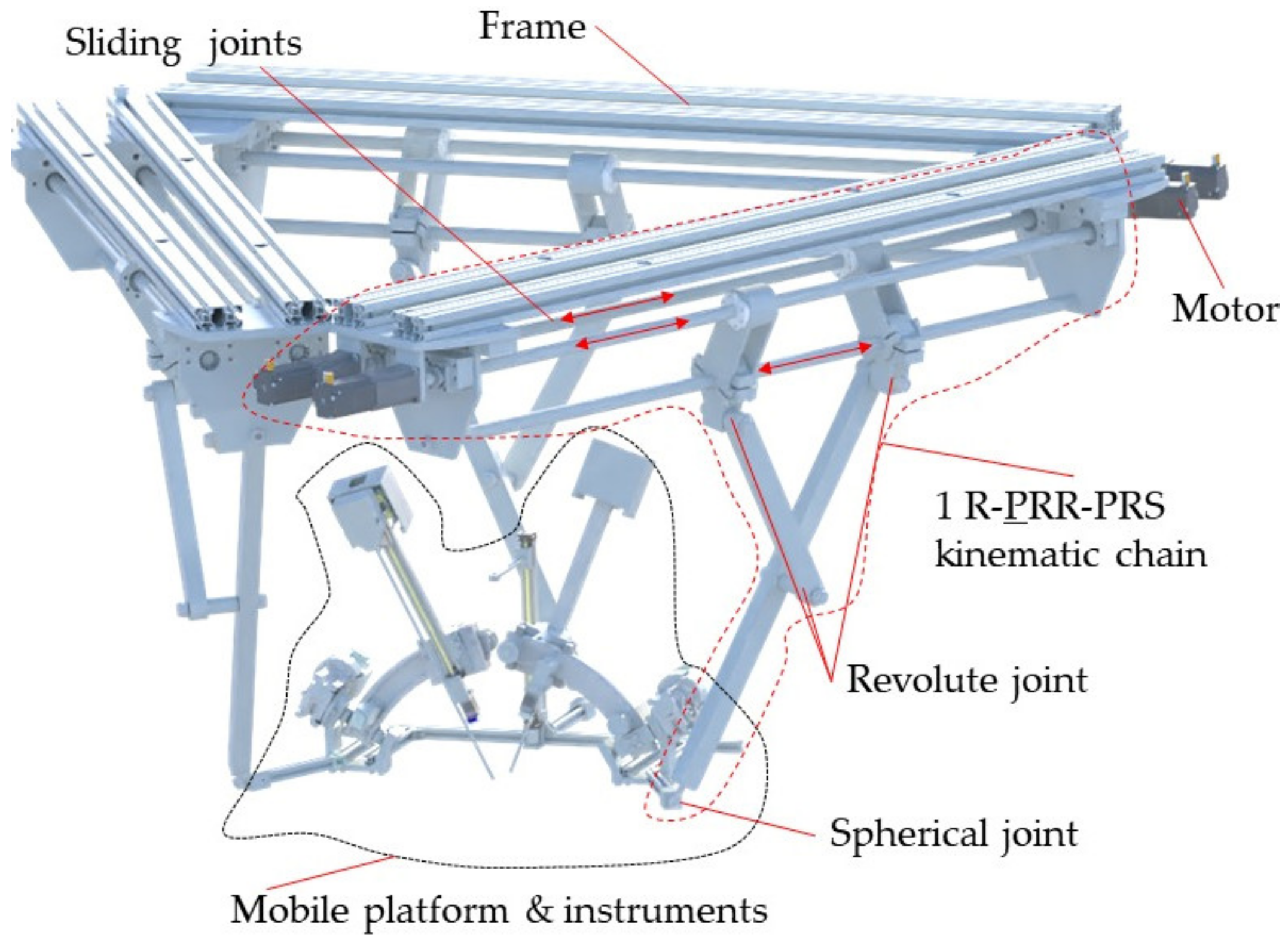
2.4. Design and Functionality of the PARA-SILSROB Robotic Structure
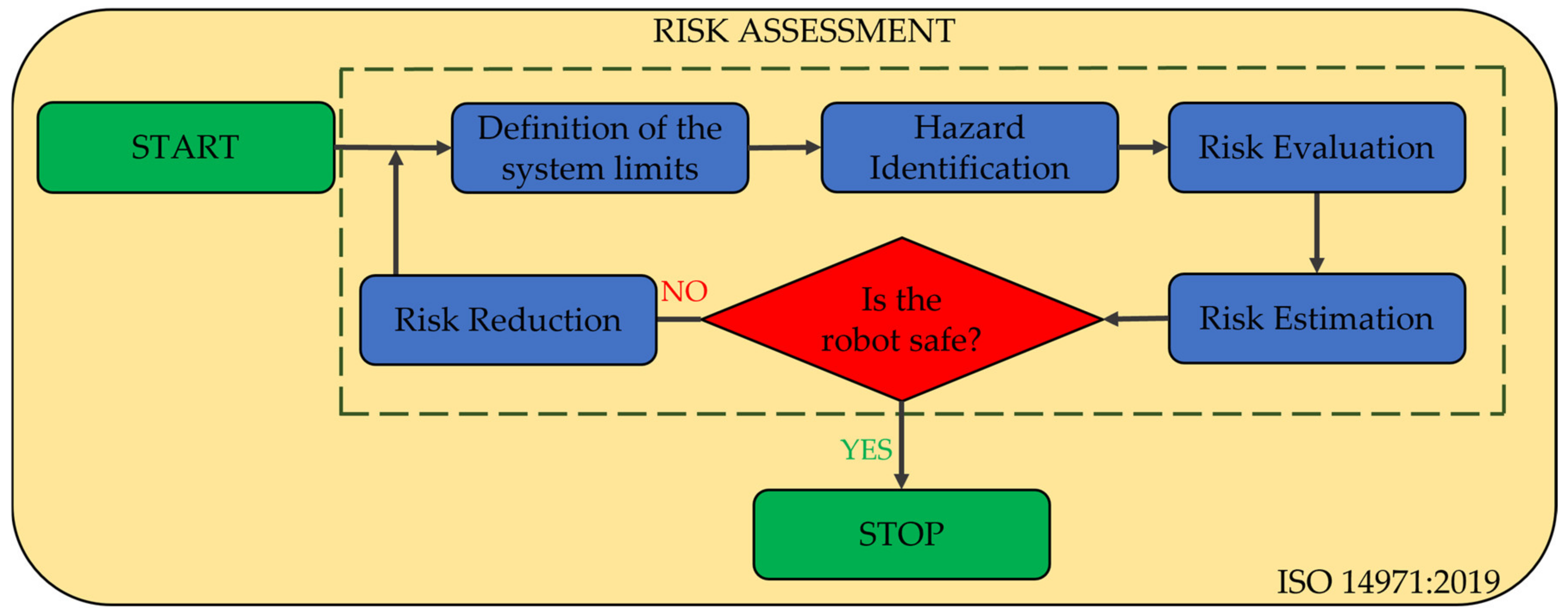
2.5. Risk Assessment of the PARA-SILSROB Robotic System
- Definition of the system limits;
- Hazard identification;
- Risk estimation;
- Risk evaluation;
- Risk reduction.
- Mechanical hazards:
- MH1: Collision between the guiding module of the laparoscope and the two active instruments. This hazard poses significant risks to the safety of the procedure, as the shocks recorded during this event may lead to mechanical damage, compromised placement accuracy, and control issues (i.e., due to sensory system physical failure).
- MH2: Collision between the mobile platform and the patient body. During a collision between the MP and the patient, the patient’s tissue may undergo deformation and damage. This has a detrimental impact on the procedure safety as it can result in improper positioning of the laparoscope and active instruments due to the displacement of their RCMs.
