Infiltrative Type I Collagen in the Treatment of Morton’s Neuroma: A Mini-Series
Abstract
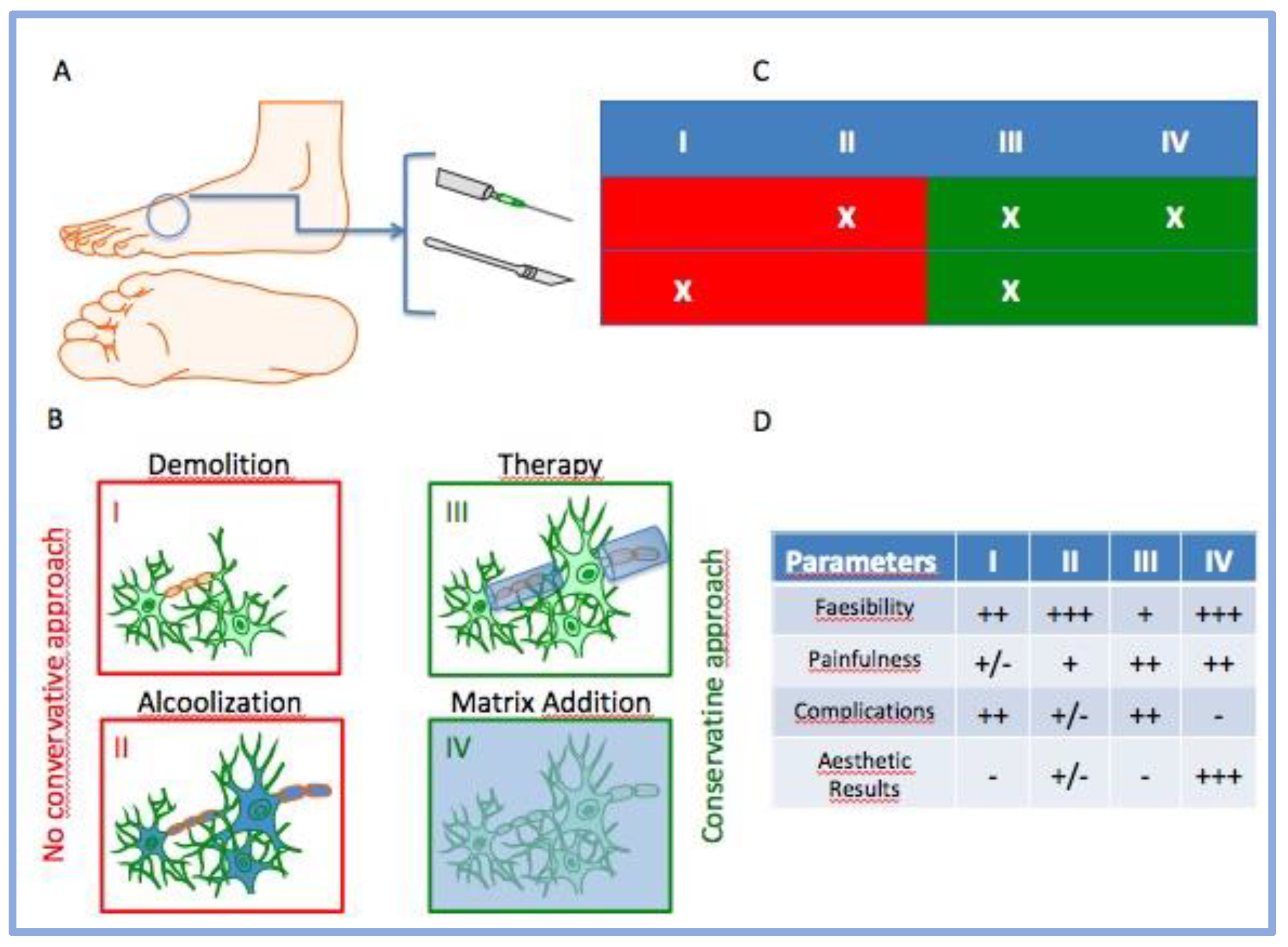
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Injection Procedure
2.3. Porcine Type 1 Collagen
2.4. Evaluation Criteria after Treatment and Follow-Up of Patients
2.5. Statistical Analyses:
3. Results
3.1. Follow-Up
3.2. AOFAS Score
3.3. VNS Score
3.3.1. VNS Score without Stimuli
3.3.2. VNS Score after Activities
4. Discussion
5. Conclusions
- The infiltrative use of porcine type I collagen could be contemplated as a promising non-surgical therapy against MN less than 10 mm in size.
