A Critical Update of the Classification of Chiari and Chiari-like Malformations
Abstract
:1. Introduction
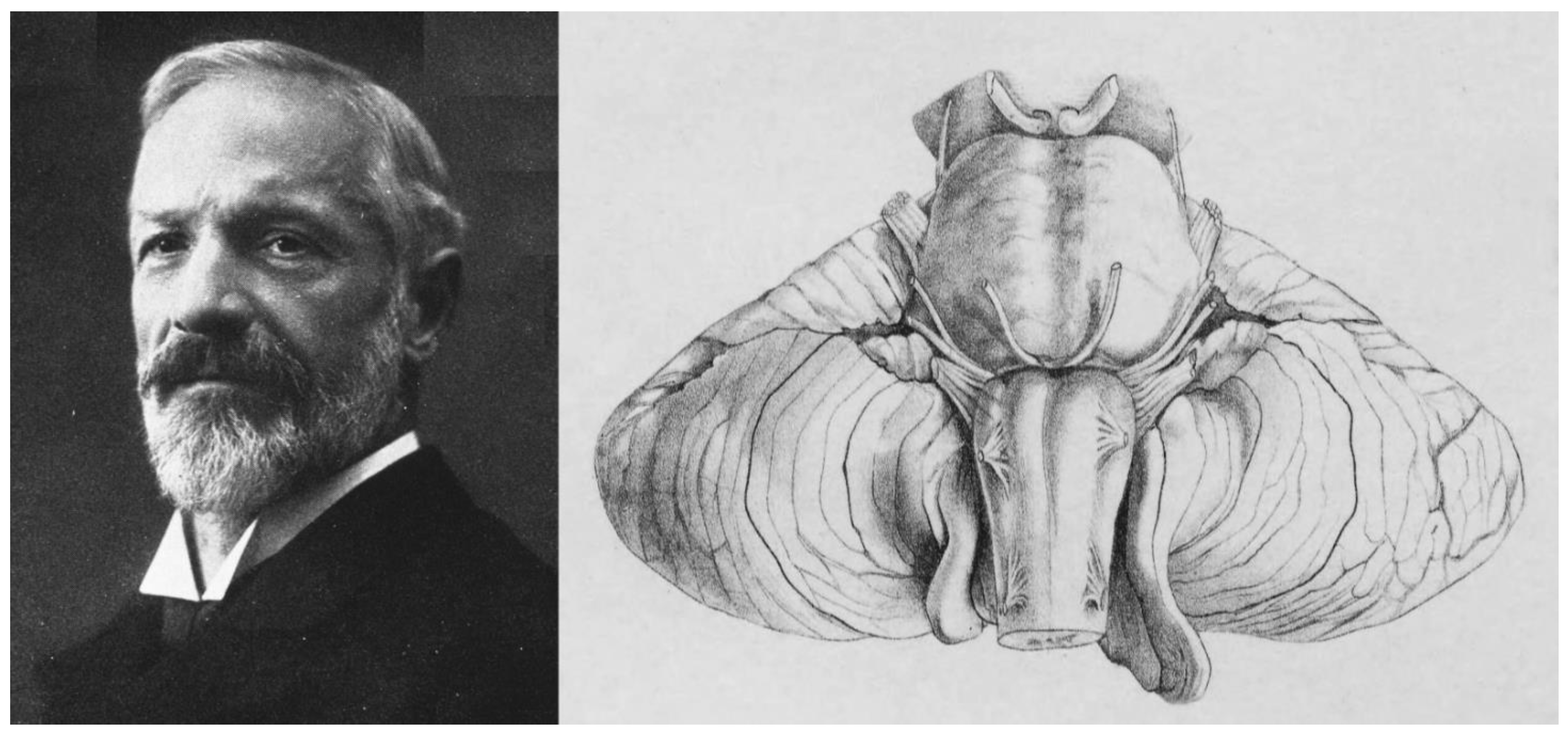
2. A Brief Historical Review of the Chiari Malformation and the ‘Arnold-Chiari’ Eponym
3. Neuroradiology. Early Radiological Classification of Chiari Malformations
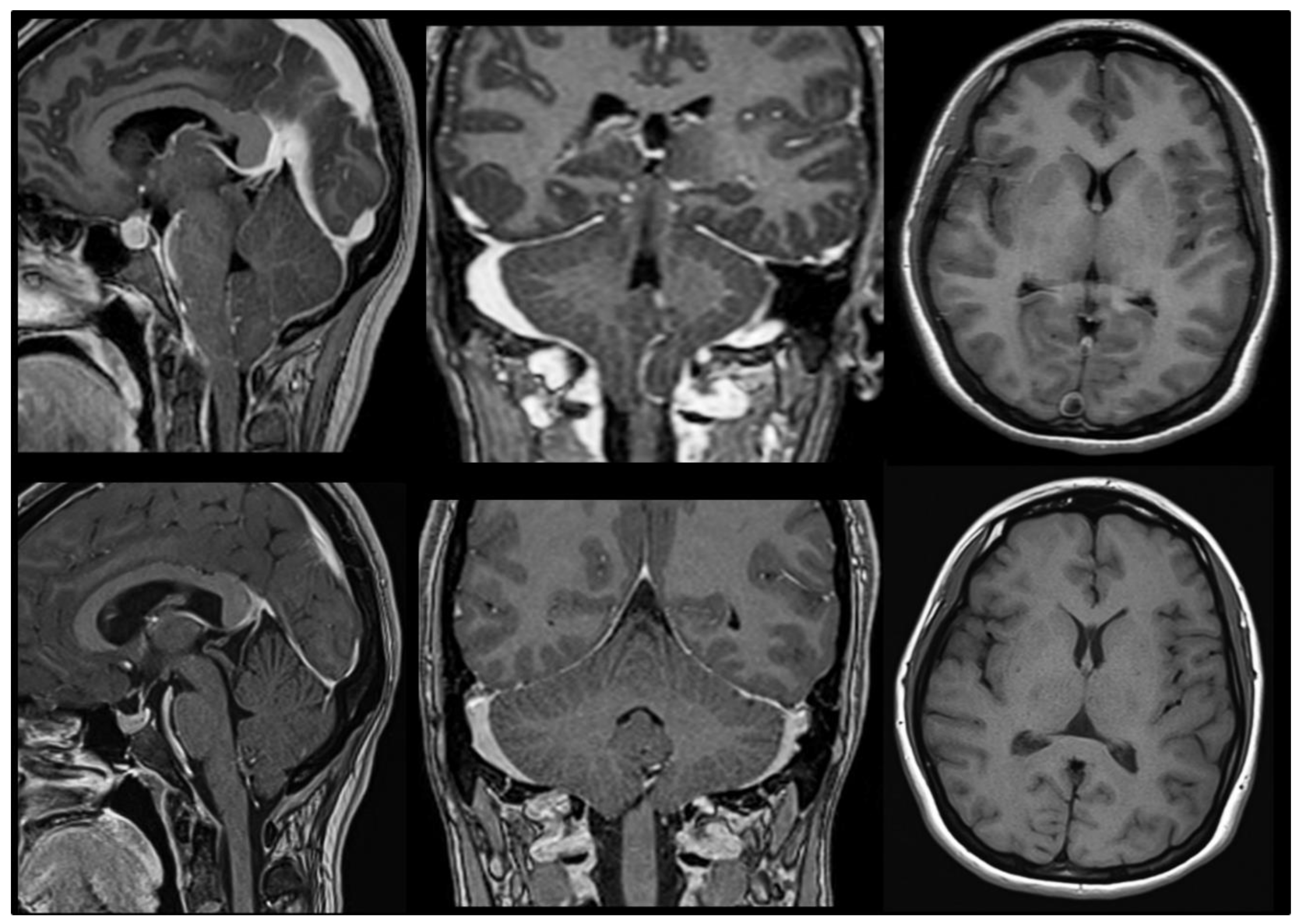
4. MRI: The Breakthrough for the Diagnosis of Chiari Malformations
5. Classification Schemes: Cerebellar Tonsillar Herniation versus Chiari Malformations
6. Primary Chiari Malformations
7. Secondary Chiari Malformations
8. Underdevelopment of the Posterior Fossa: The Original Sin in Primary CMs
9. Chiari-like Malformations in the Cavalier King Charles Spaniel Dog
10. Morphometric Studies of the Posterior Fossa in Humans
Posterior Fossa Boundaries
- McRae’s line (McRL). McRae’s and McGregor’s lines were described in the late 1940s to assess basilar impression in plain radiographs [102,103]. In 1953, McRae used Chamberlain’s line to evaluate different bony abnormalities in the FM region [103]; however, in a second paper, studying the occipitalization of the atlas, he defined the McRL, which has been extensively used in many studies to describe the position of the normal odontoid peg and/or cerebellar tonsils [102,103]. This line is drawn from the basion to the opisthion and represents the planum of the FM, and its length the anteroposterior width of the FM (Figure 7, line 2). In most studies and clinical practice, TH is evaluated by measuring the distance from the most caudal aspect of the tonsils to a line running perpendicular to the McRL [6,99]. However, since TH is usually asymmetrical, coronal slices are the best method to measure and evaluate it. As indicated by Raybaud and Jallo, in normal individuals, both the McRae and Chamberlain lines are superimposed but diverge when the clivus is hypoplastic [6] (Figure 8).
