Utility of Serum Ferritin for Predicting Myalgic Encephalomyelitis/Chronic Fatigue Syndrome in Patients with Long COVID
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Design and Patients
2.2. Clinical Characteristics
2.3. Laboratory Examinations
2.4. Statistical Analysis
2.5. Ethical Approval
3. Results
3.1. Patients’ Backgrounds
3.2. Severity of Fatigue-Related Symptoms
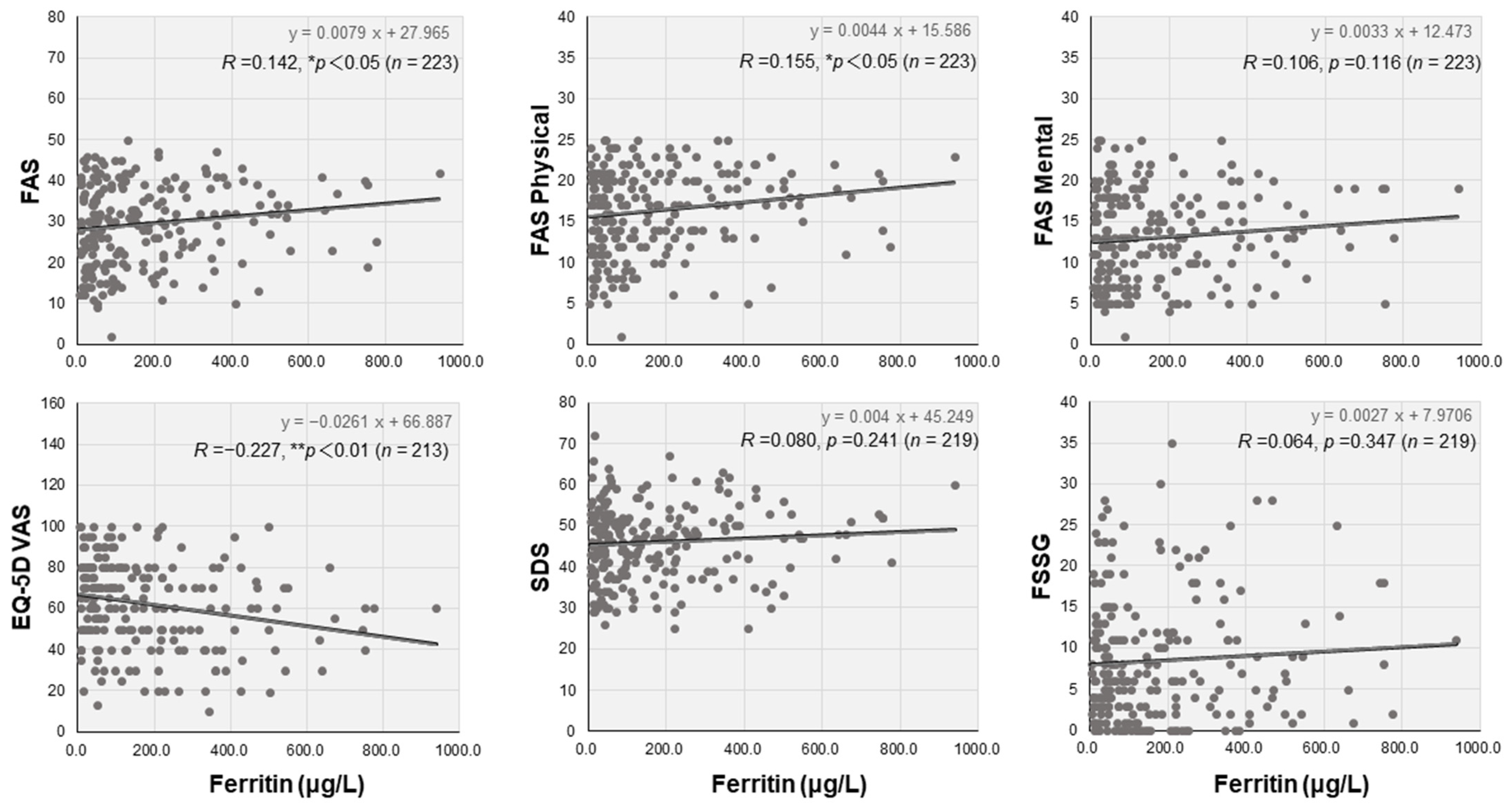
3.3. Laboratory Data and Correlations with the Self-Rating Scales
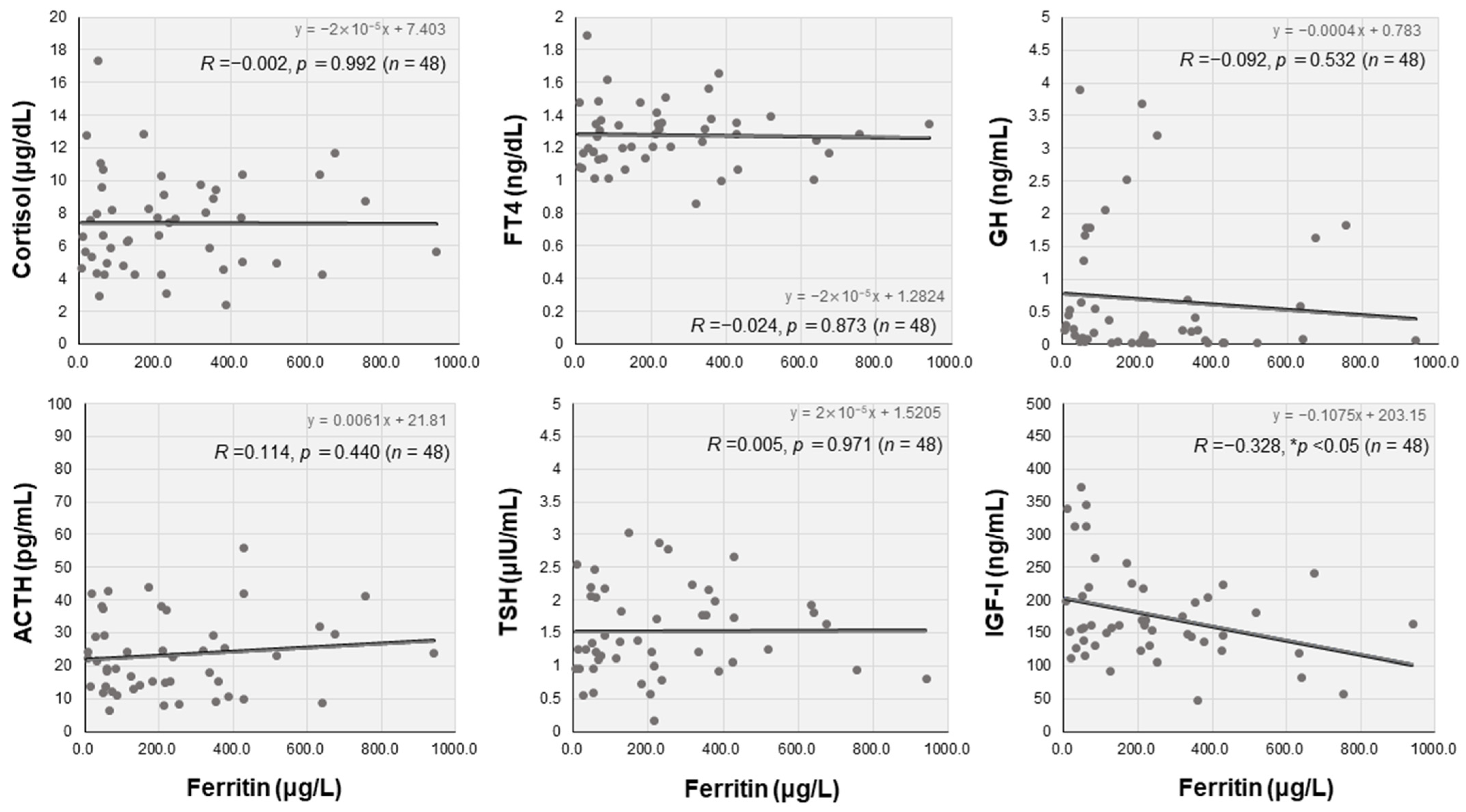
3.4. Endocrine Characteristics and Correlations with Serum Ferritin Levels
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lam, I.C.H.; Wong, C.K.H.; Zhang, R.; Chui, C.S.L.; Lai, F.T.T.; Li, X.; Chan, E.W.Y.; Luo, H.; Zhang, Q.; Man, K.K.C.; et al. Long-term post-acute sequelae of COVID-19 infection: A retrospective, multi-database cohort study in Hong Kong and the UK. EClinicalMedicine 2023, 60, 102000. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, A.V.; Jayadevan, R.; Sashidharan, S. Long COVID: An overview. Diabetes Metab. Syndr. 2021, 15, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef]
- Otsuka, Y.; Tokumasu, K.; Nakano, Y.; Honda, H.; Sakurada, Y.; Sunada, N.; Omura, D.; Hasegawa, K.; Hagiya, H.; Obika, M.; et al. Clinical characteristics of Japanese patients who visited a COVID-19 aftercare clinic for post-acute sequelae of COVID-19/long COVID. Cureus 2021, 13, e18568. [Google Scholar] [CrossRef] [PubMed]
- Nasserie, T.; Hittle, M.; Goodman, S.N. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: A systematic review. JAMA Netw. Open 2021, 4, e2111417. [Google Scholar] [CrossRef]
