Significance of Early Postoperative Magnetic Resonance Imaging following Intracranial Meningioma Resection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Enrollment and Protocol for MRI
2.2. Data Collection
2.3. Statistical Analysis
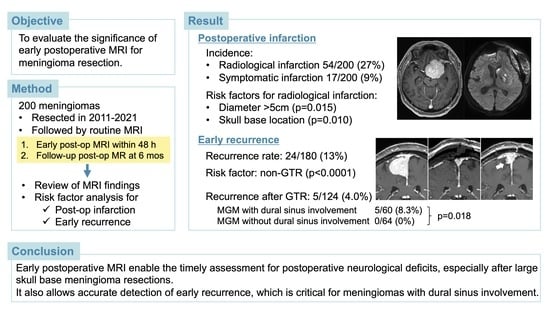
3. Results
3.1. Patient and Tumor Characteristics
3.2. Early Postoperative MRI Findings and Risk Factors for Postoperative Infarction
3.3. Late Postoperative MRI Findings and Risk Factors for Early Recurrence
4. Discussion
4.1. Significance of the Early Postoperative MRI in Brain Tumor Surgery
4.2. Role of Early Postoperative MRI to Detect Ischemia after Meningioma Resection
4.3. Role of Early Postoperative MRI to Evaluate Recurrence of Meningiomas
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albert, F.K.; Forsting, M.; Sartor, K.; Adams, H.P.; Kunze, S. Early postoperative magnetic resonance imaging after resection of malignant glioma: Objective evaluation of residual tumor and its influence on regrowth and prognosis. Neurosurgery 1994, 34, 45–60, discussion 60–61. [Google Scholar] [CrossRef] [PubMed]
- Gempt, J.; Förschler, A.; Buchmann, N.; Pape, H.; Ryang, Y.M.; Krieg, S.M.; Zimmer, C.; Meyer, B.; Ringel, F. Postoperative ischemic changes following resection of newly diagnosed and recurrent gliomas and their clinical relevance. J. Neurosurg. 2013, 118, 801–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olesrud, I.C.; Schulz, M.K.; Marcovic, L.; Kristensen, B.W.; Pedersen, C.B.; Kristiansen, C.; Poulsen, F.R. Early postoperative MRI after resection of brain metastases-complete tumour resection associated with prolonged survival. Acta Neurochir. 2019, 161, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Gempt, J.; Gerhardt, J.; Toth, V.; Hüttinger, S.; Ryang, Y.M.; Wostrack, M.; Krieg, S.M.; Meyer, B.; Förschler, A.; Ringel, F. Postoperative ischemic changes following brain metastasis resection as measured by diffusion-weighted magnetic resonance imaging. J. Neurosurg. 2013, 119, 1395–1400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhilali, L.M.; Little, A.S.; Yuen, K.C.J.; Lee, J.; Ho, T.K.; Fakhran, S.; White, W.L. Early postoperative MRI and detection of residual adenoma after transsphenoidal pituitary surgery. J. Neurosurg. 2020, 134, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2015–2019. Neuro Oncol. 2022, 24 (Suppl. S5), v1–v95. [Google Scholar] [CrossRef] [PubMed]
- Jakola, A.S.; Berntsen, E.M.; Christensen, P.; Gulati, S.; Unsgård, G.; Kvistad, K.A.; Solheim, O. Surgically acquired deficits and diffusion weighted MRI changes after glioma resection—A matched case-control study with blinded neuroradiological assessment. PLoS ONE 2014, 9, e101805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, R.B.; Gutin, P.H.; Rai, S.N.; Zhang, L.; Krol, G.; DeAngelis, L.M. Use of diffusion weighted MRI in predicting early post-operative outcome of a new neurological deficit after brain tumor resection. Neurosurgery 2006, 59, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Dützmann, S.; Geßler, F.; Bink, A.; Quick, J.; Franz, K.; Seifert, V.; Senft, C. Risk of ischemia in glioma surgery: Comparison of first and repeat procedures. J. Neurooncol. 2012, 107, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Strand, P.S.; Berntsen, E.M.; Fyllingen, E.H.; Sagberg, L.M.; Reinertsen, I.; Gulati, S.; Bouget, D.; Solheim, O. Brain infarctions after glioma surgery: Prevalence, radiological characteristics and risk factors. Acta Neurochir. 2021, 163, 3097–3108. [Google Scholar] [CrossRef] [PubMed]