- Clival and Wackenheim lines. The clival line is the distance between the top of the dorsum sellae and the basion and is a measure of clivus length [6,25]. If the line is extended postero-inferiorly to the upper cervical canal, it forms the Wackenheim line, which is usually tangential to the posterior margin of the odontoid tip (Figure 8, lines 3 and 4).
- Incisural line. This line is drawn from the tip of the dorsum sellae to the union of the vein of Galen with the straight sinus. Its length is equivalent to the antero-posterior length of the tentorial notch (Figure 8, line 5).
- Supraoccipital line. This line is drawn from the internal occipital protuberance to the opisthion and represents the length of the supraocciput (Figure 8, line 7).
11. Do I Have a Chiari Malformation 1? The Origin of the 3–5 mm Magic Rule
12. The Canonical Chiari Types and New Phenotypes
12.1. CM1 (‘Classic CM1′)
12.2. CM2 and Spina Bifida
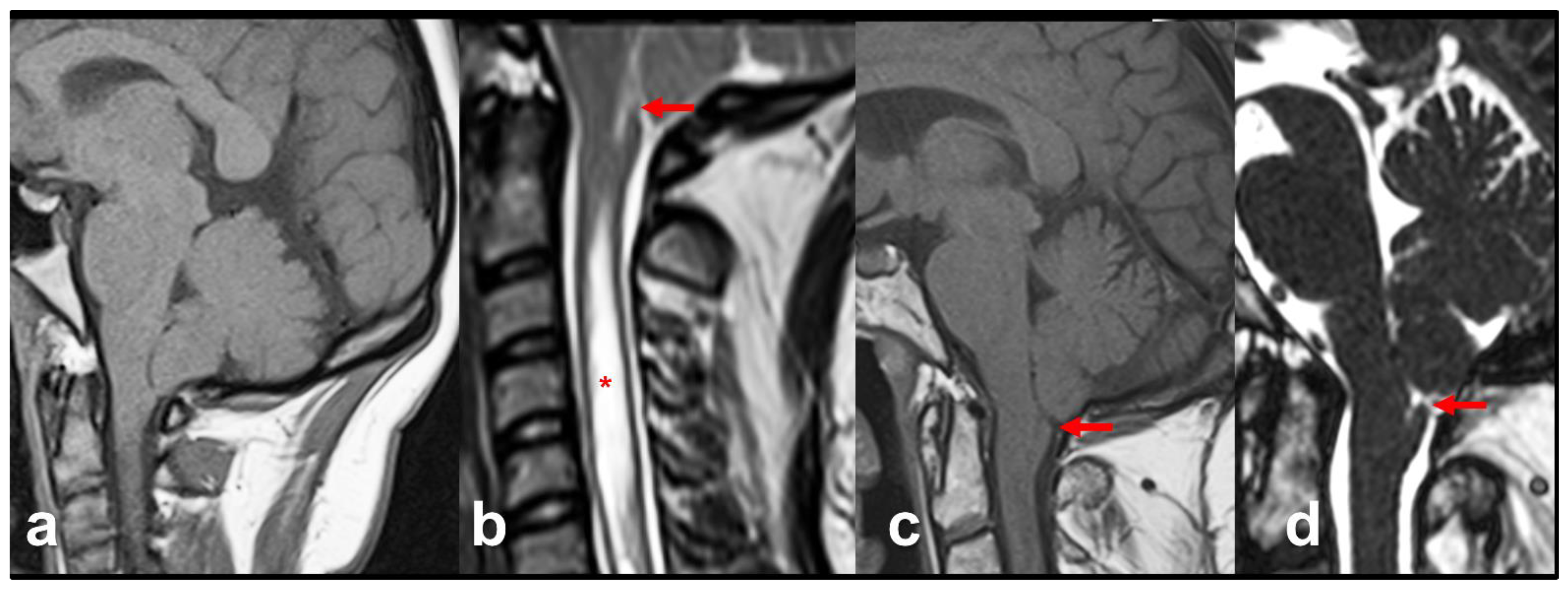
13. The Chiari 0 Malformation (CM0)
14. The Chiari 1.5 Malformation (CM1.5)
15. Complex Malformations: A Puzzle of Mesodermic Disorders
16. Syringomyelia in Chiari Malformations: An Elusive Pathophysiology
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Poretti, A.; Ashmawy, R.; Garzon-Muvdi, T.; Jallo, G.I.; Huisman, T.A.; Raybaud, C. Chiari Type 1 Deformity in Children: Pathogenetic, Clinical, Neuroimaging, and Management Aspects. Neuropediatrics 2016, 47, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.N.P. Chiari I—A ‘not so’ congenital malformation? Childs Nerv. Syst. 2019, 35, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Chiari, H. Über Veränderungen des Kleinhirns, des Pons und der Medulla Oblongata in Folge von Congenitaler Hydrocephalie; K.K. Hof- und Staatsdruckerei: Wien, Austria, 1895; Volume 63, pp. 71–116. [Google Scholar]
- Chiari, H. Über Veränderungen des Kleinhirns Infolge von Hydrocephalie des Grosshirns. Denkschr. Der Kais. Akad. Der Wiss. /Math.-Naturwissenschaftliche Cl. 1891, 17, 1172–1175. [Google Scholar]
- van Dellen, J.R. Chiari Malformation: An Unhelpful Eponym. World Neurosurg. 2021, 156, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Raybaud, C.; Jallo, G.I. Chiari 1 deformity in children: Etiopathogenesis and radiologic diagnosis. Handb. Clin. Neurol. 2018, 155, 25–48. [Google Scholar] [CrossRef] [PubMed]
- Fiaschi, P.; Morana, G.; Anania, P.; Rossi, A.; Consales, A.; Piatelli, G.; Cama, A.; Pavanello, M. Tonsillar herniation spectrum: More than just Chiari I. Update and controversies on classification and management. Neurosurg. Rev. 2020, 43, 1473–1492. [Google Scholar] [CrossRef]
- Ciaramitaro, P.; Massimi, L.; Bertuccio, A.; Solari, A.; Farinotti, M.; Peretta, P.; Saletti, V.; Chiapparini, L.; Barbanera, A.; Garbossa, D.; et al. Diagnosis and treatment of Chiari malformation and syringomyelia in adults: International consensus document. Neurol. Sci. 2022, 43, 1327–1342. [Google Scholar] [CrossRef] [PubMed]
- Massimi, L.; Peretta, P.; Erbetta, A.; Solari, A.; Farinotti, M.; Ciaramitaro, P.; Saletti, V.; Caldarelli, M.; Canheu, A.C.; Celada, C.; et al. Diagnosis and treatment of Chiari malformation type 1 in children: The International Consensus Document. Neurol. Sci. 2021, 43, 1311–1326. [Google Scholar] [CrossRef] [PubMed]
- Ciaramitaro, P.; Garbossa, D.; Peretta, P.; Piatelli, G.; Massimi, L.; Valentini, L.; Migliaretti, G.; Baldovino, S.; Roccatello, D.; Kodra, Y.; et al. Syringomyelia and Chiari Syndrome Registry: Advances in epidemiology, clinical phenotypes and natural history based on a North Western Italy cohort. Ann. Ist. Super. Sanita 2020, 56, 48–58. [Google Scholar] [CrossRef]
- Tubbs, R.S.; Turgut, M. Defining the Chiari Malformations: Past and Newer Classifications. In The Chiari Malformations, 2nd ed.; Tubbs, R.S., Turgut, M., Oakes, W.J., Tubbs, R.S., Turgut, M., Oakes, W.J., Eds.; Springer: New York, NY, USA, 2020; pp. 21–39. [Google Scholar]
- Fisahn, C.; Shoja, M.M.; Turgut, M.; Oskouian, R.J.; Oakes, W.J.; Tubbs, R.S. The Chiari 3.5 malformation: A review of the only reported case. Childs Nerv. Syst. 2016, 32, 2317–2319. [Google Scholar] [CrossRef]
- Tubbs, R.S.; Muhleman, M.; Loukas, M.; Oakes, W.J. A new form of herniation: The Chiari V malformation. Childs Nerv. Syst. 2012, 28, 305–307. [Google Scholar] [CrossRef]
- Cools, M.J.; Wellons, J.C., 3rd; Iskandar, B.J. The Nomenclature of Chiari Malformations. Neurosurg. Clin. N. Am. 2023, 34, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pearce, J.M.S. Historical note. Arnold Chiari, or “Cruveilhier Cleland Chiari” malformation. J. Neurol. Neurosurg. Psychiat. 2000, 68, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solt, I. Chiari malformation eponym- time for historical justice (letter). Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2011, 37, 250–251. [Google Scholar] [CrossRef] [PubMed]
- Azahraa Haddad, F.; Qaisi, I.; Joudeh, N.; Dajani, H.; Jumah, F.; Elmashala, A.; Adeeb, N.; Chern, J.J.; Tubbs, R.S. The newer classifications of the chiari malformations with clarifications: An anatomical review. Clin. Anat. 2018, 31, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Tubbs, R.S.; Turgut, M.; Oakes, W.J. A History of the Chiari Malformations. In Chiari Malformations, 2nd ed.; Tubbs, R.S., Turgut, M., Oakes, W.J., Eds.; Springer: New York, NY, USA, 2020; pp. 3–20. [Google Scholar]
- PubMed. Arnold-Chiari Malformation MeSH Descriptor Data 2022. Available online: https://meshb.nlm.nih.gov/record/ui?ui=D001139 (accessed on 3 July 2023).