- The procedure is safe and easy to perform.
- The combined indirect US-guided injection (IUSGI) approach can allow a more precise subministration of medical devices, where the point of application fits perfectly with the point of maximum pain.
- The proposed approach places itself exactly between the conservative approach and nerve-ablative treatments, as well up-front surgery and alcohol injection.
- Collagen treatment could play an important pivotal role in the positive modulation of the inflammatory process as well as steroid treatments but avoid their side effects (probably) physically acting on the fibrosis process.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pasero, G.; Marson, P. Filippo Civinini (1805–1844) and the discovery of plantar neuroma. Reum.-Ital. J. Rheumatol. 2006, 58, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Morton, T.G. A Peculiar and Painful Affection of the Fourth Metatarso-Phalangeal Articulation. Am. J. Med. Sci. 1876, 71, 37–45. [Google Scholar] [CrossRef]
- Stecco, C.; Fantoni, I.; Macchi, V.; Del Borrello, M.; Porzionato, A.; Biz, C.; De Caro, R. The Role of Fasciae in Civinini-Morton’s Syndrome. J. Anat. 2015, 227, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Biz, C.; Bonvicini, B.; Sciarretta, G.; Pendin, M.; Cecchetto, G.; Ruggieri, P. Digital Ischemia after Ultrasound-Guided Alcohol Injection for Morton’s Syndrome: Case Report and Review of the Literature. J. Clin. Med. 2022, 11, 6263. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.K. Morton’s Interdigital Neuroma: A Clinical Review of Its Etiology, Treatment, and Results. J. Foot Ankle Surg. 1996, 35, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Gougoulias, N.; Lampridis, V.; Sakellariou, A. Morton’s Interdigital Neuroma: Instructional Review. EFORT Open Rev. 2019, 4, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Biz, C.; Stecco, C.; Fantoni, I.; Aprile, G.; Giacomini, S.; Pirri, C.; Ruggieri, P. Fascial Manipulation Technique in the Conservative Management of Morton’s Syndrome: A Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 7952. [Google Scholar] [CrossRef]
- del Mar Ruiz-Herrera, M.; Marcos-Tejedor, F.; Aldana-Caballero, A.; Calvo-Lobo, C.; Rodriguez-Sanz, D.; Moroni, S.; Konschake, M.; Mohedano-Moriano, A.; Aceituno-Gómez, J.; Criado-Álvarez, J.J. Novel Ultrasound Anatomical Measurement of the Deep Transverse Metatarsal Ligament: An Intra-Rater Reliability and Inter-Rater Concordance Study. J. Clin. Med. 2022, 11, 2553. [Google Scholar] [CrossRef]
- Bignotti, B.; Signori, A.; Sormani, M.P.; Molfetta, L.; Martinoli, C.; Tagliafico, A. Ultrasound versus Magnetic Resonance Imaging for Morton Neuroma: Systematic Review and Meta-Analysis. Eur. Radiol. 2015, 25, 2254–2262. [Google Scholar] [CrossRef]
- Matthews, B.G.; Hurn, S.E.; Harding, M.P.; Henry, R.A.; Ware, R.S. The Effectiveness of Non-Surgical Interventions for Common Plantar Digital Compressive Neuropathy (Morton’s Neuroma): A Systematic Review and Meta-Analysis. J. Foot Ankle Res. 2019, 12, 12. [Google Scholar] [CrossRef]
- Klontzas, M.E.; Koltsakis, E.; Kakkos, G.A.; Karantanas, A.H. Ultrasound-Guided Treatment of Morton’s Neuroma. J. Ultrason. 2021, 21, e134–e138. [Google Scholar] [CrossRef] [PubMed]
- Santiago, F.R.; Muñoz, P.T.; Ramos-Bossini, A.J.L.; Martínez, A.M.; Olleta, N.P. Long-Term Comparison between Blind and Ultrasound-Guided Corticoid Injections in Morton Neuroma. Eur. Radiol. 2022, 32, 8414–8422. [Google Scholar] [CrossRef] [PubMed]