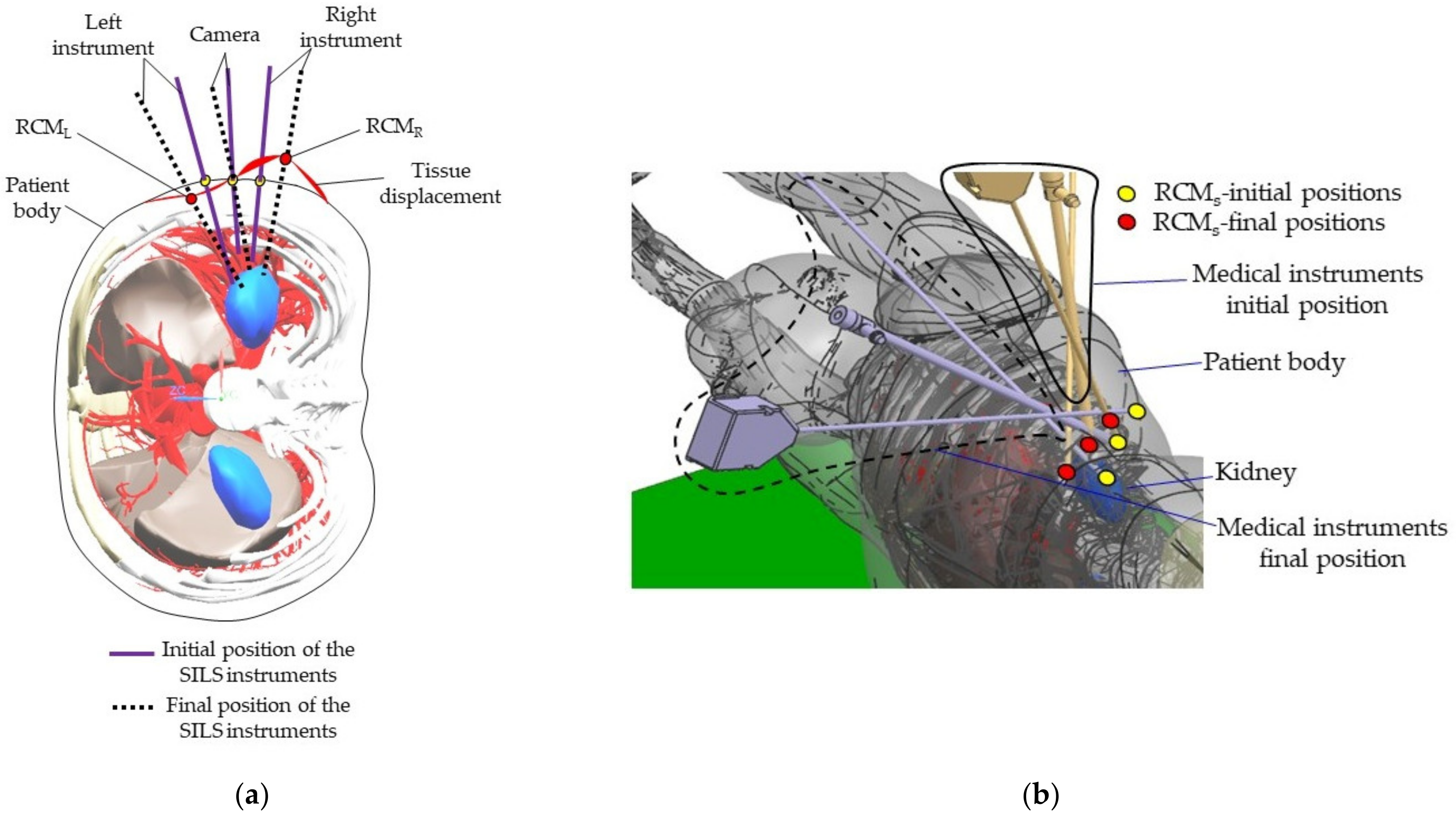
- MH3: Patient tissue may experience excessive strain due to the displacement of the Remote Center of Motion when orienting the endoscopic camera. As mentioned in Section 2.2, the orientation of the endoscopic camera is achieved using the PARA-SILSROB parallel robot, which possesses 6-DOF. Consequently, the architecturally constrained RCMs of the two modules guiding the SILS instruments will shift in accordance with the camera’s orientation angle. Section 2.7 presents an experimental setup designed to evaluate the risk of the RCM displacement for the instruments during the SILS procedure.
- Electrical hazards:
- EH1: Risk of power outage.
- EH2: Risk of electrocution of the patient. Improper grounding may lead to such risks.
- EH3: Risk of short circuit.
- EH4: Risk of sensors system malfunction.
- EH5: Risk of communication protocol malfunction (cabling issues).
- Thermal hazard:
- TH1: Tissue burns caused by wrong manipulation of active instruments (Permanent Cautery Hook).
- TH2: Burns caused by overheating of the active instruments (Permanent Cautery Hook).
- Vibration hazards:
- VH1: High level of tremor at the tip of the instruments (and the laparoscope) due to vibration modalities of the robotic system.
- Noise hazard:
- NH1: Acoustic discomfort caused by driving the motion of the mobile components when the robotic system is operated.
- High: over 151;
- Medium: 101–150;
- Low: 61–100;
- Negligible: 0–50.
2.6. Failure Mode and Effects Analysis for the PARA-SILSROB System
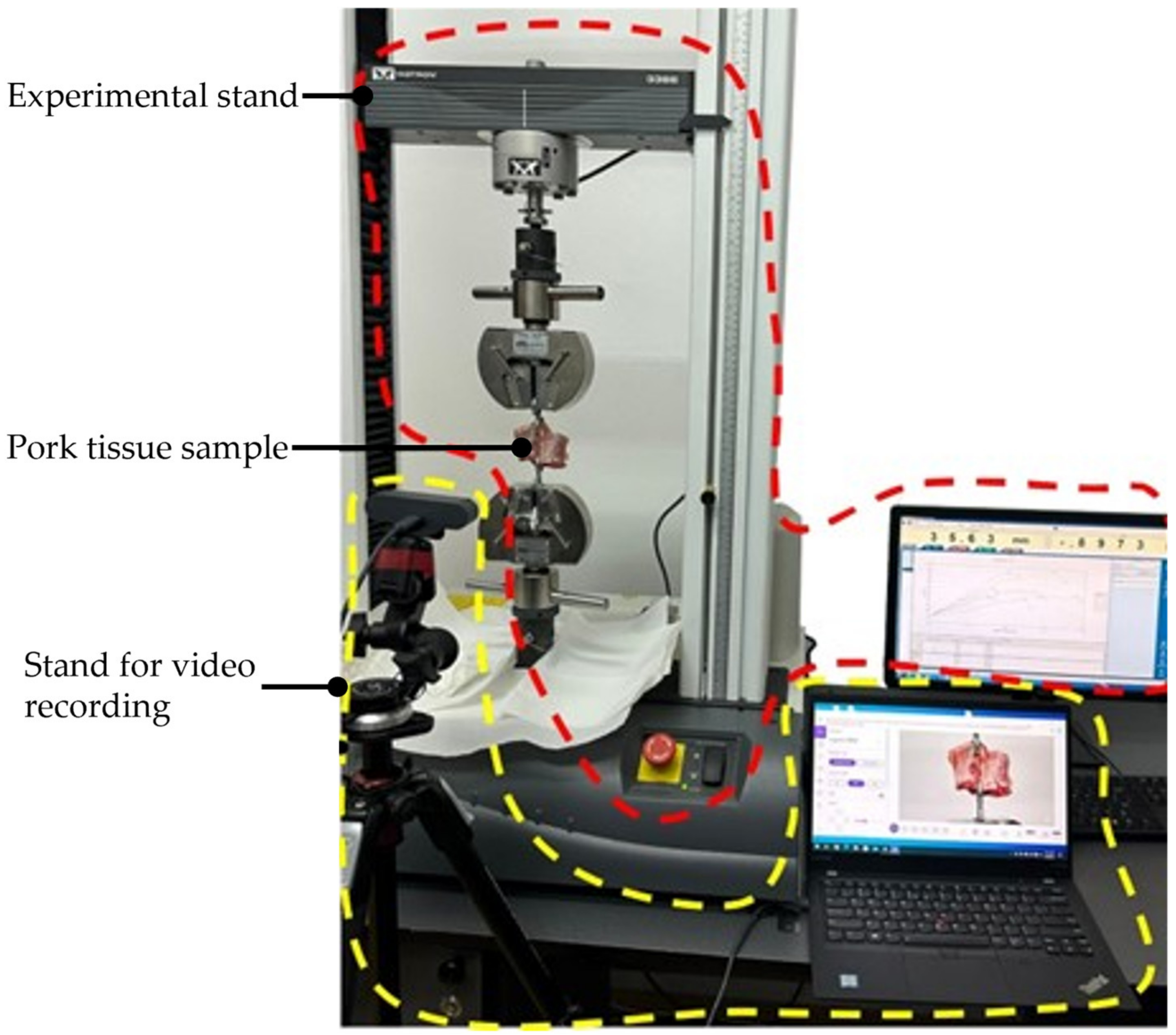
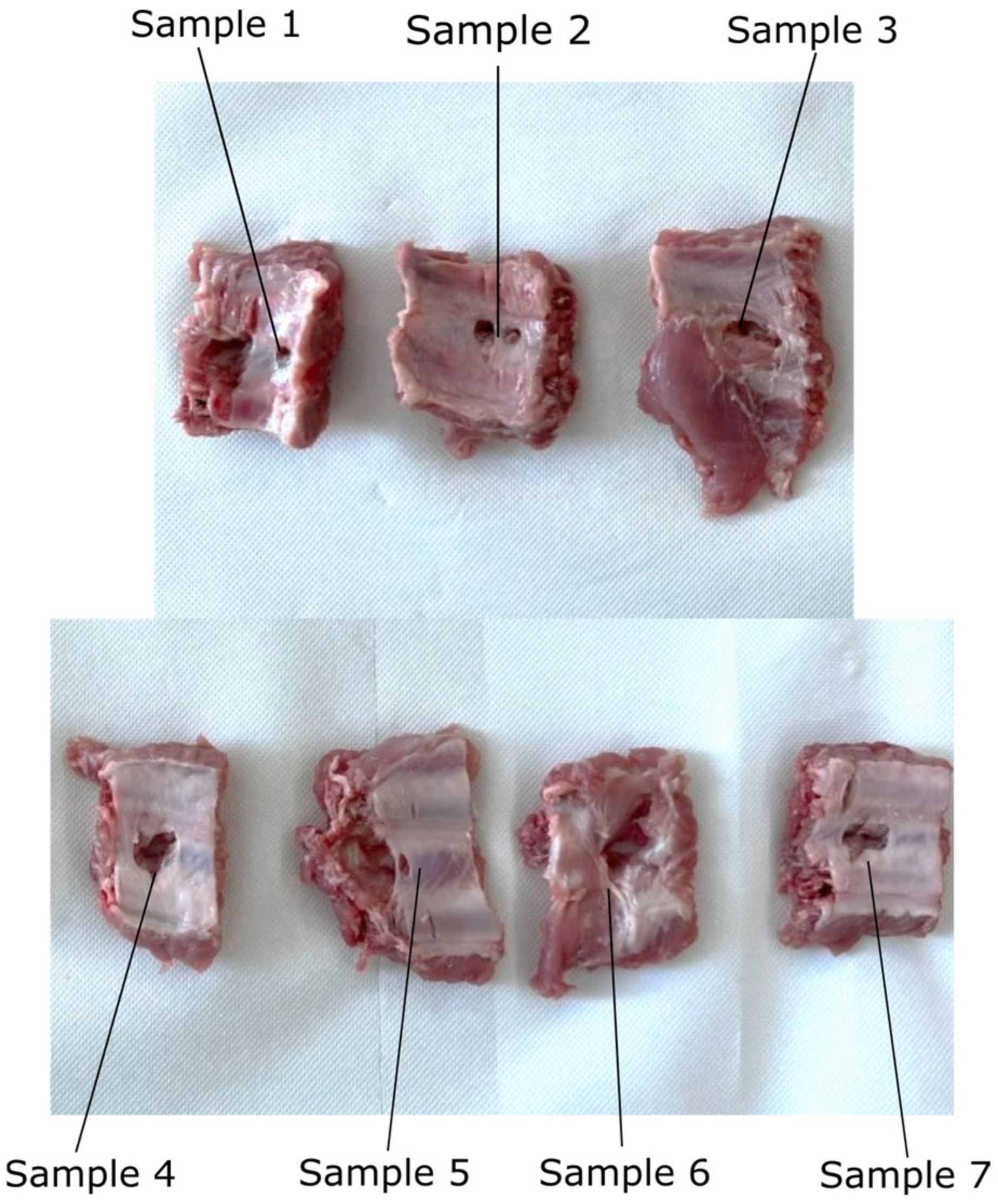
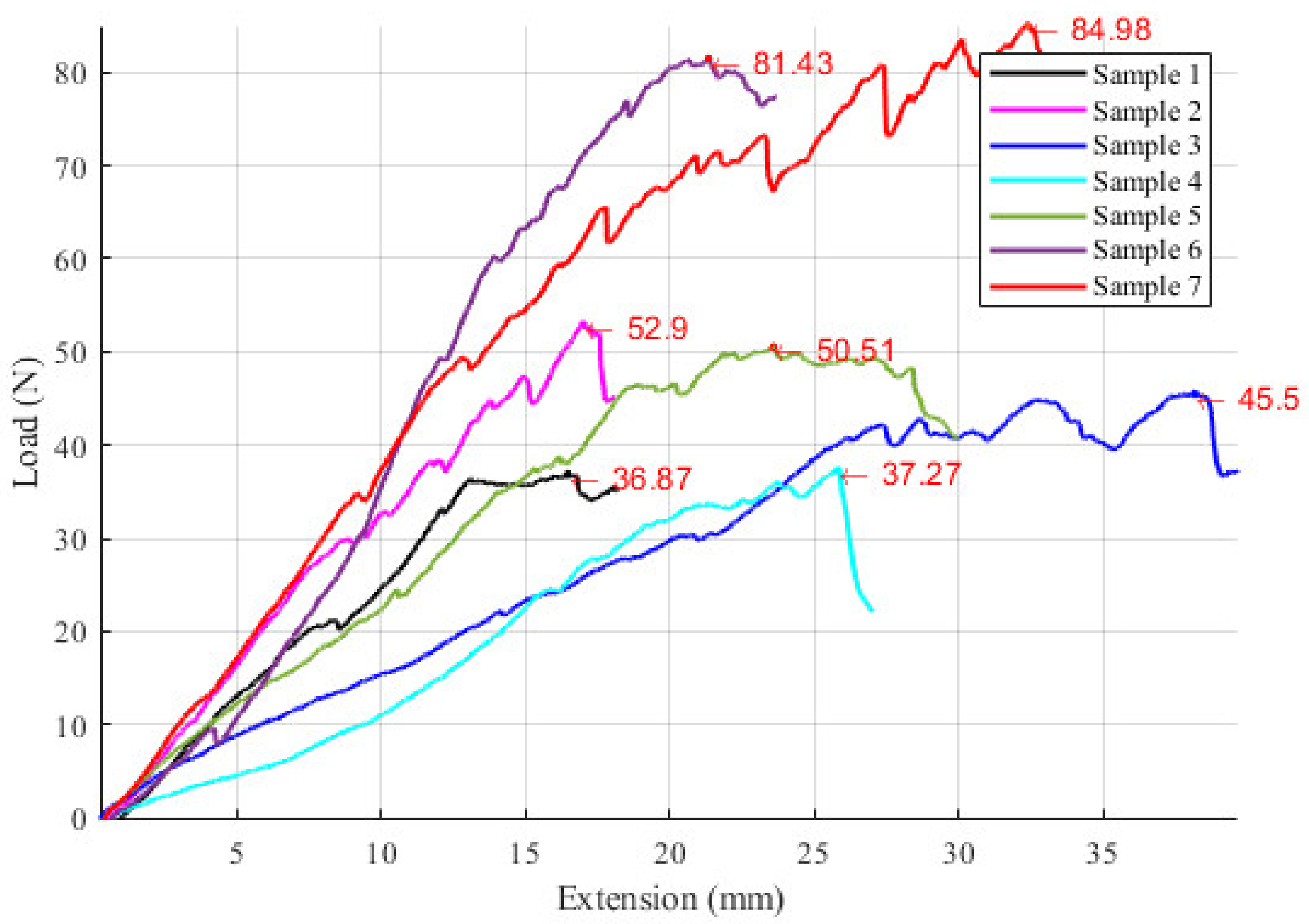
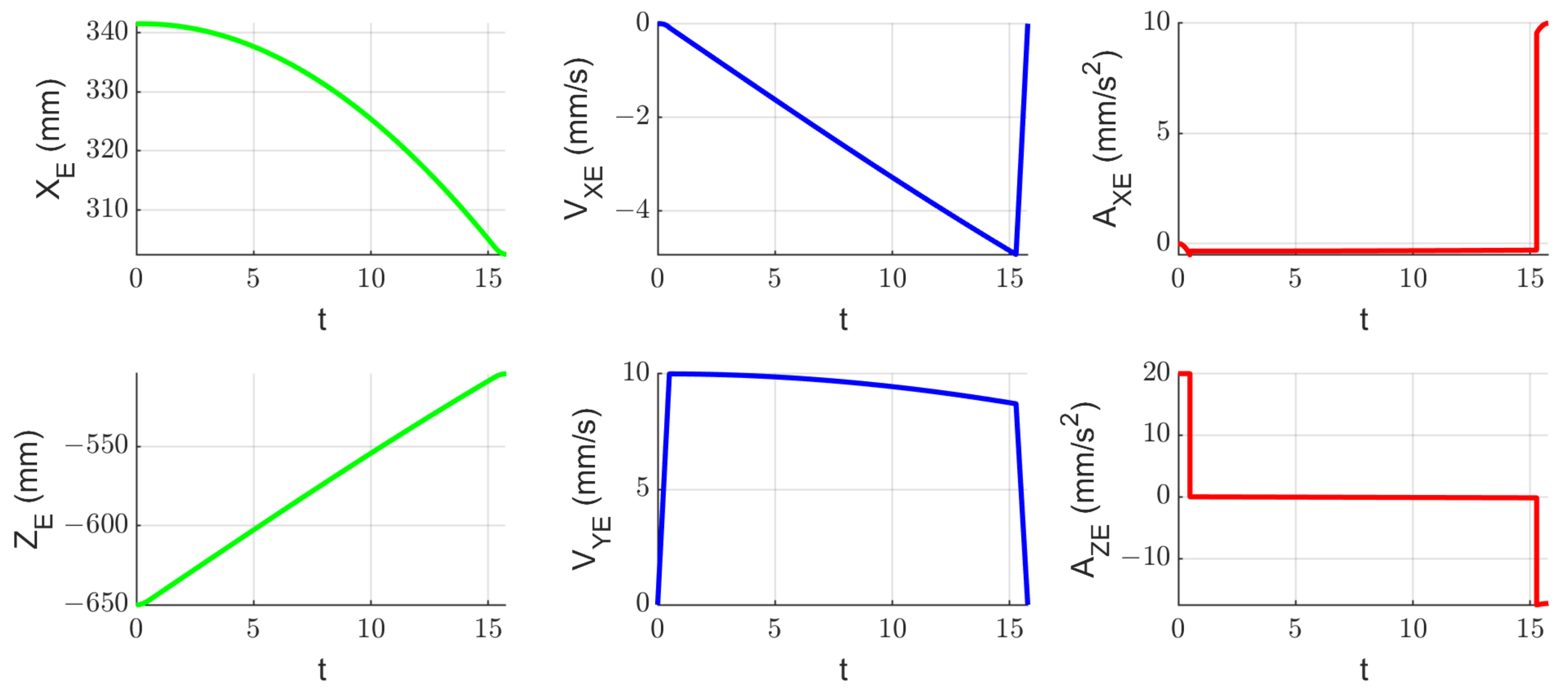
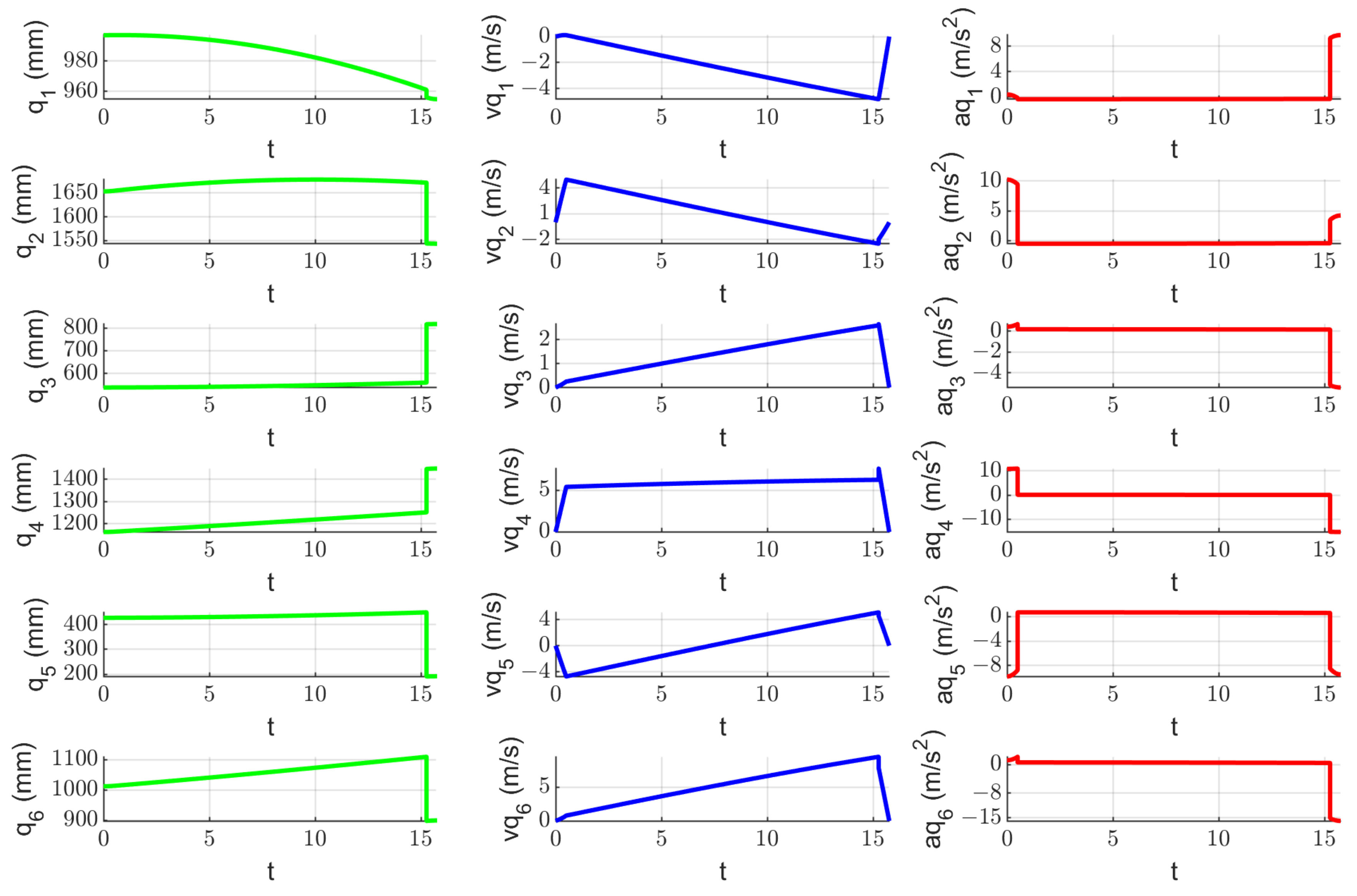
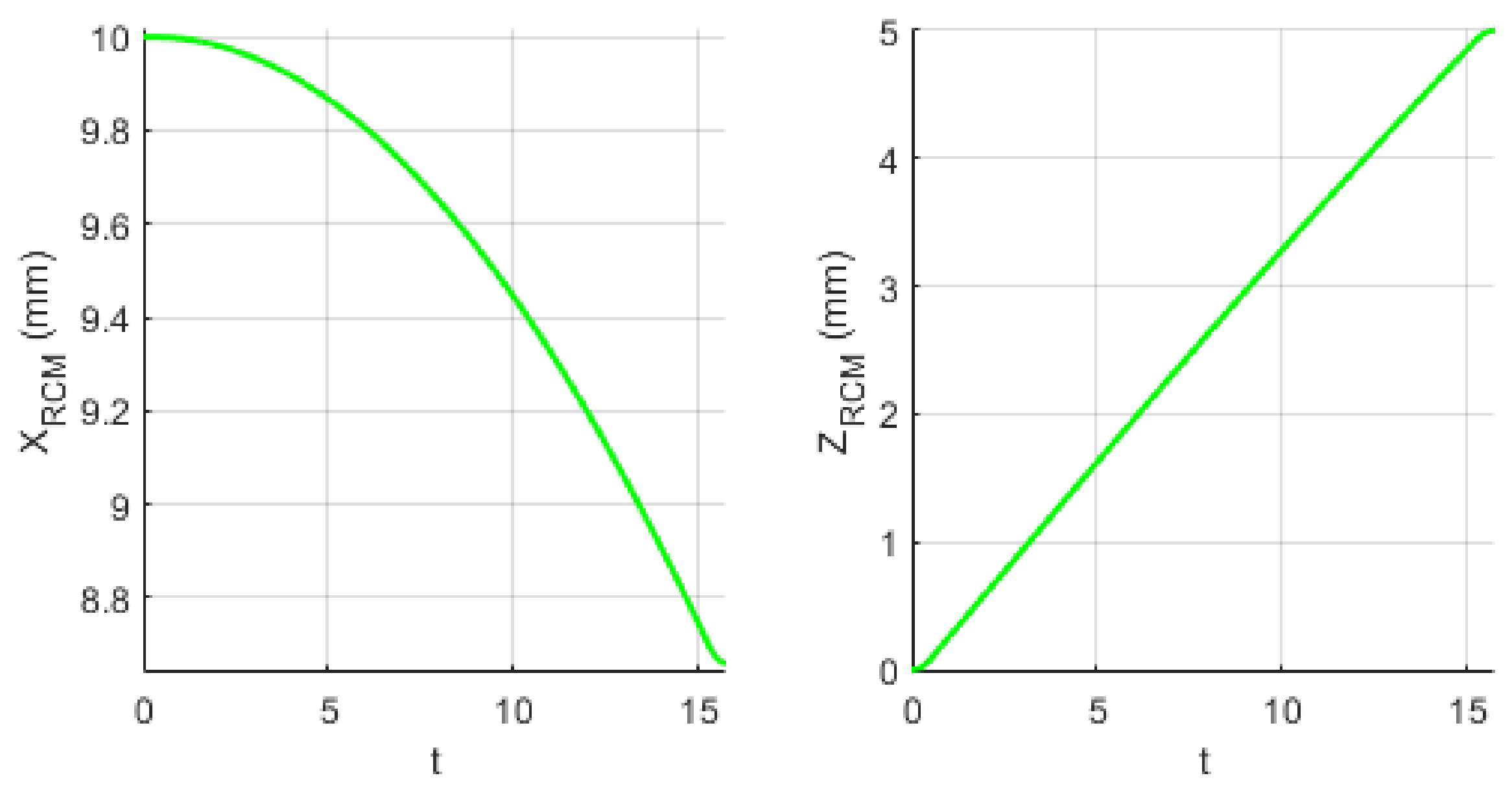
2.7. Experimental Tests to Assess the Instruments RCM Displacement Risk (MH3)
3. Results
3.1. Risk Reduction Solutions for the PARA-SILSROB System
3.2. Results of FMEA for PARA-SILSROB System
3.3. Results Regarding the Experimental Tests for the Instruments RCM Displacement Risk (MH3)
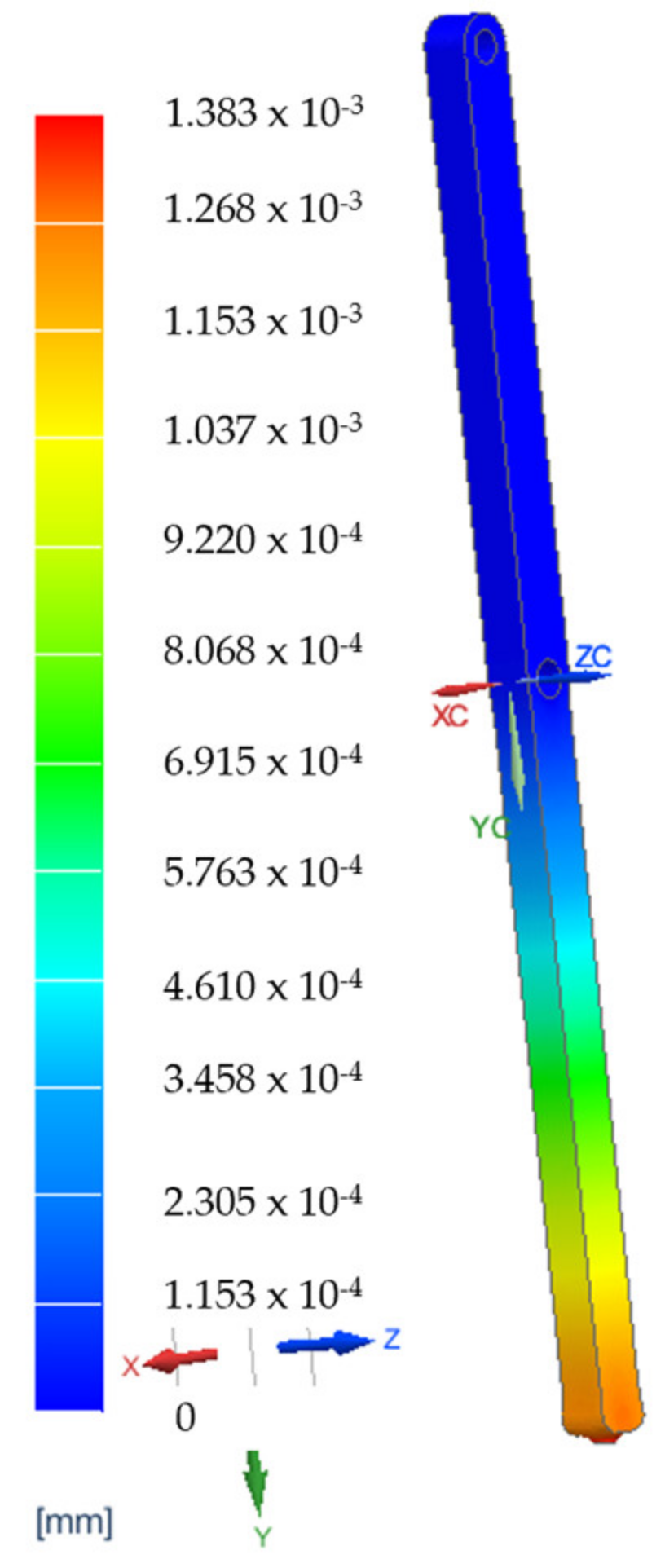
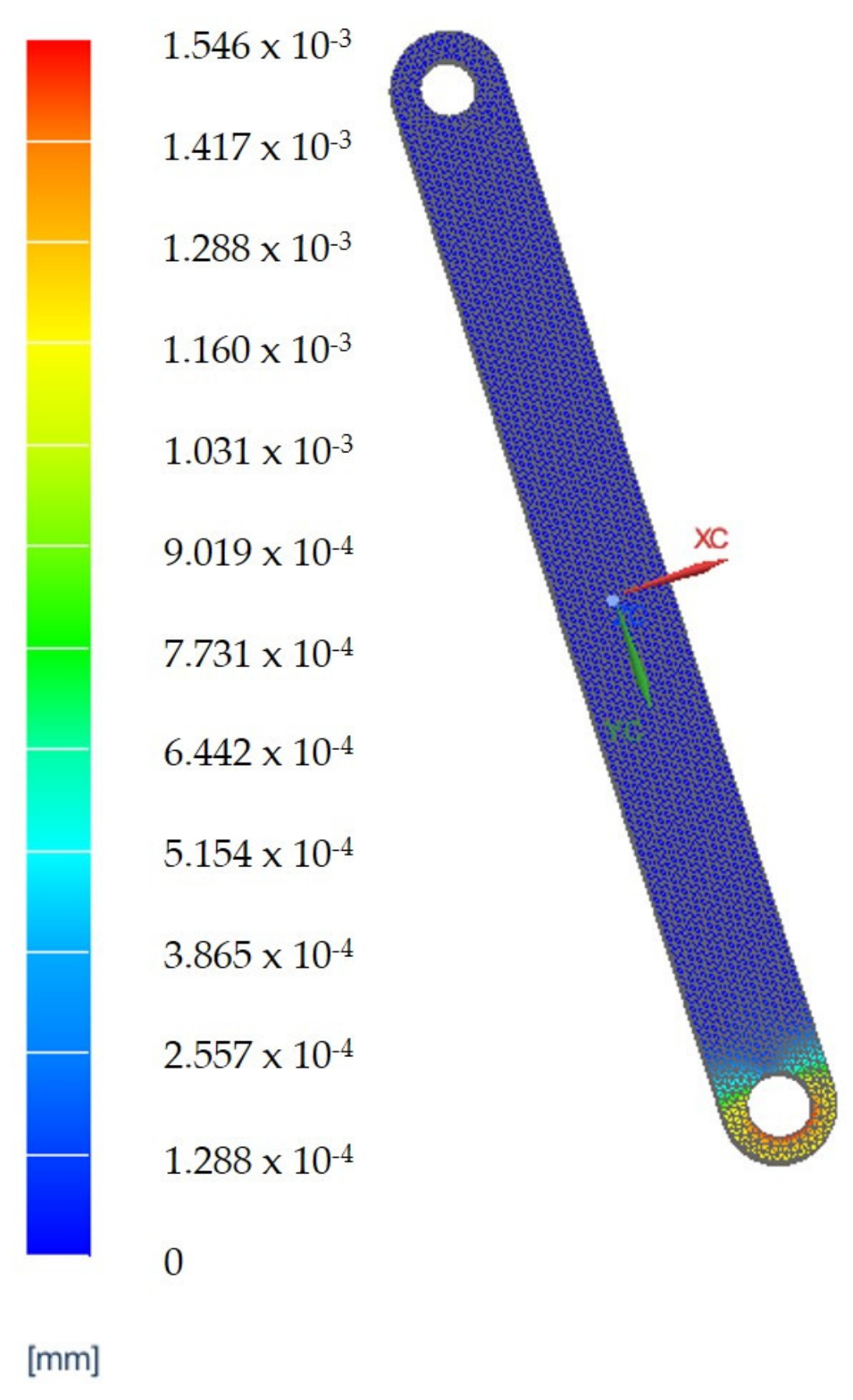
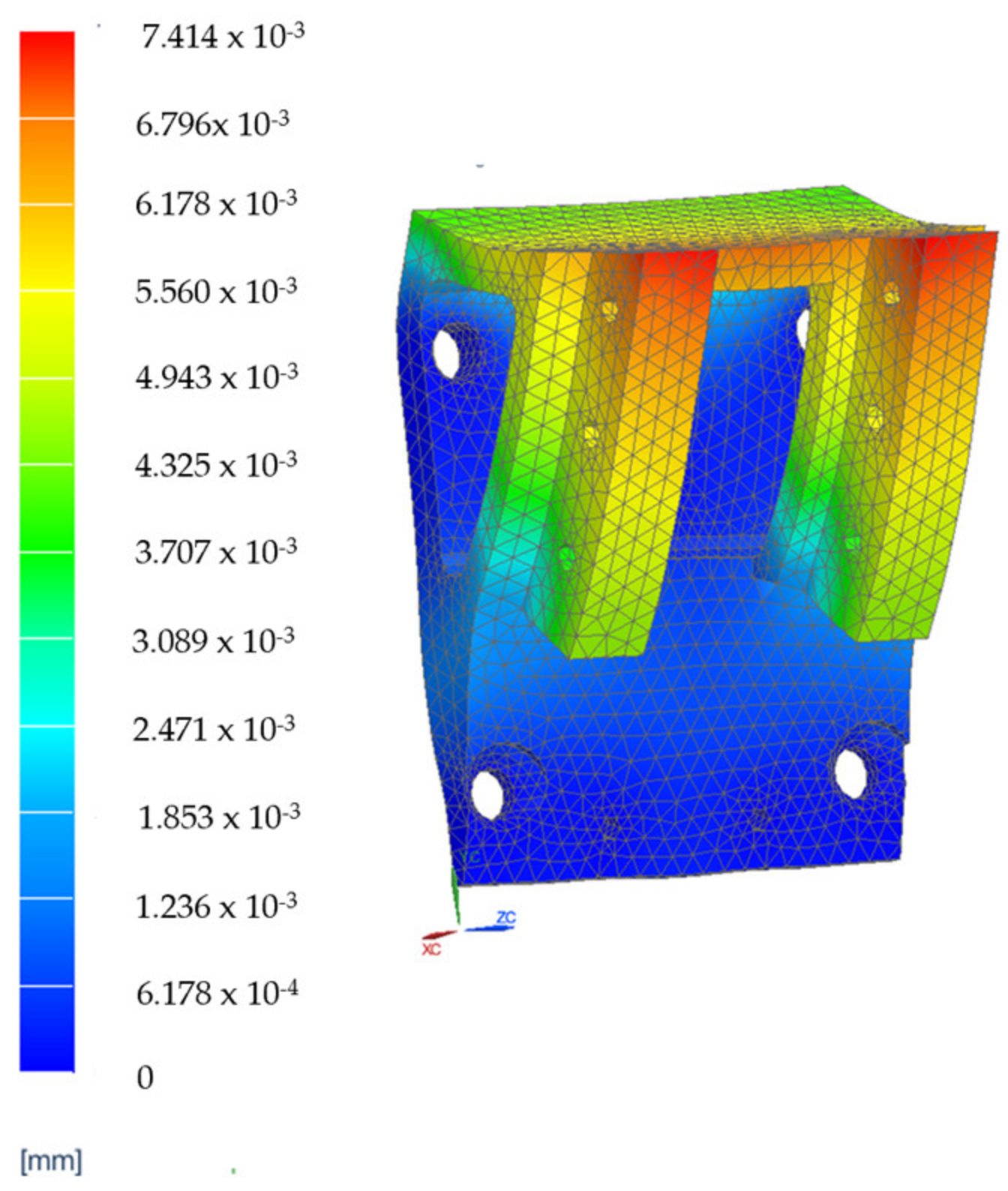
3.4. Finite Element Analysis Results for the Main Component of the PARA-SILSROB System
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Questionnaire
| Catastrophic [100] | Serious [90–99] | Moderate [30–89] | Minor [0–29] | ||
|---|---|---|---|---|---|
| MH1 | Robotic arms crushing due to wrong motion of the linear actuating joints (this hazard can be created if the robot is wrongly programmed) | ||||
| MH2 | Cuts/scratches caused by wrong insertion of the instruments and the endoscopic camera in the patient body (this hazard can be created through incorrect definition of the insertion point in the patient’s body or incorrect assessment of the patient’s anthropomorphic characteristics) | ||||
| MH3 | Impact between the mobile platform and the patient body (this hazard can be created if the surgeon wrongly operates the master console) | ||||
| MH4 | The mechanical locking of modules used for handling active instruments or the mobile platform when the endoscopic camera is reoriented (this hazard can be created by incorrect control of the master console or improper programming of the robotic system) | ||||
| MH5 | Impact between the active instruments and the endoscopic camera (this hazard can be created due to a malfunction of orientation modules attached to the mobile platform or improper handling of active instruments by the surgeon using the master console) | ||||
| MH6 | Locking of the active translational joints (this hazard can be created by overloading of the robot structure or master console malfunction) | ||||
| MH7 | Over the limit movements of the robot arms and the mobile platform (this hazard can be created if the robotic system is wrongly teleoperated) | ||||