- Orphanet Encyclopedia. Arnold-Chiari Malformation Type I. Available online: https://www.orpha.net (accessed on 8 August 2022).
- Orphanet. The Portal for Rare Diseases and Orphan Drugs; Orphanet: Paris, France, 2023. [Google Scholar]
- Ask, O. The Arnold-Chiari malformation; a morphogenetic study. Upsala Lakareforen Forh 1945, 51, 259–275. [Google Scholar] [PubMed]
- Loukas, M.; Shayota, B.J.; Oelhafen, K.; Miller, J.H.; Chern, J.J.; Tubbs, R.S.; Oakes, W.J. Associated disorders of Chiari Type I malformations: A review. Neurosurg. Focus 2011, 31, E3. [Google Scholar] [CrossRef] [Green Version]
- Capra, V.; Iacomino, M.; Accogli, A.; Pavanello, M.; Zara, F.; Cama, A.; De Marco, P. Chiari malformation type I: What information from the genetics? Childs Nerv. Syst. 2019, 35, 1665–1671. [Google Scholar] [CrossRef]
- Markunas, C.A.; Enterline, D.S.; Dunlap, K.; Soldano, K.; Cope, H.; Stajich, J.; Grant, G.; Fuchs, H.; Gregory, S.G.; Ashley-Koch, A.E. Genetic Evaluation and Application of Posterior Cranial Fossa Traits as Endophenotypes for Chiari Type I Malformation. Ann. Hum. Genet. 2014, 78, 1–12. [Google Scholar] [CrossRef]
- Urbizu, A.; Khan, T.N.; Ashley-Koch, A.E. Genetic dissection of Chiari malformation type 1 using endophenotypes and stratification. J. Rare Dis. Res. Treat. 2017, 2, 35–42. [Google Scholar]
- Urbizu, A.; Garrett, M.E.; Soldano, K.; Drechsel, O.; Loth, D.; Marcé-Grau, A.; Mestres, I.S.O.; Poca, M.A.; Ossowski, S.; Macaya, A.; et al. Rare functional genetic variants in COL7A1, COL6A5, COL1A2 and COL5A2 frequently occur in Chiari Malformation Type 1. PLoS ONE 2021, 16, e0251289. [Google Scholar] [CrossRef] [PubMed]
- Miro, X.; Zhou, X.; Boretius, S.; Michaelis, T.; Kubisch, C.; Alvarez-Bolado, G.; Gruss, P. Haploinsufficiency of the murine polycomb gene Suz12 results in diverse malformations of the brain and neural tube. Dis. Model Mech. 2009, 2, 412–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordin, M.D. Scientific Babel: How Science Was Done Before and After Global English; The University of Chicago Press: Chicago, IL, USA; London, UK, 2015; 415p. [Google Scholar]
- Cruveilhier, J. Anatomie Pathologique du Corps Humain ou Descriptions avec Figures Litographiées et Coloriées des Diverses Altération Morbides dont le Corps Humain est Susceptible; J.B. Bailliere: Paris, France, 1829; pp. 1829–1842. [Google Scholar]
- Arnold, J. Myelocytes, Transposition von Gewebskeimen und Sympodie. Beitr. Pathol. Anat. 1894, 16, 1–28. [Google Scholar]
- Cleland, J. Contribution to the Study of Spina Bifida, Encephalocele, and Anencephalus. J. Anat. Physiol. 1883, 17, 257–292. [Google Scholar]
- Black, A. John Cleland 1835–1925. Scott. Med. J. 2002, 47, 140–142. [Google Scholar] [CrossRef]
- Fries, F.N.; Hendrix, P.; Brinker, T.J.; Loukas, M.; Tubbs, R.S. Otto Mennicke (1876-) and the first description of skull base anomalies causing cerebellar tonsillar ectopia: One of the first mentions of the Chiari I malformation. Childs Nerv. Syst. 2017, 33, 825–827. [Google Scholar] [CrossRef]
- Mennicke, O. Ueber Syringomyelie Syringomyelie Mit Anatomischer Untersuchung Zweier Fälle; University of Marburg, Universitäts- Buchdruckerei (R. Friedrich): Maburg, Germany, 1891. [Google Scholar]
- Ollivier, C.P. Traité de la Moelle Épinière et de ses Maladies: Contenant L’histoire Anatomique, Physiologique et Pathologique de ce Centre Nerveux chez L’homme; Crevot: Paris, France, 1827; Volume 1. [Google Scholar]
- Radkowski, M.A. Hans Chiari. Concerning alterations in the cerebellum resulting from cerebral hydrocephalus (English translation). Pediatr. Neurosci. 1987, 13, 3–8. [Google Scholar]
- Bejjani, G.K. Definition of the adult Chiari malformation: A brief historical overview. Neurosurg. Focus 2001, 11, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Schwalbe, E.; Gredig, M. Ueber Entwicklungsstorungen des Kleinhirns, Hirnstamms und Halsmarks bei Spina Bifida (Arnold’sche und Chiari’sche Missbildung). Beitr. Path. Anat. 1907, 40, 132–194. [Google Scholar]
- Sarnat, H.B. Semantics do matter! Precision in scientific communication in pediatric neurology. J. Child Neurol. 2007, 22, 1245–1251. [Google Scholar] [CrossRef]
- Paul, K.S.; Lye, R.H.; Strang, F.A.; Dutton, J. Arnold-Chiari malformation. Review of 71 cases. J. Neurosurg. 1983, 58, 183–187. [Google Scholar] [CrossRef]
- Spinos, E.; Laster, D.W.; Moody, D.M.; Ball, M.R.; Witcofski, R.L.; Kelly, D.L., Jr. MR evaluation of Chiari I malformations at 0.15 T. AJR Am. J. Roentgenol. 1985, 144, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Gilroy, J.; Meyer, J.S. Medical Neurology, 3rd ed.; Macmillan Publishing Co., Inc.: London, UK, 1979. [Google Scholar]
- O’Connor, S.; du Boulay, G.; Logue, V. The normal position of the cerebellar tonsils as demonstrated by myelography. J. Neurosurg. 1973, 39, 387–389. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, G. Anomalies of the cranioverteebral junction. In Neurological Surgery: A Comprehensive Reference Guide to the Diagnosis and Management of Neurosurgical Problems, 2nd ed.; Youmans, J.R., Ed.; WB Saunders Company: Philadelphia, PA, USA, 1982; Volume 3, pp. 1482–1508. [Google Scholar]
- Viard, A.; Eustache, F.; Segobin, S. History of Magnetic Resonance Imaging: A Trip Down Memory Lane. Neuroscience 2021, 474, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Barkovich, A.J.; Wippold, F.J.; Sherman, J.L.; Citrin, C.M. Significance of cerebellar tonsillar position on MR. AJNR Am. J. Neuroradiol. 1986, 7, 795–799. [Google Scholar] [PubMed]