- Sandler, C.X.; Wyller, V.B.B.; Moss-Morris, R.; Buchwald, D.; Crawley, E.; Hautvast, J.; Katz, B.Z.; Knoop, H.; Little, P.; Taylor, R.; et al. Long COVID and post-infective fatigue syndrome: A review. Open Forum Infect. Dis. 2021, 8, ofab440. [Google Scholar] [CrossRef]
- Soejima, Y.; Otsuka, Y.; Tokumasu, K.; Nakano, Y.; Harada, K.; Nakamoto, K.; Sunada, N.; Sakurada, Y.; Hasegawa, K.; Hagiya, H.; et al. Late-onset hypogonadism in a male patient with long COVID diagnosed by exclusion of ME/CFS. Medicina 2022, 58, 536. [Google Scholar] [CrossRef] [PubMed]
- Sunada, N.; Honda, H.; Nakano, Y.; Yamamoto, K.; Tokumasu, K.; Sakurada, Y.; Matsuda, Y.; Hasegawa, T.; Otsuka, Y.; Obika, M.; et al. Hormonal trends in patients suffering from long COVID symptoms. Endocr. J. 2022, 69, 1173–1181. [Google Scholar] [CrossRef]
- Tokumasu, K.; Honda, H.; Sunada, N.; Sakurada, Y.; Matsuda, Y.; Yamamoto, K.; Nakano, Y.; Hasegawa, T.; Yamamoto, Y.; Otsuka, Y.; et al. Clinical characteristics of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) diagnosed in patients with long COVID. Medicina 2022, 58, 850. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Otsuka, Y.; Sunada, N.; Tokumasu, K.; Nakano, Y.; Honda, H.; Sakurada, Y.; Hagiya, H.; Hanayama, Y.; Otsuka, F. Detection of male hypogonadism in patients with post COVID-19 condition. J. Clin. Med. 2022, 11, 1955. [Google Scholar] [CrossRef]
- Boaventura, P.; Macedo, S.; Ribeiro, F.; Jaconiano, S.; Soares, P. Post-COVID-19 condition: Where are we now? Life 2022, 12, 517. [Google Scholar] [CrossRef]