| MH8 | Cuts and scratches caused by edges of the metal parts (this hazard can be created by faulty design and manufacturing of the robotic structure and may occur when the robot is sterilized) | ||||
| EH1 | Risk of power outage. | ||||
| EH2 | Risk of electrocution of the patient. | ||||
| EH3 | Motors in overload. | ||||
| EH4 | Risk of short circuit. | ||||
| EH5 | Risk of sensors system malfunction. | ||||
| EH6 | Risk of communication protocol malfunction. | ||||
| TH1 | Tissue burns caused by wrong manipulation of active instruments (Permanent Cautery Hook). | ||||
| TH2 | Burns caused by overheating of the active instruments (Permanent Cautery Hook). | ||||
| VH1 | Patient harm by robot structure entry in singularity positions. | ||||
| VH2 | Patient harm caused by uncontrolled vibrations of active instruments and the endoscopic camera generated by malfunctions of the modules attached to the mobile platform or overloading of the active instruments. | ||||
| NH1 | Acoustic discomfort caused by driving the motion of the mobile components when the robotic system is operated. | ||||
| ERH1 | Risk to the medical act failing (this hazard can be created when the surgeon is improperly positioned at the master console) |
| Very Likely [100] | Likely [90–99] | Unlikely [30–89] | Remote [0–29] | ||
|---|---|---|---|---|---|
| MH1 | Robotic arms crushing due to wrong motion of the linear actuating joints (this hazard can be created if the robot is wrongly programmed) | ||||
| MH2 | Cuts/scratches caused by wrong insertion of the instruments and the endoscopic camera in the patient body (this hazard can be created through incorrect definition of the insertion point in the patient’s body or incorrect assessment of the patient’s anthropomorphic characteristics) | ||||
| MH3 | Impact between the mobile platform and the patient body (this hazard can be created if the surgeon wrongly operates the master console) | ||||
| MH4 | The mechanical locking of modules used for handling active instruments or the mobile platform when the endoscopic camera is reoriented (this hazard can be created by incorrect control of the master console or improper programming of the robotic system) | ||||
| MH5 | Impact between the active instruments and the endoscopic camera (this hazard can be created due to a malfunction of orientation modules attached to the mobile platform or improper handling of active instruments by the surgeon using the master console) | ||||
| MH6 | Locking of the active translational joints (this hazard can be created by overloading of the robot structure or master console malfunction) | ||||
| MH7 | Over the limit movements of the robot arms and the mobile platform (this hazard can be created if the robotic system is wrongly teleoperated) | ||||
| MH8 | Cuts and scratches caused by edges of the metal parts (this hazard can be created by faulty design and manufacturing of the robotic structure and may occur when the robot is sterilized) | ||||
| EH1 | Risk of power outage. | ||||
| EH2 | Risk of electrocution of the patient. | ||||
| EH3 | Motors in overload. | ||||
| EH4 | Risk of short circuit. | ||||
| EH5 | Risk of sensors system malfunction. | ||||
| EH6 | Risk of communication protocol malfunction. | ||||
| TH1 | Tissue burns caused by wrong manipulation of active instruments (Permanent Cautery Hook). | ||||
| TH2 | Burns caused by overheating of the active instruments (Permanent Cautery Hook). | ||||
| VH1 | Patient harm by robot structure entry in singularity positions. | ||||
| VH2 | Patient harm caused by uncontrolled vibrations of active instruments and the endoscopic camera generated by malfunctions of the modules attached to the mobile platform or overloading of the active instruments. | ||||
| NH1 | Acoustic discomfort caused by driving the motion of the mobile components when the robotic system is operated. | ||||
| ERH1 | Risk to the medical act failing (this hazard can be created when the surgeon is improperly positioned at the master console) |