- Magill, S.T.; Nguyen, M.P.; Aghi, M.K.; Theodosopoulos, P.V.; Villanueva-Meyer, J.E.; McDermott, M.W. Postoperative diffusion-weighted imaging and neurological outcome after convexity meningioma resection. J. Neurosurg. 2021, 135, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Strand, P.S.; Sagberg, L.M.; Gulati, S.; Solheim, O. Brain infarction following meningioma surgery-incidence, risk factors, and impact on function, seizure risk, and patient-reported quality of life. Neurosurg. Rev. 2022, 45, 3237–3244. [Google Scholar] [CrossRef] [PubMed]
- Geßler, F.; Dützmann, S.; Quick, J.; Tizi, K.; Voigt, M.A.; Mutlak, H.; Vatter, H.; Seifert, V.; Senft, C. Is Postoperative Imaging Mandatory after Meningioma Removal? Results of a Prospective Study. PLoS ONE 2015, 10, e0124534. [Google Scholar] [CrossRef] [PubMed]
- Materi, J.; Mampre, D.; Ehresman, J.; Rincon-Torroella, J.; Chaichana, K.L. Predictors of recurrence and high growth rate of residual meningiomas after subtotal resection. J. Neurosurg. 2021, 134, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, T.H.; McDermott, M.W. The Simpson grade: Abandon the scale but preserve the message. J. Neurosurg. 2020, 135, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Driver, J.; Hoffman, S.E.; Tavakol, S.; Woodward, E.; Maury, E.A.; Bhave, V.; Greenwald, N.F.; Nassiri, F.; Aldape, K.; Zadeh, G.; et al. A molecularly integrated grade for meningioma. Neuro Oncol. 2022, 24, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Cha, S.; Mayo, M.C.; McDermott, M.W.; Parsa, A.T.; Chang, S.M.; Dillon, W.P.; Berger, M.S. Serial diffusion-weighted magnetic resonance imaging in cases of glioma: Distinguishing tumor recurrence from postresection injury. J. Neurosurg. 2005, 103, 428–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oya, S.; Kim, S.H.; Sade, B.; Lee, J.H. The natural history of intracranial meningiomas. J. Neurosurg. 2011, 114, 1250–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiller, M.; Tenner, M.S.; Couldwell, W.T. Effect of absorbable topical hemostatic agents on the relaxation time of blood: An in vitro study with implications for postoperative magnetic resonance imaging. J. Neurosurg. 2001, 95, 687–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Patient Characteristics (n = 200) | ||
| Mean age (years) | 61.7 (SD 12.8) | |
| Sex | Male | 71 (36%) |
| Female | 129 (65%) | |
| Initial or Re-do | Initial | 174 (87%) |
| Re-do | 26 (13%) | |
| Prior radiation | 17 (9%) | |
| Pre-op symptoms | Symptomatic | 159 (80%) |
| Asymptomatic | 41 (21%) | |
| Tumor Characteristics (n = 200) | ||
| Mean maximum diameter (cm) | 4.1 (SD 1.6) | |
| Location | Skull base | 124 (62%) |
| Non skull base | 76 (38%) | |
| Dural Sinus involvement | Involved | 114 (57%) |
| Not involved | 86 (43%) | |
| PTBE | Present | 107 (54%) |
| Absent | 93 (47%) | |
| WHO grade | I | 167 (84%) |
| II | 28 (14%) | |
| III | 5 (3%) | |
| Extent of resection | GTR | 134 (67%) |
| STR or PR | 66 (33%) | |
| Early Postoperative MRI Findings (n = 200) | ||
|---|---|---|
| Infarction | Overall | 54 (27%) |
| Symptomatic | 17 (9%) | |
| Contusion | 130 (65%) | |
| Dural sinus problems | Thrombosis | 7 (4%) |
| Stenosis | 11 (6%) | |
| Residual on MRI | 66 (33%) | |
| Additional treatment for residual | 7 (4%) | |