- DeLaPaz, R.L.; Brady, T.J.; Buonanno, F.S.; New, P.F.; Kistler, J.P.; McGinnis, B.D.; Pykett, I.L.; Taveras, J.M. Nuclear magnetic resonance (NMR) imaging of Arnold-Chiari type I malformation with hydromyelia. J. Comput. Assist. Tomogr. 1983, 7, 126–129. [Google Scholar] [CrossRef]
- Elster, A.D.; Chen, M.Y.M. Chiari I malformations: Clinical and radiological reappraisal. Radiology 1992, 183, 347–353. [Google Scholar] [CrossRef]
- Aboulezz, A.; Sartor, K.; Geyer, C.A.; Gado, M.H. Position of cerebellar tonsils in the normal population and in patients with Chiari malformation: A quantitive approach with MR imaging. J. Comput. Assist. Tomogr. 1985, 9, 1033–1036. [Google Scholar] [CrossRef]
- Cinalli, G.; Spennato, P.; Sainte-Rose, C.; Arnaud, E.; Aliberti, F.; Brunelle, F.; Cianciulli, E.; Renier, D. Chiari malformation in craniosynostosis. Childs Nerv. Syst. 2005, 21, 889–901. [Google Scholar] [CrossRef]
- Caldarelli, M.; Novegno, F.; Di Rocco, C. A late complication of CSF shunting: Acquired Chiari I malformation. Childs Nerv. Syst. 2009, 25, 443–452. [Google Scholar] [CrossRef]
- Arnautovic, A.; Pojskić, M.; Arnautović, K.I. Adult Chiari Malformation Type I: Surgical Anatomy, Microsurgical Technique, and Patient Outcomes. Neurosurg. Clin. N. Am. 2023, 34, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Buell, T.J.; Heiss, J.D.; Oldfield, E.H. Pathogenesis and Cerebrospinal Fluid Hydrodynamics of the Chiari I Malformation. Neurosurg. Clin. N. Am. 2015, 26, 495–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knight, J.; De Jesus, O. Tonsillar Herniation. In StatPearls; StatPearls Publishing Copyright© 2022; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Cushing, H. Some experimental and clinical observations concerning states of increased intracranial tension. Am. J. Med. Sci. 1902, 124, 375–400. [Google Scholar] [CrossRef]
- Galarza, M.; Lopez-Guerrero, A.L.; Martinez-Lage, J.F. Posterior fossa arachnoid cysts and cerebellar tonsillar descent: Short review. Neurosurg. Rev. 2010, 33, 305–314. [Google Scholar] [CrossRef]
- Schievink, W.I.; Meyer, F.B.; Atkinson, J.L.D.; Mokri, B. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. J. Neurosurg. 1996, 84, 598–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samii, C.; Mobius, E.; Weber, W.; Heienbrok, H.W.; Berlit, P. Pseudo Chiari type I malformation secondary to cerebrospinal fluid leakage. J. Neurol. 1999, 246, 162–164. [Google Scholar] [CrossRef]
- Sugrue, P.A.; Hsieh, P.C.; Getch, C.C.; Batjer, H.H. Acute symptomatic cerebellar tonsillar herniation following intraoperative lumbar drainage. J. Neurosurg. 2009, 110, 800–803. [Google Scholar] [CrossRef]
- Sporns, P.B.; Schwindt, W.; Cnyrim, C.D.; Heindel, W.; Zoubi, T.; Zimmer, S.; Hanning, U.; Niederstadt, T.U. Undetected Dural Leaks Complicated by Accidental Drainage of Cerebrospinal Fluid (CSF) can Lead to Severe Neurological Deficits. Rofo 2016, 188, 451–458. [Google Scholar] [CrossRef]
- Snow, R.B.; Kuhel, W.; Martin, S.B. Prolonged lumbar spinal drainage after the resection of tumors of the skull base: A cautionary note. Neurosurgery 1991, 28, 880–882. [Google Scholar] [CrossRef]
- Payner, T.D.; Prenger, E.; Berger, T.S.; Crone, K.R. Acquired Chiari malformations: Incidence, diagnosis, and management. Neurosurgery 1994, 34, 429–434. [Google Scholar] [CrossRef]
- Chumas, P.D.; Drake, J.M.; DelBigio, M.R. Death from chronic tonsillar herniation in a patient with lumboperitoneal shunt and Crouzon’s disease. Br. J. Neurosurg. 1992, 6, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Chumas, P.D.; Armstrong, D.C.; Drake, J.M.; Kulkarni, A.V.; Hoffman, H.J.; Humphreys, R.P.; Rutka, J.T.; Hendrick, E.B. Tonsillar herniation: The rule rather than the exception after lumboperitoneal shunting in the pediatric population. J. Neurosurg. 1993, 78, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Milhorat, T.H.; Chou, M.W.; Trinidad, E.M.; Kula, R.W.; Mandell, M.; Wolpert, C.; Speer, M.C. Chiari I malformation redefined: Clinical and radiographic findings for 364 symptomatic patients. Neurosurgery 1999, 44, 1005–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cesmebasi, A.; Loukas, M.; Hogan, E.; Kralovic, S.; Tubbs, R.S.; Cohen-Gadol, A.A. The Chiari malformations: A review with emphasis on anatomical traits. Clin. Anat. 2015, 28, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Leikola, J.; Koljonen, V.; Valanne, L.; Hukki, J. The incidence of Chiari malformation in nonsyndromic, single suture craniosynostosis. Childs Nerv. Syst. 2010, 26, 771–774. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, A.; Giammalva, G.R.; Furlanis, G.M.; Emanuelli, E.; Maugeri, R.; Baro, V.; Denaro, L. Acquired Chiari type I malformation: A late and misunderstood supratentorial over-drainage complication. Childs Nerv. Syst. 2023, 39, 343–351. [Google Scholar] [CrossRef]
- Potgieser, A.R.E.; Hoving, E.W. A novel technique to treat acquired Chiari I malformation after supratentorial shunting. Childs Nerv. Syst. 2016, 32, 1721–1725. [Google Scholar] [CrossRef] [Green Version]
- Poretti, A.; Mall, V.; Smitka, M.; Grunt, S.; Risen, S.; Toelle, S.P.; Benson, J.E.; Yoshida, S.; Jung, N.H.; Tinschert, S.; et al. Macrocerebellum: Significance and Pathogenic Considerations. Cerebellum 2012, 11, 1026–1036. [Google Scholar] [CrossRef] [Green Version]
- Conway, R.L.; Danielpour, M.; Graham, J.M., Jr. Surgical management of cerebellar tonsillar herniation in three patients with macrocephaly-cutis marmorata telangiectatica congenita. Report of three cases. J. Neurosurg. 2007, 106, 296–301. [Google Scholar]