References
- Saidy, M.N.; Tessier, M.; Tessier, D. Single-incision laparoscopic surgery-hype or reality: A historical control study. Perm. J. 2012, 16, 47–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuang, S.H.; Chuang, S.C. Single-incision laparoscopic surgery to treat hepatopancreatobiliary cancer: A technical review. World J. Gastroenterol. 2022, 28, 3359–3369. [Google Scholar] [CrossRef] [PubMed]
- Kiblawi, R.; Zoeller, C.; Zanini, A.; Kuebler, J.F.; Dingemann, C.; Ure, B.; Schukfeh, N. Laparoscopic versus Open Pediatric Surgery: Three Decades of Comparative Studies. Eur. J. Pediatr. Surg. 2022, 32, 9–25. [Google Scholar] [CrossRef]
- Vlădescu, C.; Copăescu, C. The Use of Minimal Invasive Surgery versus Open Approach in Hospitalized Cases. Chirurgia 2021, 116, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rieder, E.; Swanstrom, L.L. Advances in cancer surgery: Natural orifice surgery (NOTES) for oncological diseases. Surg. Oncol. 2011, 20, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Mouret, P. How I developed laparoscopic cholecystectomy. Ann. Acad. Med. Singap. 1996, 25, 744–747. [Google Scholar] [PubMed]
- Litynski, G.S. Erich Muhe and the rejection of laparoscopic cholecystectomy (1985): A surgeon ahead of his time. J. Soc. Laparoendosc. Surg. 1998, 2, 341–346. [Google Scholar]
- Vaida, C.; Andras, I.; Birlescu, I.; Crisan, N.; Plitea, N.; Pisla, D. Preliminary control design of a Single-Incision Laparoscopic Surgery Robotic System. In Proceedings of the 2021 25th International Conference on System Theory, Control and Computing (ICSTCC), Iasi, Romania, 20–23 October 2021; pp. 384–389. [Google Scholar] [CrossRef]
- Gherman, B.; Vaida, C.; Pisla, D.; Plitea, N.; Gyurka, B.; Lese, D.; Glogoveanu, M. Singularities and Workspace Analysis for a Parallel Robot for Minimally Invasive Surgery. In Proceedings of the 2010 IEEE International Conference on Automation, Quality and Testing, Robotics (AQTR), Cluj-Napoca, Romania, 28–30 May 2010; pp. 1–6. [Google Scholar] [CrossRef]
- Alimoglu, O.; Sagiroglu, J.; Atak, I.; Kilic, A.; Eren, T.; Caliskan, M.; Bas, G. Robot-assisted laparoscopic (RAL) procedures in general surgery. Int. J. Med. Robot. 2016, 12, 427–430. [Google Scholar] [CrossRef]
- Plewe, J.M.; Wabitsch, S.; Krenzien, F.; Haber, P.; Denecke, T.; Gebauer, B.; Öllinger, R.; Pratschke, J.; Schmelzle, M. Single-incision laparoscopic surgery portal vein embolisation before extended hepatectomy. J. Minim. Access Surg. 2020, 16, 185–189. [Google Scholar] [CrossRef]
- Wang, W.; Sun, X.; Wei, F. Laparoscopic surgery and robotic surgery for single-incision cholecystectomy: An updated systematic review. Updat. Surg. 2021, 73, 2039–2046. [Google Scholar] [CrossRef]
- Antoniou, S.A.; Morales-Conde, S.; Antoniou, G.A.; Granderath, F.A.; Berrevoet, F.; Muysoms, F.E.; The Bonham Group. Single-incision laparoscopic surgery through the umbilicus is associated with a higher incidence of trocar-site hernia than conventional laparoscopy: A meta-analysis of randomized controlled trials. Hernia 2016, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Runge, J.J. Single-Incision Laparoscopic Surgery. In Small Animal Laparoscopy and Thoracoscopy; Wiley: Hoboken, NJ, USA, 2015; pp. 65–72. [Google Scholar] [CrossRef]
- Arkenbout, E.A.; Henselmans, P.W.J.; Jelínek, F.; Breedveld, P. A state of the art review and categorization of multi-branched instruments for NOTES and SILS. Surg. Endosc. 2015, 29, 1281–1296. [Google Scholar] [CrossRef] [PubMed]
- Karabicak, I.; Karabulut, K. Single port laparoscopic liver surgery: A minireview. World J. Gastrointest. Endosc. 2016, 8, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Parekattil, S.J.; Moran, M.E. Robotic instrumentation: Evolution and microsurgical applications. Indian J. Urol. 2010, 26, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Leal Ghezzi, T.; Campos Corleta, O. 30 Years of Robotic Surgery. World J. Surg. 2016, 40, 2550–2557. [Google Scholar] [CrossRef]