- Lorenzon, P.; Rettore, C.; Scalvi, A. Infiltrative Therapy of Morton’s Neuroma: A Systematic Review of Different Treatment Options. Acta Bio Med. Atenei Parm. 2022, 92, e2021556. [Google Scholar] [CrossRef]
- Samaila, E.M.; Ambrosini, C.; Negri, S.; Maluta, T.; Valentini, R.; Magnan, B. Can Percutaneous Alcoholization of Morton’s Neuroma with Phenol by Electrostimulation Guidance Be an Alternative to Surgical Excision? Long-Term Results. Foot Ankle Surg. 2020, 26, 314–319. [Google Scholar] [CrossRef]
- Lizano-Díez, X.; Ginés-Cespedosa, A.; Alentorn-Geli, E.; Pérez-Prieto, D.; González-Lucena, G.; Gamba, C.; de Zabala, S.; Solano-López, A.; Rigol-Ramón, P. Corticosteroid Injection for the Treatment of Morton’s Neuroma: A Prospective, Double-Blinded, Randomized, Placebo-Controlled Trial. Foot Ankle Int. 2017, 38, 944–951. [Google Scholar] [CrossRef]
- Samaila, E.; Colò, G.; Rava, A.; Negri, S.; Valentini, R.; Felli, L.; Magnan, B. Effectiveness of Corticosteroid Injections in Civinini–Morton’s Syndrome: A Systematic Review. Foot Ankle Surg. 2021, 27, 357–365. [Google Scholar] [CrossRef]
- Liebendorfer, A.; Ochoa, E.; Dy, C.J. Changes in Patient-Reported Pain Intererence After Surgical Treatment of Painful Lower Extremity Neuromas. J. Hand Surg. Glob. Online 2023, 5, 97–101. [Google Scholar] [CrossRef]
- Rajan, L.; Mizher, R.; Srikumar, S.; Fuller, R.; Ellis, S.J. Clinical Outcomes of Revision Surgery Using a Dorsal Approach After Failed Primary Interdigital Neuroma Excision. Foot Ankle Spec. 2022, 193864002211164. [Google Scholar] [CrossRef]
- Valisena, S.; Petri, G.J.; Ferrero, A. Treatment of Morton’s Neuroma: A Systematic Review. Foot Ankle Surg. 2018, 24, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Pace, A.; Scammell, B.; Dhar, S. The Outcome of Morton’s Neurectomy in the Treatment of Metatarsalgia. Int. Orthop. 2010, 34, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhang, N.; Li, Z.; Wang, Y.; Li, X.; Wang, Y.; Si, H.; Hu, Y. Plantar and Dorsal Approaches for Excision of Morton’s Neuroma: A Comparison Study. BMC Musculoskelet. Disord. 2022, 23, 898. [Google Scholar] [CrossRef]
- Ortu, S.; Fiori, E.; Bagnoli, I.; Valente, A.; Pisanu, F.; Caggiari, G.; Doria, C.; Milano, L. Complications of Alcohol Injections for Morton’s Neuroma. J. Orthop. Trauma Rehabil. 2022, 29, 221049172211163. [Google Scholar] [CrossRef]
- Choi, J.Y.; Lee, H.I.; Hong, W.H.; Suh, J.S.; Hur, J.W. Corticosteroid Injection for Morton’s Interdigital Neuroma: A Systematic Review. Clin. Orthop. Surg. 2021, 13, 266. [Google Scholar] [CrossRef] [PubMed]
- Thomson, L.; Aujla, R.S.; Divall, P.; Bhatia, M. Non-Surgical Treatments for Morton’s Neuroma: A Systematic Review. Foot Ankle Surg. 2020, 26, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Corrado, B.; Bonini, I.; Chirico, V.A.; Filippini, E.; Liguori, L.; Magliulo, G.; Mazzuocco, G.; Rosano, N.; Gisonni, P. Ultrasound-Guided Collagen Injections in the Treatment of Supraspinatus Tendinopathy: A Case Series Pilot Study. J. Biol. Regul. Homeost. Agents 2020, 34, 33–39. [Google Scholar]
- Micarelli, A.; Viziano, A.; Granito, I.; Antonuccio, G.; Felicioni, A.; Loberti, M.; Carlino, P.; Micarelli, R.X.; Alessandrini, M. Combination of In-Situ Collagen Injection and Rehabilitative Treatment in Long-Lasting Facial Nerve Palsy: A Pilot Randomized Controlled Trial. Eur. J. Phys. Rehabil. Med. 2021, 57, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Godek, P. Collagen Therapy in Lumbar Spondylosis—A Pilot Study. Does the Route of Administration Matter? Ortop. Traumatol. Rehabil. 2019, 21, 427–436. [Google Scholar] [CrossRef]