- Sadler, T.W. Langman’s Medical Embryology, 14th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2019; p. 22. 432p. [Google Scholar]
- Maschner, A.; Krück, S.; Draga, M.; Pröls, F.; Scaal, M. Developmental dynamics of occipital and cervical somites. J. Anat. 2016, 229, 601–609. [Google Scholar] [CrossRef] [Green Version]
- Marin-Padilla, M. Cephalic axial skeletal-neural dysraphic disorders: Embryology and pathology. Can. J. Neurol. Sci. 1991, 18, 153–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamilton, W. Mossman’s Human Embryology: Prenatal Development of Form and Function; Hoffer: Cambridge, UK, 1972. [Google Scholar]
- Marin-Padilla, M.; Marin-Padilla, T.M. Morphogenesis of experimentally induced Arnold—Chiari malformation. J. Neurol. Sci. 1981, 50, 29–55. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, R.; Robinson, F. Anomalies of the craniovertebral border. AJR Am. J. Roentgenol. 1976, 127, 281–287. [Google Scholar] [CrossRef]
- Marin-Padilla, M. Mesodermal alterations induced by hypervitaminosis A. J. Embryol. Exp. Morphol. 1966, 15, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Bastir, M.; Rosas, A. Cranial base topology and basic trends in the facial evolution of Homo. J. Hum. Evol. 2016, 91, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Ferre, A.; Poca, M.A.; de la Calzada, M.D.; Moncho, D.; Romero, O.; Sampol, G.; Sahuquillo, J. Sleep-Related Breathing Disorders in Chiari Malformation Type 1: A Prospective Study of 90 Patients. Sleep 2017, 40, zsx069. [Google Scholar] [CrossRef] [Green Version]
- Ferre-Maso, A.; Poca, M.A.; de la Calzada, M.D.; Solana, E.; Romero-Tomas, O.; Sahuquillo, J. Sleep disturbance: A forgotten syndrome in patients with Chiari I malformation. Neurologia 2014, 29, 294–304. [Google Scholar] [CrossRef]
- Amin-Hanjani, S.; Sathi, S.; Scott, R.M. De novo Chiari-I malformation in infants demonstrated by sequential magnetic resonance imaging scans. Report of two cases. Pediatr. Neurosurg. 1995, 22, 299–302. [Google Scholar] [CrossRef]
- Ellis, R. Norms for some structural changes in the human cerebellum from birth to old age. J. Comp. Neurol. 1920, 32, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Marino, D.J.; Dewey, C.W. Chiari-Like Malformation in Dogs. In The Chiari Malformations; Tubbs, R.S., Turgut, M., Oakes, W.J., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 529–547. [Google Scholar]
- Rusbridge, C.; MacSweeny, J.; Davies, J.; Chandler, K.; Fitzmaurice, S.; Dennis, R.; Cappello, R.; Wheeler, S. Syringohydromyelia in Cavalier King Charles spaniels. J. Am. Anim. Hosp. Assoc. 2000, 36, 34–41. [Google Scholar] [CrossRef]
- Rusbridge, C.; McFadyen, A.K.; Knower, S.P. Behavioral and clinical signs of Chiari-like malformation-associated pain and syringomyelia in Cavalier King Charles spaniels. J. Vet. Intern. Med. 2019, 33, 2138–2150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cappello, R.; Rusbridge, C.; Chiari-Like, M.; Syringomyelia Working, G. Report from the Chiari-Like Malformation and Syringomyelia Working Group round table. Vet. Surg. 2007, 36, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Driver, C.J.; Volk, H.A.; Rusbridge, C.; Van Ham, L.M. An update on the pathogenesis of syringomyelia secondary to Chiari-like malformations in dogs. Vet. J. 2013, 198, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Nyland, H.; Krogness, K.G. Size of posterior fossa in Chiari type 1 malformation in adults. Acta Neurochir. 1978, 40, 233–242. [Google Scholar] [CrossRef]
- Krogness, K.G.; Nyland, H. Posterior fossa measurements. II. Size of the posterior fossa in myelomeningocele. Pediatr. Radiol. 1978, 6, 198–202. [Google Scholar] [CrossRef]
- Krogness, K.G. Posterior fossa measurements. I. The normal size of the posterior fossa. Pediatr. Radiol. 1978, 6, 193–197. [Google Scholar] [CrossRef]
- Schijman, E. History, anatomic forms, and pathogenesis of Chiari I malformations. Childs Nerv. Syst. 2004, 20, 323–328. [Google Scholar] [CrossRef]
- Schady, W.; Metcalfe, R.A.; Butler, P. The incidence of craniocervical bony anomalies in the adult Chiari malformation. J. Neurol. Sci. 1987, 82, 193–203. [Google Scholar] [CrossRef]
- Stovner, L.J.; Bergan, U.; Nilsen, G.; Sjaastad, O. Posterior cranial fossa dimensions in the Chiari I malformation: Relation to pathogenesis and clinical presentation. Neuroradiology 1993, 35, 113–118. [Google Scholar] [CrossRef]
- Vega, A.; Quintana, F.; Berciano, J. Basichondrocranium anomalies in adult Chiari type I malformation: A morphometric study. J. Neurol. Sci. 1990, 99, 137–145. [Google Scholar] [CrossRef]
- Nemzek, W.R.; Brodie, H.A.; Hecht, S.T.; Chong, B.W.; Babcook, C.J.; Seibert, J.A. MR, CT, and Plain Film Imaging of the Developing Skull Base in Fetal Specimens. Am. J. Neuroradiol. 2000, 21, 1699–1706. [Google Scholar] [PubMed]
- Carreiro, J.E. Chapter 3—Development of the cranium. In An Osteopathic Approach to Children, 2nd ed.; Carreiro, J.E., Ed.; Churchill Livingstone: Edinburgh, Scotland, 2009; pp. 53–72. [Google Scholar]
- Urbizu, A.; Poca, M.A.; Vidal, X.; Rovira, A.; Sahuquillo, J.; Macaya, A. MRI-based morphometric analysis of posterior cranial fossa in the diagnosis of chiari malformation type I. J. Neuroimaging 2014, 24, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Ferre, A.; Poca, M.A.; de la Calzada, M.D.; Moncho, D.; Urbizu, A.; Romero, O.; Sampol, G.; Sahuquillo, J. A Conditional Inference Tree Model for Predicting Sleep-Related Breathing Disorders in Patients With Chiari Malformation Type 1: Description and External Validation. J. Clin. Sleep Med. 2019, 15, 89–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chamberlain, W.E. Basilar Impression (Platybasia): A Bizarre Developmental Anomaly of the Occipital Bone and Upper Cervical Spine with Striking and Misleading Neurologic Manifestations. Yale J. Biol. Med. 1939, 11, 487–496. [Google Scholar] [PubMed]