- Liu, R.R.; Wandeto, J.; Nageotte, F.; Zanne, P.; de Mathelin, M.; Dresp-Langley, B. Spatiotemporal Modeling of Grip Forces Captures Proficiency in Manual Robot Control. Bioengineering 2023, 10, 59. [Google Scholar] [CrossRef]
- Farooq, M.U.; Ko, S.Y. An automated Extracorporeal Knot-tying System Using Two Concentric Tube Robotic Arms for Deployment through a 30 mm Port. Int. J. Control Autom. Syst. 2020, 18, 2–9. [Google Scholar] [CrossRef]
- Datta, R.R.; Dieplinger, G.; Wahba, R.; Kleinert, R.; Thomas, M.; Gebauer, F.; Schiffmann, L.; Stippel, D.L.; Bruns, C.J.; Fuchs, H.F. True single-port cholecystectomy with ICG cholangiography through a single 15-mm trocar using the new surgical platform ”symphonX”: First case study with commercialy available device. Surg. Endosc. Other Interv. Tech. 2019, 34, 2722–2729. [Google Scholar] [CrossRef]
- Pisla, D.; Birlescu, I.; Crisan, N.; Pusca, A.; Andras, I.; Tucan, P.; Radu, C.; Gherman, B.; Vaida, C. Singularity Analysis and Geometric Optimization of a 6-DOF Parallel Robot for SILS. Machines 2022, 10, 764. [Google Scholar] [CrossRef]
- Pisla, D.; Andras, I.; Vaida, C.; Crisan, N.; Ulinici, I.; Birlescu, I.; Plitea, N. New Approach to Hybrid Robotic System Application in Single Incision Laparoscopic Surgery. Acta Tech. Napoc. 2021, 64, 369–378. [Google Scholar]
- Chen, Y.; Zhang, S.; Wu, Z.; Yang, B.; Luo, Q.; Xu, K. Review of surgical robotic systems for keyhole and endoscopic procedures: State of the art and perspectives. Front Med. 2020, 14, 382–403. [Google Scholar] [CrossRef] [PubMed]
- Piozzi, G.N.; Kim, J.S.; Choo, J.M. şi colab. Abordarea robotică Da Vinci SP a chirurgiei colorectale: Două indicații specifice și rezultate pe termen scurt. Tech. Coloproctol. 2022, 26, 461–470. [Google Scholar] [CrossRef]
- Pisla, D.; Plitea, N.; Gherman, B.; Vaida, C.; Pisla, A.; Suciu, M. Kinematics and design of a 5-DOF parallel robot used in minimally invasive surgery. In Advances in Robot Kinematics: Motion in Man and Machine; Springer: Berlin/Heidelberg, Germany, 2010; pp. 99–106. [Google Scholar]
- Harky, A.; Chaplin, G.; Chan, J.S.; Eriksen, P.; MacCarthy-Ofosu, B.; Theologou, T.; Muir, A.D. The Future of Open Heart Surgery in the Era of Robotic and Minimal Surgical Interventions. Heart Lung Circ. 2022, 29, 49–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Covas Moschovas, M.; Bhat, S.; Rogers, T.; Reddy, S.; Noel, J.; Corder, C.; Patel, V. Da Vinci SP platform updates and modifications: The first impression of new settings. J. Robot. Surg. 2021, 15, 977–979. [Google Scholar] [CrossRef]
- Pisla, D.; Gherman, B.; Tucan, P.; Birlescu, I.; Pusca, A.; Rus, G.; Pisla, A.; Vaida, C. Application Oriented Modelling and Simulation of an Innovative Parallel Robot for Single Incision Laparoscopic Surgery. In Proceedings of the ASME 2022 International Design Engineering Technical Conferences and Computers and Information in Engineering Conference, Volume 7: 46th Mechanisms and Robotics Conference (MR), St. Louis, MO, USA, 14–17 August 2022. [Google Scholar]
- Alip, S.L.; Kim, J.; Rha, K.H.; Han, W.K. Future Platforms of Robotic Surgery. Urol. Clin. N. Am. 2022, 49, 23–38. [Google Scholar] [CrossRef]
- Bindi, E.; Todesco, C.; Nino, F.; Torino, G.; Gentilucci, G.; Cobellis, G. Robotic Surgery: Is There a Possibility of Increasing Its Application in Pediatric Settings? A Single-Center Experience. Children 2022, 9, 1021. [Google Scholar] [CrossRef]
- Molinaro, F.; Krasniqi, P.; Scolletta, S.; Giuntini, L.; Navarra, C.; Puzzutiello, R.; Fusi, G.; Angotti, R.; Bindi, E.; Zanaboni, C.; et al. Considerations regarding pain management and anesthesiological aspects in pediatric patients undergoing minimally invasive surgery: Robotic vs laparoscopic-thoracoscopic approach. J. Robot. Surg. 2020, 14, 423–430. [Google Scholar] [CrossRef]