| Factor | Radiological Infarction | Symptomatic Infarction | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Infarction (n = 54) | No Infarction (n = 146) | p Value, Univariate | p Value, Multivariate | OR (95% CI) | Infarction (n = 17) | No Infarction (n = 183) | p Value, Univariate | p Value, Multivariate | OR (95% CI) | |
| Age > 60 years | 37 (69%) | 83 (57%) | 0.13 | 0.13 | 1.77 (0.84–3.72) | 8 (47%) | 112 (61%) | 0.25 | 0.11 | 0.40 (0.13–1.24) |
| Male sex | 23 (43%) | 48 (33%) | 0.20 | 0.34 | 1.43 (0.69–2.94) | 9 (53%) | 62 (34%) | 0.12 | 0.34 | 1.70 (0.58–5.00) |
| Redo surgeries | 10 (19%) | 16 (11%) | 0.16 | 0.89 | 0.91 (0.22–3.66) | 2 (12%) | 24 (13%) | 0.87 | 0.44 | 0.30 (0.01–6.26) |
| Prior radiation | 8 (15%) | 9 (6%) | 0.051 | 0.53 | 1.68 (0.33–8.45) | 2 (12%) | 15 (8%) | 0.61 | 0.50 | 2.86 (0.13–60.8) |
| Pre-op symptoms | 49 (91%) | 110 (75%) | 0.017 | 0.098 | 2.60 (0.84–8.06) | 16 (94%) | 143 (78%) | 0.12 | 0.27 | 3.47 (0.39–31.1) |
| Size > 5 cm | 22 (41%) | 30 (21%) | 0.004 | 0.015 | 2.56 (1.20–5.44) | 9 (53%) | 43 (24%) | 0.008 | 0.063 | 2.85 (0.95–8.57) |
| Skull base location | 40 (74%) | 84 (58%) | 0.032 | 0.010 | 3.03 (1.31–7.03) | 10 (59%) | 114 (62%) | 0.78 | 0.89 | 0.92 (0.28–3.05) |
| PTBE | 33 (61%) | 74 (51%) | 0.19 | 0.51 | 1.29 (0.61–2.71) | 12 (71%) | 95 (52%) | 0.14 | 0.37 | 1.78 (0.51–6.24) |
| Dural sinus involvement | 33 (61%) | 81 (55%) | 0.48 | 0.83 | 0.92 (0.44–1.95) | 10 (59%) | 104 (57%) | 0.87 | 0.56 | 0.70 (0.21–2.33) |
| Non-GTR | 25 (46%) | 41 (28%) | 0.015 | 0.50 | 1.29 (0.62–2.69) | 9 (53%) | 57 (31%) | 0.068 | 0.15 | 2.32 (0.73–7.37) |
| WHO grade II or III | 15 (28%) | 18 (12%) | 0.009 | 0.098 | 2.27 (0.86–6.01) | 6 (35%) | 27 (15%) | 0.029 | 0.28 | 2.06 (0.56–7.61) |
| Factor | Recurrence (n = 24) | No Recurrence (n = 156) | p Value, Univariate | p Value, Multivariate | OR (95% CI) |
|---|---|---|---|---|---|
| Age > 60 years | 16 (67%) | 91 (58%) | 0.44 | 0.99 | 1.00 (0.34–2.92) |
| Male sex | 10 (42%) | 53 (34%) | 0.46 | 0.56 | 1.36 (0.49–3.79) |
| Redo surgeries | 6 (25%) | 15 (10%) | 0.029 | 0.18 | 3.34 (0.58–19.1) |
| Prior radiation | 3 (13%) | 9 (6%) | 0.22 | 0.47 | 0.45 (0.05–4.02) |
| Size > 5 cm | 9 (38%) | 36 (23%) | 0.13 | 0.41 | 1.58 (0.54–4.62) |
| Skull base location | 16 (67%) | 94 (60%) | 0.55 | 0.57 | 0.72 (0.23–2.23) |
| Dural sinus involvement | 18 (75%) | 81 (52%) | 0.03 | 0.38 | 1.66 (0.54–5.08) |
| Non-GTR | 19 (79%) | 37 (24%) | <0.0001 | <0.0001 | 11.4 (3.64–36.0) |
| WHO grade II or III | 5 (21%) | 22 (14%) | 0.39 | 0.67 | 0.74 (0.19–2.90) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, M.; Miyazaki, M.; Oya, S. Significance of Early Postoperative Magnetic Resonance Imaging following Intracranial Meningioma Resection. J. Clin. Med. 2023, 12, 4733. https://doi.org/10.3390/jcm12144733
Inoue M, Miyazaki M, Oya S. Significance of Early Postoperative Magnetic Resonance Imaging following Intracranial Meningioma Resection. Journal of Clinical Medicine. 2023; 12(14):4733. https://doi.org/10.3390/jcm12144733
Chicago/Turabian StyleInoue, Mizuho, Masaya Miyazaki, and Soichi Oya. 2023. "Significance of Early Postoperative Magnetic Resonance Imaging following Intracranial Meningioma Resection" Journal of Clinical Medicine 12, no. 14: 4733. https://doi.org/10.3390/jcm12144733
APA StyleInoue, M., Miyazaki, M., & Oya, S. (2023). Significance of Early Postoperative Magnetic Resonance Imaging following Intracranial Meningioma Resection. Journal of Clinical Medicine, 12(14), 4733. https://doi.org/10.3390/jcm12144733