- Cronin, C.G.; Lohan, D.G.; Mhuircheartigh, J.N.; Meehan, C.P.; Murphy, J.M.; Roche, C. MRI evaluation and measurement of the normal odontoid peg position. Clin. Radiol. 2007, 62, 897–903. [Google Scholar] [CrossRef]
- McRae, D.L.; Barnum, A.S. Occipitalization of the atlas. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1953, 70, 23–46. [Google Scholar] [PubMed]
- Tubbs, S.R.; Pugh, J.A.; Oakes, W.J. Chiari Malformations. In Youmans and Winn Neurological Surgery, 6th ed.; Winn, H.R., Ed.; Elsevier Saunders: Amsterdam, The Netherlands, 2011; Volume 2, p. 4. [Google Scholar]
- Davidson, L.; Phan, T.N.; Myseros, J.S.; Magge, S.N.; Oluigbo, C.; Sanchez, C.E.; Keating, R.F. Long-term outcomes for children with an incidentally discovered Chiari malformation type 1: What is the clinical significance? Childs Nerv. Syst. 2021, 37, 1191–1197. [Google Scholar] [CrossRef]
- Meadows, J.; Kraut, M.; Guarnieri, M.; Haroun, R.I.; Carson, B.S. Asymptomatic Chiari Type I malformations identified on magnetic resonance imaging. J. Neurosurg. 2000, 92, 920–926. [Google Scholar] [CrossRef] [Green Version]
- Savy, L.E.; Stevens, J.M.; Taylor, D.J.; Kendall, B.E. Apparent cerebellar ectopia: A reappraisal using volumetric MRI. Neuroradiology 1994, 36, 360–363. [Google Scholar] [CrossRef]
- Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [CrossRef]
- Whyte, M.B.; Kelly, P. The normal range: It is not normal and it is not a range. Postgrad. Med. J. 2018, 94, 613–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceriotti, F.; Henny, J. “Are my Laboratory Results Normal?” Considerations to be Made Concerning Reference Intervals and Decision Limits. Ejifcc 2008, 19, 106–114. [Google Scholar] [PubMed]
- Żytkowski, A.; Tubbs, R.S.; Iwanaga, J.; Clarke, E.; Polguj, M.; Wysiadecki, G. Anatomical normality and variability: Historical perspective and methodological considerations. Transl. Res. Anat. 2021, 23, 100105. [Google Scholar] [CrossRef]
- Barros, D.P.M.; Ribeiro, E.C.O.; Nascimento, J.J.C.; Silva-Neto, E.J.; Araujo-Neto, S.A. Reliability and Agreement in the Cerebellar Tonsil Tip Localization: Two Methods Using the McRae Line Concept in MRI. World Neurosurg. 2022, 165, e611–e618. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, B.J.; Urbizu, A.; Allen, P.A.; Loth, F.; Tubbs, R.S.; Bunck, A.C.; Kroger, J.R.; Rocque, B.G.; Madura, C.; Chen, J.A.; et al. Cerebellar tonsil ectopia measurement in type I Chiari malformation patients show poor inter-operator reliability. Fluids Barriers CNS 2018, 15, 33. [Google Scholar] [CrossRef] [Green Version]
- American Medical Association. ICD-10-CM 2023: The Complete Official Codebook; American Medical Association: Philadelphia, PA, USA, 2022; p. 1250. [Google Scholar]
- Bogdanov, E.I. Clinical significance of subthreshold cerebellar tonsil ectopia into foramen magnum and Chiari malformation type 0. Burdenko’s J. Neurosurg. 2022, 86, 92–98. [Google Scholar] [CrossRef]
- Botelho, R.V.; Bittencourt, L.R.; Rotta, J.M.; Tufik, S. Adult Chiari malformation and sleep apnoea. Neurosurg. Rev. 2005, 28, 169–176. [Google Scholar] [CrossRef]
- Milhorat, T.H.; Nishikawa, M.; Kula, R.W.; Dlugacz, Y.D. Mechanisms of cerebellar tonsil herniation in patients with Chiari malformations as guide to clinical management. Acta Neurochir. 2010, 152, 1117–1127. [Google Scholar] [CrossRef] [Green Version]
- Pueyrredon, F.; Spaho, N.; Arroyave, I.; Vinters, H.; Lazareff, J. Histological findings in cerebellar tonsils of patients with Chiari type I malformation. Childs Nerv. Syst. 2007, 23, 427–429. [Google Scholar] [CrossRef]
- Urbizu, A.; Martin, B.A.; Moncho, D.; Rovira, A.; Poca, M.A.; Sahuquillo, J.; Macaya, A.; Espanol, M.I. Machine learning applied to neuroimaging for diagnosis of adult classic Chiari malformation: Role of the basion as a key morphometric indicator. J. Neurosurg. 2017, 129, 779–791. [Google Scholar] [CrossRef] [Green Version]
- Moncho, D.; Poca, M.A.; Minoves, T.; Ferre, A.; Rahnama, K.; Sahuquillo, J. Brainstem auditory and somatosensory evoked potentials in relation to clinical and neuroimaging findings in Chiari type 1 malformation. J. Clin. Neurophysiol. 2015, 32, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.W.A. An encephalographic ratio for estimating ventricular enlargement and cerebral atrophy. Arch. Neurol. Psychiatry 1942, 47, 931–937. [Google Scholar] [CrossRef]
- Moncho, D.; Poca, M.A.; Minoves, T.; Ferre, A.; Canas, V.; Sahuquillo, J. Are evoked potentials clinically useful in the study of patients with Chiari malformation Type 1? J. Neurosurg. 2017, 126, 606–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gagnadoux, F.; Meslier, N.; Svab, I.; Menei, P.; Racineux, J.L. Sleep-disordered breathing in patients with Chiari malformation: Improvement after surgery. Neurology 2006, 66, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, P.F.; Tosi, U.; Uribe-Cardenas, R.; Greenfield, J.P. Ventrolateral Tonsillar Position Defines Novel Chiari 0.5 Classification. World Neurosurg. 2020, 136, 444–453. [Google Scholar] [CrossRef]
- Kunpalin, Y.; Richter, J.; Mufti, N.; Bosteels, J.; Ourselin, S.; De Coppi, P.; Thompson, D.; David, A.L.; Deprest, J. Cranial findings detected by second-trimester ultrasound in fetuses with myelomeningocele: A systematic review. BJOG 2021, 128, 366–374. [Google Scholar] [CrossRef]