- Sang, H.; Wang, S.; Li, J.; He, C.; Zhang, L.; Wang, X. Control design and implementation of a novel master-slave surgery robot system, MicroHand A. Int. J. Med. Robot. 2011, 7, 334–347. [Google Scholar] [CrossRef]
- Pisla, D.; Birlescu, I.; Pusca, A.; Tucan, P.; Gherman, B.; Vaida, C. Kinematics and Workspace Analysis of an Innovative 6-Dof Parallel Robot for SILS. In Proceedings of the Romanian Academy; Series A; The Publishing House of the Romanian Academy: Bucharest, Romania, 2022; Volume 23, pp. 277–286. [Google Scholar]
- Pisla, D.; Andras, I.; Rus, G.; Moldovanu, C.; Crisan, N.; Antal, T.; Vaida, C. U-net Network Optimization For 3D Reconstruction in Robotic SILS Pre-Planning Phase. In Proceedings of the MEDITECH, Advancements of Medicine and Health Care Through Technology 2022, Cluj-Napoca, Romania, 20–22 October 2022. [Google Scholar]
- Moschovas, M.C.; Seetharam Bhat, K.R.; Onol, F.F.; Rogers, T.; Ogaya-Pinies, G.; Roof, S.; Patel, V.R. Single-port technique evolution and current practice in urologic procedures. Asian J. Urol. 2021, 8, 100–104. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, H.; Feng, M.; Zhao, J.; Fu, Y. New remote centre of motion mechanism for robot-assisted minimally invasive surgery. BioMed. Eng. OnLine 2018, 17, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaida, C.; Birlescu, I.; Pusca, A.; Gherman, B.; Tucan, P.; Antal, T.A.; Pisla, D. Geometric Modeling of a New Modular Spherical Robotic System for Single Incision Laparoscopic Surgery. In Advances in Service and Industrial Robotics; Müller, A., Brandstötter, M., Eds.; RAAD 2022; Mechanisms and Machine Science Book Series; Springer: Cham, Switzerland, 2022; Volume 120. [Google Scholar]
- Vaida, C.; Rus, G.; Gherman, B.; Pusca, A.; Tucan, P.; Ulinici, I.; Pisla, D. Development of an augmented reality simulator for a robotic system used in single incision laparoscopic surgery. Rom. J. Tech. Sci. Appl. Mech. 2023, 68, 3–19. [Google Scholar] [CrossRef]
- Pisla, D.; Birlescu, I.; Vaida, C.; Tucan, P.; Gherman, B.; Plitea, N. Family of modular parallel robots with active translational joints for Single Incision Laparoscopic Surgery. Patent No. OSIM A00733/03.12.2021, 3 December 2021. [Google Scholar]
- International Organization for Standardization (ISO:31000). Available online: https://www.iso.org/iso-31000-risk-management.html (accessed on 4 April 2023).
- Meyer, T.; Reniers, G. Engineering Risk Management; De Gruyter: Berlin, Germany; Boston, MA, USA, 2022. [Google Scholar]
- International Organization for Standardization (ISO 14971:2019). Available online: https://www.iso.org/standard/72704.html (accessed on 6 April 2023).
- Tucan, P.; Vaida, C.; Plitea, N.; Pisla, A.; Carbone, G.; Pisla, D. Risk-Based Assessment Engineering of a Parallel Robot Used in Post-Stroke Upper Limb Rehabilitation. Sustainability 2019, 11, 2893. [Google Scholar] [CrossRef] [Green Version]
- Pisla, D.; Calin, V.; Birlescu, I.; Hajjar, N.A.; Gherman, B.; Radu, C.; Plitea, N. Risk Management for the Reliability of Robotic Assisted Treatment of Non-resectable Liver Tumors. Appl. Sci. 2020, 10, 52. [Google Scholar] [CrossRef] [Green Version]
- Buescu, C.T.; Dudescu, C.M.; Caterev, S.; Lucaciu, D.G.O. Double whip stitch for free graft femoral fixation in ACL reconstruction: Experimental study on porcine model. Clujul Med. 2018, 91, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
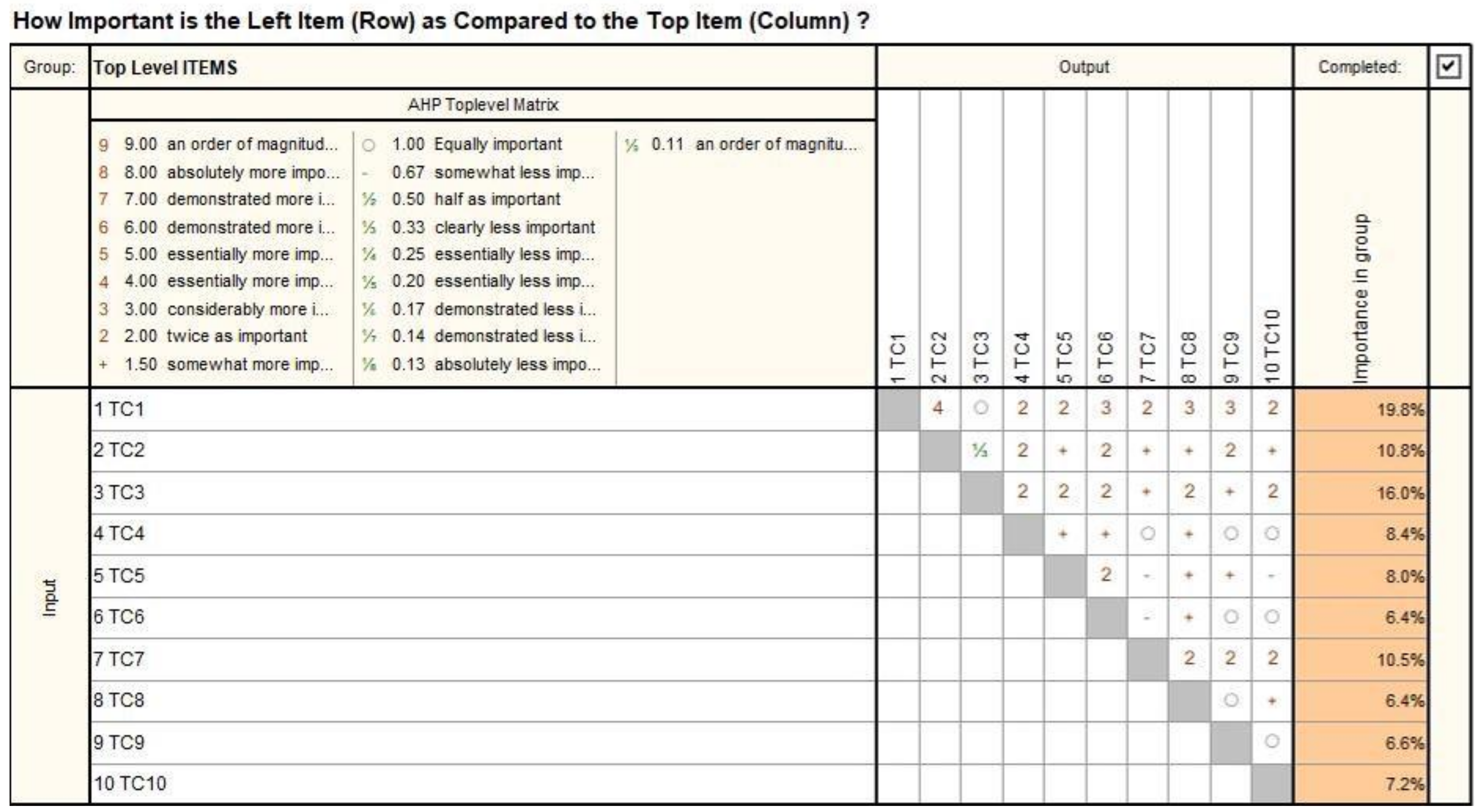




| Codification | Technical Characteristics |
|---|---|
| TC1 | Safety. The robotic-assisted SILS procedure needs to be performed in completely safe conditions for the patient. |
| TC2 | Versatility. Although the SILS procedure is usually limited to the umbilicus area, it should be compliant with other approaches as well. In this paper, the robotic proposed concept targets kidney treatment using the retroperitoneal approach. |
| TC3 | Emergency compliance. The system and its components (instruments, modules) must be quickly removable from the operation site in case of emergency. |