- Pavelka, K.; Jarosova, H.; Milani, L.; Prochazka, Z.; Kostiuk, P.; Kotlarova, L.; Meroni, A.M.; Sliva, J. Efficacy and Tolerability of Injectable Collagen-Containing Products in Comparison to Trimecaine in Patients with Acute Lumbar Spine Pain (Study FUTURE-MD-Back Pain). Physiol. Res. 2019, 68, S65–S74. [Google Scholar] [CrossRef]
- Godek, P.; Szczepanowska-Wolowiec, B.; Golicki, D. Collagen and PRP in Partial Thickness Rotator Cuff Injuries. Friends Or Only Indifferent Neighbours? Randomized Controlled Trial. BMC Musculoskelet. Disord. 2022, 23, 1109. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, M.-W.; Park, D.Y. Indirect Ultrasound Guidance Increased Accuracy of the Glenohumeral Injection Using the Superior Approach: A Cadaveric Study of Injection Accuracy. Ann. Rehabil. Med. 2013, 37, 202. [Google Scholar] [CrossRef]
- Smith, N.; Liew, Z.; Johnson, S.; Ellard, D.R.; Underwood, M.; Kearney, R. A Systematic Review of the Methods and Drugs Used for Performing Suprascapular Nerve Block Injections for the Non-Surgical Management of Chronic Shoulder Pain. Br. J. Pain 2021, 15, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Thomajan, C.H. A Method for Entubulating Exposed Nerve Ends Following Neurectomy Using a Porcine Extracellular Matrix Nerve Cap. Foot Ankle Spec. 2022, 15, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Tork, S.; Faleris, J.; Engemann, A.; Deister, C.; DeVinney, E.; Valerio, I.L. Application of a Porcine Small Intestine Submucosa Nerve Cap for Prevention of Neuromas and Associated Pain. Tissue Eng. Part A 2020, 26, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Randelli, F.; Menon, A.; Giai Via, A.; Mazzoleni, M.; Sciancalepore, F.; Brioschi, M.; Gagliano, N. Effect of a Collagen-Based Compound on Morpho-Functional Properties of Cultured Human Tenocytes. Cells 2018, 7, 246. [Google Scholar] [CrossRef]
- Randelli, F.; Sartori, P.; Carlomagno, C.; Bedoni, M.; Menon, A.; Vezzoli, E.; Sommariva, M.; Gagliano, N. The Collagen-Based Medical Device MD-Tissue Acts as a Mechanical Scaffold Influencing Morpho-Functional Properties of Cultured Human Tenocytes. Cells 2020, 9, 2641. [Google Scholar] [CrossRef]





| ID_Code | Sex | Age (yo) |
|---|---|---|
| MN_001 | F | 61 |
| MN_002 | F | 62 |
| MN_003 | F | 51 |
| MN_004 | F | 55 |
| MN_005 | F | 44 |
| ID_Code | Imaging | Site | MN Dimension |
|---|---|---|---|
| MN_001 | MRI/US | SIS | <10 mm |
| MN_002 | US | SIS | <10 mm |
| MN_003 | MRI/US | SIS | <10 mm |
| MN_004 | US | SIS | <10 mm |
| MN_005 | US | SIS | <10 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giarda, F.; Agostini, A.; Colonna, S.; Sciumè, L.; Meroni, A.; Beretta, G.; Dalla Costa, D. Infiltrative Type I Collagen in the Treatment of Morton’s Neuroma: A Mini-Series. J. Clin. Med. 2023, 12, 4640. https://doi.org/10.3390/jcm12144640
Giarda F, Agostini A, Colonna S, Sciumè L, Meroni A, Beretta G, Dalla Costa D. Infiltrative Type I Collagen in the Treatment of Morton’s Neuroma: A Mini-Series. Journal of Clinical Medicine. 2023; 12(14):4640. https://doi.org/10.3390/jcm12144640
Chicago/Turabian StyleGiarda, Federico, Adele Agostini, Stefano Colonna, Luciana Sciumè, Alberto Meroni, Giovanna Beretta, and Davide Dalla Costa. 2023. "Infiltrative Type I Collagen in the Treatment of Morton’s Neuroma: A Mini-Series" Journal of Clinical Medicine 12, no. 14: 4640. https://doi.org/10.3390/jcm12144640
APA StyleGiarda, F., Agostini, A., Colonna, S., Sciumè, L., Meroni, A., Beretta, G., & Dalla Costa, D. (2023). Infiltrative Type I Collagen in the Treatment of Morton’s Neuroma: A Mini-Series. Journal of Clinical Medicine, 12(14), 4640. https://doi.org/10.3390/jcm12144640