- Worley, G.; Schuster, J.M.; Oakes, W.J. Survival at 5 years of a cohort of newborn infants with myelomeningocele. Dev. Med. Child Neurol. 1996, 38, 816–822. [Google Scholar] [CrossRef]
- Danzer, E.; Johnson, M.P. Fetal surgery for neural tube defects. Semin. Fetal. Neonatal. Med. 2014, 19, 2–8. [Google Scholar] [CrossRef]
- Russell, D.S.; Donald, C. The mechanism of internal hydrocephalus in spina bifida. Brain A J. Neurol. 1935, 58, 203. [Google Scholar] [CrossRef]
- Ogryzlo, M.A. The Arnold-Chiari Malformation. Arch. Neurol. Psychiatry 1942, 48, 30–46. [Google Scholar] [CrossRef]
- McLone, D.G.; Dias, M.S. The Chiari II malformation: Cause and impact. Childs Nerv. Syst. 2003, 19, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Adzick, N.S.; Thom, E.A.; Spong, C.Y.; Brock, J.W.; Burrows, P.K.; Johnson, M.P.; Howell, L.J.; Farrell, J.A.; Dabrowiak, M.E.; Sutton, L.N.; et al. A Randomized Trial of Prenatal versus Postnatal Repair of Myelomeningocele. N. Engl. J. Med. 2011, 364, 993–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iskandar, B.J.; Hedlund, G.L.; Grabb, P.A.; Oakes, W.J. The resolution of syringohydromyelia without hindbrain herniation after posterior fossa decompression. J. Neurosurg. 1998, 89, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Tubbs, R.S.; Elton, S.; Grabb, P.; Dockery, S.E.; Bartolucci, A.A.; Oakes, W.J. Analysis of the posterior fossa in children with the Chiari 0 malformation. Neurosurgery 2001, 48, 1050–1054. [Google Scholar]
- Kyoshima, K.; Kuroyanagi, T.; Oya, F.; Kamijo, Y.; El Noamany, H.; Kobayashi, S. Syringomyelia without hindbrain herniation: Tight cisterna magna. Report of four cases and a review of the literature. J. Neurosurg. 2002, 96, 239–249. [Google Scholar] [CrossRef]
- Chern, J.J.; Gordon, A.J.; Mortazavi, M.M.; Tubbs, R.S.; Oakes, W.J. Pediatric Chiari malformation Type 0: A 12-year institutional experience. J. Neurosurg. Pediatr. 2011, 8, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Bogdanov, E.I.; Faizutdinova, A.T.; Heiss, J.D. The Small Posterior Cranial Fossa Syndrome and Chiari Malformation Type 0. J. Clin. Med. 2022, 11, 5472. [Google Scholar] [CrossRef]
- Bagci, A.M.; Lee, S.H.; Nagornaya, N.; Green, B.A.; Alperin, N. Automated Posterior Cranial Fossa Volumetry by MRI: Applications to Chiari Malformation Type I. Am. J. Neuroradiol. 2013, 34, 1758–1763. [Google Scholar] [CrossRef] [Green Version]
- Tubbs, R.S.; Iskandar, B.J.; Bartolucci, A.A.; Oakes, W.J. A critical analysis of the Chiari 1.5 malformation. J. Neurosurg. 2004, 101, 179–183. [Google Scholar] [CrossRef]
- Lang, J. Clinical Anatomy of the Posterior Cranial Fossa and Its Foramina; George Thieme Verlag: Stuttgart, Germany, 1991; pp. 112–118. [Google Scholar]
- Quisling, R.G.; Quisling, S.G.; Mickle, J.P. Obex/nucleus gracilis position—Its role as a marker for the cervicomedullary junction. Pediatr. Neurosurg. 1993, 19, 143–150. [Google Scholar] [CrossRef]
- Tubbs, R.S.; McGirt, M.J.; Oakes, W.J. Surgical experience in 130 pediatric patients with Chiari I malformations. J. Neurosurg. 2003, 99, 291–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markunas, C.A.; Tubbs, R.S.; Moftakhar, R.; Ashley-Koch, A.E.; Gregory, S.G.; Oakes, W.J.; Speer, M.C.; Iskandar, B.J. Clinical, radiological, and genetic similarities between patients with Chiari Type I and Type 0 malformations. J. Neurosurg. Pediatr. 2012, 9, 372–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Creuzet, S.; Couly, G.; Le Douarin, N.M. Patterning the neural crest derivatives during development of the vertebrate head: Insights from avian studies. J. Anat. 2005, 207, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Cook, G.M.W.; Lewis, K.E.; Keynes, R.J. Neural Patterning: Spinal Cord Segmentation and Somite Patterning. In Reference Module in Neuroscience and Biobehavioral Psychology; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Menezes, A.H. Craniocervical developmental anatomy and its implications. Childs Nerv. Syst. 2008, 24, 1109–1122. [Google Scholar] [CrossRef]
- Menezes, A.H. Chiari I malformations and hydromyelia—Complications. Pediatr. Neurosurg. 1991, 17, 146–154. [Google Scholar] [CrossRef]
- Bollo, R.J.; Riva-Cambrin, J.; Brockmeyer, M.M.; Brockmeyer, D.L. Complex Chiari malformations in children: An analysis of preoperative risk factors for occipitocervical fusion. J. Neurosurg. Pediatr. 2012, 10, 134–141. [Google Scholar] [CrossRef]
- Ravindra, V.M.; Brockmeyer, D.L. Complex Chiari Malformations: Diagnosis, Evaluation, and Treatment. Neurosurg. Clin. N. Am. 2023, 34, 143–150. [Google Scholar] [CrossRef]
- Goel, A.; Bhatjiwale, M.; Desai, K. Basilar invagination: A study based on 190 surgically treated patients. J. Neurosurg. 1998, 88, 962–968. [Google Scholar] [CrossRef]
- Sánchez-Pernaute, R.; Berciano, J.; Rebollo, M.; Pascual, J. Intramedullary tuberculoma of the spinal cord with syringomyelia. Neuroradiology 1996, 38, S105–S106. [Google Scholar] [CrossRef]
- Nagashima, C.; Kubota, S. Craniocervical abnormalities. Modern diagnosis and a comprehensive surgical approach. Neurosurg. Rev. 1983, 6, 187–197. [Google Scholar] [CrossRef]