| TC4 | Stiffness. Since stiffness is closely related to the positioning accuracy of surgical instruments, a stiff architecture and mechanical structure is desirable. |
| TC5 | Fast initialization procedure. A setup time of 25–35 min is specified in [28,31,32]. Placing the SILS instruments into a commercially available SILS port designed for manual intervention is not always an easy task, which is why the system should be fitted with additional devices to speed up the setup time. |
| TC6 | Sterilization. The instruments need to be easily sterilizable, while the non-sterile equipment should be isolated from the operating field. |
| TC7 | Collisions avoidance. The robotic systems modules should not collide during the intervention. Instruments collision might be inevitable, but the surgeon should be notified regarding this event. |
| TC8 | Fit into the operating room. The robotic system should fit into the existing operating rooms, considering the other medical equipment required in a SILS procedure. |
| TC9 | Tremor management. The tremor recorded at the tip of the SILS instruments has various sources. One of them is the natural vibration of the robot during the SILS instrument positioning. This aspect should be controlled well during the design phase. |
| TC10 | Reachability. The tip of the medical instruments should be able to reach the lesion area with accuracy. This further means that the insertion length of the instruments should not negatively affect the positioning accuracy and the motion resolution [22,28]. |
| Hazard | E1 | E2 | E3 | E4 | E5 | E6 | E7 | E8 | E9 | E10 | Mean Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MH1 | 98 | 100 | 98 | 100 | 99 | 100 | 90 | 96 | 98 | 100 | 97.9 |
| MH2 | 93 | 98 | 95 | 98 | 96 | 90 | 98 | 99 | 100 | 90 | 95.7 |
| MH3 | 97 | 100 | 100 | 90 | 98 | 95 | 100 | 98 | 95 | 98 | 97.1 |
| EH1 | 95 | 90 | 95 | 95 | 90 | 95 | 90 | 90 | 98 | 94 | 93.2 |
| EH2 | 99 | 99 | 96 | 98 | 95 | 96 | 98 | 100 | 98 | 95 | 97.4 |
| EH3 | 55 | 70 | 55 | 65 | 40 | 45 | 55 | 35 | 60 | 55 | 53.5 |
| EH4 | 80 | 90 | 80 | 75 | 85 | 70 | 75 | 60 | 70 | 70 | 75.5 |
| EH5 | 95 | 95 | 97 | 94 | 98 | 95 | 95 | 98 | 95 | 97 | 95.9 |
| TH1 | 96 | 90 | 95 | 97 | 95 | 85 | 98 | 95 | 90 | 95 | 93.6 |
| TH2 | 90 | 95 | 80 | 88 | 89 | 95 | 85 | 90 | 87 | 95 | 89.4 |
| VH1 | 99 | 95 | 100 | 97 | 95 | 90 | 95 | 99 | 95 | 90 | 95.5 |
| NH1 | 15 | 55 | 25 | 35 | 20 | 25 | 35 | 25 | 40 | 20 | 29.5 |
| Hazard | E1 | E2 | E3 | E4 | E5 | E6 | E7 | E8 | E9 | E10 | Mean Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MH1 | 60 | 60 | 70 | 70 | 60 | 55 | 60 | 55 | 65 | 60 | 61.5 |
| MH2 | 70 | 65 | 60 | 55 | 70 | 60 | 60 | 55 | 70 | 50 | 61.5 |
| MH3 | 60 | 65 | 55 | 60 | 65 | 75 | 65 | 65 | 60 | 60 | 63 |
| EH1 | 45 | 65 | 50 | 45 | 35 | 45 | 55 | 45 | 60 | 55 | 50 |
| EH2 | 25 | 10 | 35 | 35 | 30 | 35 | 20 | 20 | 35 | 45 | 29 |
| EH3 | 15 | 20 | 10 | 20 | 15 | 20 | 10 | 15 | 10 | 15 | 15 |
| EH4 | 15 | 30 | 15 | 25 | 15 | 25 | 25 | 35 | 20 | 25 | 23 |
| EH5 | 45 | 30 | 35 | 25 | 20 | 35 | 40 | 30 | 25 | 20 | 30.5 |
| TH1 | 60 | 50 | 55 | 60 | 55 | 60 | 65 | 55 | 50 | 70 | 58 |
| TH2 | 25 | 20 | 15 | 30 | 35 | 20 | 20 | 25 | 30 | 25 | 24.5 |
| VH1 | 25 | 20 | 30 | 35 | 45 | 35 | 45 | 20 | 35 | 30 | 32 |
| NH1 | 20 | 10 | 20 | 25 | 10 | 10 | 10 | 20 | 25 | 30 | 18 |
| Function | Potential Failure Mode | Hazard Potential | Potential Cause of the Failure | Safety Action | Codification |
|---|---|---|---|---|---|
| Positioning | Wrong manipulation Reach the maximum range of motion | Possible patient injury Surgery failing System damage | Lack of end-stroke limiters Flawed system manipulation Wrong RCM definition | Mount end-stroke limiters Check the system functionality before performing the surgery (pre-planning) | F1 |
| Orientation | Wrong manipulation Reach the maximum range of motion | Possible patient injury Surgery failing System damage | Lack of end-stroke limiters Flawed system manipulation | Mount end-stroke limiters Check the system functionality before performing the surgery (pre-planning) | F2 |
| Instruments attach/detach | Attach/detach mechanism does not work properly | Possible patient injury | Failure of the mechanical interface | Design of a reconfigurable case equipped with a locking system for the instruments | F3 |
| Sensor system | Incorrect feedback | Possible patient injury Incorrect robot motion | Sensor’s malfunction Encoder failure Power outage | Redundant sensor system Check the system functionality in the pre-planning stage Implement the homing position in the control unit | F4 |
| Control system | Delays between the master console and the slave system Communication errors based on protocol communication failing | Possible patient injury Incorrect robot motion Surgery failing | Power supply failure Processor unit failure Wrong programming or failure communication system | Safety loop control Error tracking using an AI agent | F5 |
| Hazard | Score | Evaluation Score |
|---|---|---|
| MH1 | 159.4 | High |
| MH2 | 157.2 | High |
| MH3 | 160.1 | High |
| EH1 | 143.2 | Medium |
| EH2 | 126.4 | Medium |
| EH3 | 68.5 | Low |
| EH4 | 98.5 | Low |
| EH5 | 126.4 | Low |
| TH1 | 151.6 | Medium |
| TH2 | 113.9 | Medium |
| VH1 | 127.5 | Medium |
| NH1 | 47.5 | Negligible |
| Hazard | Risk Reduction Method |
|---|---|
| MH1 | Design the laparoscope and instrument’s guiding modules to reduce the collision probability. Mounting the proximity sensors on the robot structure in areas with higher collision probability. Implementation of software limitation for the range of motion from the control unit. Monitoring in real time the position of each arm using the data from the motor encoders. |
| MH2 | Definition of motion limits for the orientation of the laparoscope. Monitor the orientation angles of the platform and define alert messages for the surgeon. |
| MH3 | Experimental tests have shown that the tissue damage due to the instrument RCM displacement is very low. |
| EH1 | Use a generator or a UPS power supply. |
| EH2 | Use low-voltage components. Reduce the direct contact between the patient and components under voltage. |
| EH3 | Use of circuits with short circuit protection. Use of electric fuses. |
| EH5 | Use secondary sensor system. Test the sensor system functionality before the medical procedure. |
| EH6 | Define two different communication protocol. |
| TH1 | Define a special motion when the hook electrode is used. Implement motion scaling in the control unit. |
| TH2 | Actuating the instruments using cables. Limit contact of heat-generating parts with the patient body. |
| VH1 | The reduction in vibrations in the robotic structure during the design phase using Finite Element Analysis (FEA) to determine the deformation of the main components. |
| NH1 | Cover the robotic structure with noise-reducing material. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pisla, D.; Crisan, N.; Gherman, B.; Andras, I.; Tucan, P.; Radu, C.; Pusca, A.; Vaida, C.; Al Hajjar, N. Safety Issues in the Development of an Innovative Medical Parallel Robot Used in Renal Single-Incision Laparoscopic Surgery. J. Clin. Med. 2023, 12, 4617. https://doi.org/10.3390/jcm12144617
Pisla D, Crisan N, Gherman B, Andras I, Tucan P, Radu C, Pusca A, Vaida C, Al Hajjar N. Safety Issues in the Development of an Innovative Medical Parallel Robot Used in Renal Single-Incision Laparoscopic Surgery. Journal of Clinical Medicine. 2023; 12(14):4617. https://doi.org/10.3390/jcm12144617
Chicago/Turabian StylePisla, Doina, Nicolae Crisan, Bogdan Gherman, Iulia Andras, Paul Tucan, Corina Radu, Alexandru Pusca, Calin Vaida, and Nadim Al Hajjar. 2023. "Safety Issues in the Development of an Innovative Medical Parallel Robot Used in Renal Single-Incision Laparoscopic Surgery" Journal of Clinical Medicine 12, no. 14: 4617. https://doi.org/10.3390/jcm12144617