- Menezes, A.H.; VanGilder, J.C.; Graf, C.J.; McDonnell, D.E. Craniocervical abnormalities. A comprehensive surgical approach. J. Neurosurg. 1980, 53, 444–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langhans, T. Ueber Höhlenbildung im Rückenmark als Folge von Blutstauung. Arch. Pathol. Anat. Und Physiol. Klin. Med. 1881, 85, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Mortazavi, M.M.; Tubbs, R.S.; Brockerhoff, M.A.; Loukas, M.; Oakes, W.J. The first description of Chiari I malformation with intuitive correlation between tonsillar ectopia and syringomyelia. J. Neurosurg. Pediatr. 2011, 7, 257–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Logue, V.; Edwards, M.R. Syringomyelia and its surgical treatment—An analysis of 75 patients. J. Neurol. Neurosurg. Psychiatry 1981, 44, 273–284. [Google Scholar] [CrossRef] [Green Version]
- Heiss, J.D. Cerebrospinal Fluid Hydrodynamics in Chiari I Malformation and Syringomyelia: Modeling Pathophysiology. Neurosurg. Clin. N. Am. 2023, 34, 81–90. [Google Scholar] [CrossRef]
- Tachibana, S.; Harada, K.; Abe, T.; Yamada, H.; Yokota, A. Syringomyelia secondary to tonsillar herniation caused by posterior fossa tumors. Surg. Neurol. 1995, 43, 470–475. [Google Scholar] [CrossRef]
- Milhorat, T.H. Classification of syringomyelia. Neurosurg. Focus 2000, 8, E1. [Google Scholar] [CrossRef]
- Gardner, W.J.; Angel, J. The mechanism of syringomyelia and its surgical correction. Clin. Neurosurg. 1958, 6, 131–140. [Google Scholar] [CrossRef]
- Gardner, W.J.; Goodall, R.J. The surgical treatment of Arnold-Chiari malformation in adults; an explanation of its mechanism and importance of encephalography in diagnosis. J. Neurosurg. 1950, 7, 199–206. [Google Scholar] [CrossRef] [Green Version]
- Leung, V.; Magnussen, J.S.; Stoodley, M.A.; Bilston, L.E. Cerebellar and hindbrain motion in Chiari malformation with and without syringomyelia. J. Neurosurg. Spine 2016, 24, 546–555. [Google Scholar] [CrossRef] [Green Version]
- Oldfield, E.H.; Muraszko, K.; Shawker, T.H.; Patronas, N.J. Pathophysiology of syringomyelia associated with Chiari I malformation of the cerebellar tonsils. Implications for diagnosis and treatment. J. Neurosurg. 1994, 80, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Milhorat, T.H.; Kotzen, R.M.; Anzil, A.P. Stenosis of central canal of spinal cord in man: Incidence and pathological findings in 232 autopsy cases. J. Neurosurg. 1994, 80, 716–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, J. On the anatomy of the calamus region in the human bulb; with an account of a hitherto undescribed “Nucleus postremus.”: Part I. J. Anat. Physiol. 1906, 40, 210. [Google Scholar] [PubMed]
- Longatti, P.; Fiorindi, A.; Marton, E.; Sala, F.; Feletti, A. Where the central canal begins: Endoscopic in vivo description. J. Neurosurg. 2022, 136, 895–904. [Google Scholar] [CrossRef]
- Allen, L.; O’Connell, A.; Kiermer, V. How can we ensure visibility and diversity in research contributions? How the Contributor Role Taxonomy (CRediT) is helping the shift from authorship to contributorship. Learn. Publ. 2019, 32, 71–74. [Google Scholar] [CrossRef] [Green Version]
- Sahuquillo, J.; Rubio, E.; Poca, M.A.; Rovira, A.; Rodriguez-Baeza, A.; Cervera, C. Posterior fossa reconstruction: A surgical technique for the treatment of Chiari I malformation and Chiari I/syringomyelia complex—Preliminary results and magnetic resonance imaging quantitative assessment of hindbrain migration. Neurosurgery 1994, 35, 874–884. [Google Scholar] [CrossRef]
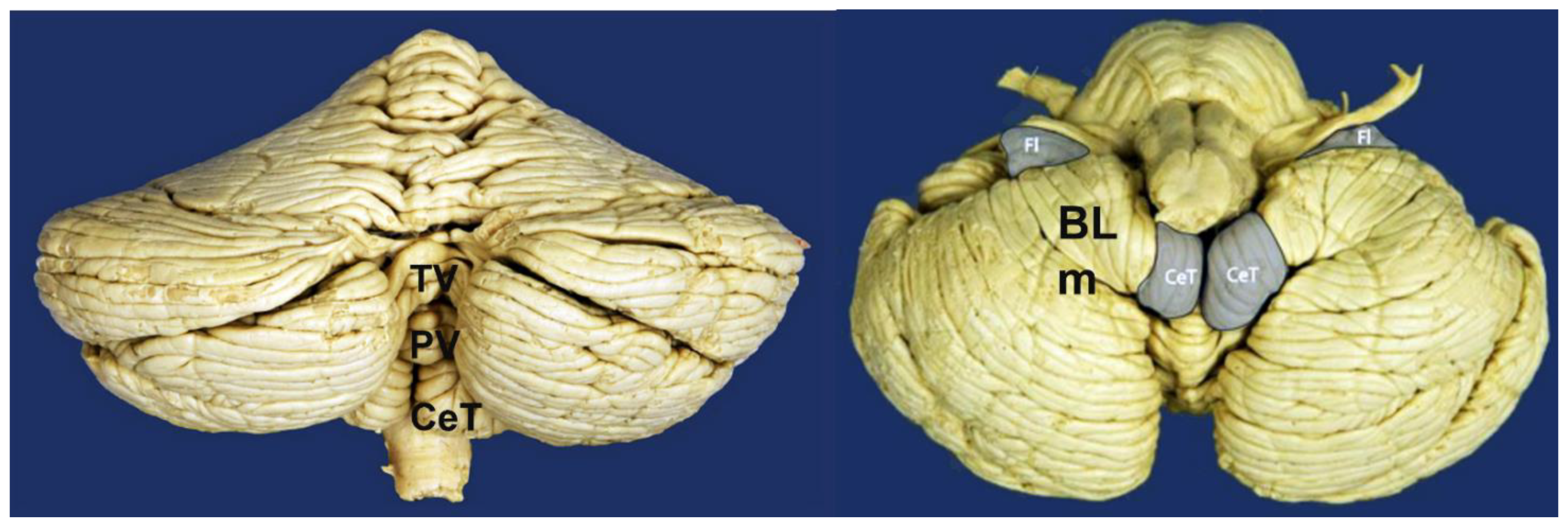





| Type | Abbreviation | Author|Year | Main Characteristic |
|---|---|---|---|
| Chiari 0 | CM0 | Iskandar et al., 1998 [1] | Posterior fossa crowding. No TH |
| Chiari 0.5 | CM0.5 | Morgenstern et al., 2020 [2] | Ventrolateral tonsillar wrapping |
| Chiari 1 | CM1 | Chiari, 1891 [3] | Tonsillar herniation (>3–5 mm) |
| Chiari 1.5 | CM1.5 | Tubbs et al., 2004 [4] | Cerebellar and brainstem herniation |
| Chiari 2 | CM2 | Chiari, 1891 [3] | Spina bifida|Cerebellar-brainstem herniation |
| Chiari 3 | CM3 | Chiari, 1891 [3] | Cerebellar-cervical encephalocele |
| Chiari 3.5 | CM3.5 | Fisahn et al., 2016 [5] | Cerebellar-cervical encephalocele connected to foregut |
| Chiari 4 | CM4 | Chiari, 1895 [6] | Cerebellar hypoplasia or aplasia. No TH |
| Chiari 5 | CM5 | Tubbs et al., 2012 [7] | Cerebellar aplasia and occipital lobe herniation |
| Category | Etiology | Pathologies |
|---|---|---|
| Tonsillar herniation | Increased volume|High ICP |
|
| Tonsillar herniation | Cranio-spinal gradients |
|
| Primary Chiari malformations | Mesodermal insuficiency |
|
| Complex CVJ malformations | Embriological mesodermal abnormalities |
|
| Secondary Chiari-like malformations | Secondary reduced volume of the skull or neural overgrowth |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahuquillo, J.; Moncho, D.; Ferré, A.; López-Bermeo, D.; Sahuquillo-Muxi, A.; Poca, M.A. A Critical Update of the Classification of Chiari and Chiari-like Malformations. J. Clin. Med. 2023, 12, 4626. https://doi.org/10.3390/jcm12144626
Sahuquillo J, Moncho D, Ferré A, López-Bermeo D, Sahuquillo-Muxi A, Poca MA. A Critical Update of the Classification of Chiari and Chiari-like Malformations. Journal of Clinical Medicine. 2023; 12(14):4626. https://doi.org/10.3390/jcm12144626
Chicago/Turabian StyleSahuquillo, Juan, Dulce Moncho, Alex Ferré, Diego López-Bermeo, Aasma Sahuquillo-Muxi, and Maria A. Poca. 2023. "A Critical Update of the Classification of Chiari and Chiari-like Malformations" Journal of Clinical Medicine 12, no. 14: 4626. https://doi.org/10.3390/jcm12144626
APA StyleSahuquillo, J., Moncho, D., Ferré, A., López-Bermeo, D., Sahuquillo-Muxi, A., & Poca, M. A. (2023). A Critical Update of the Classification of Chiari and Chiari-like Malformations. Journal of Clinical Medicine, 12(14), 4626. https://doi.org/10.3390/jcm12144626