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long covid-mechanisms, risk factors, and management. BMJ 2021, 374, n1648. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.J.; Son, C.G. Review of case definitions for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J. Transl. Med. 2020, 18, 289. [Google Scholar] [CrossRef] [PubMed]
- Deumer, U.S.; Varesi, A.; Floris, V.; Savioli, G.; Mantovani, E.; Lopez-Carrasco, P.; Rosati, G.M.; Prasad, S.; Ricevuti, G. Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): An overview. J. Clin. Med. 2021, 10, 4786. [Google Scholar] [CrossRef] [PubMed]
- Sotzny, F.; Blanco, J.; Capelli, E.; Castro-Marrero, J.; Steiner, S.; Murovska, M.; Scheibenbogen, C.; European Network on ME/CFS. Myalgic encephalomyelitis/chronic fatigue syndrome—Evidence for an autoimmune disease. Autoimmun. Rev. 2018, 17, 601–609. [Google Scholar] [CrossRef]
- Lim, E.J.; Ahn, Y.C.; Jang, E.S.; Lee, S.W.; Lee, S.H.; Son, C.G. Systematic review and meta-analysis of the prevalence of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). J. Transl. Med. 2020, 18, 100. [Google Scholar] [CrossRef]
- Dibble, J.J.; McGrath, S.J.; Ponting, C.P. Genetic risk factors of ME/CFS: A critical review. Hum. Mol. Genet. 2020, 29, R117–R124. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Straus, S.E.; Hickie, I.; Sharpe, M.C.; Dobbins, J.G.; Komaroff, A. The chronic fatigue syndrome: A comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann. Intern. Med. 1994, 121, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, B.M.; Jain, A.K.; De Meirleir, K.L.; Peterson, D.L.; Klimas, N.G.; Lerner, A.M.; Bested, A.C.; Flor-Henry, P.; Joshi, P.; Powles, A.P. Myalgic encephalomyelitis/chronic fatigue syndrome: Clinical working case definition, diagnostic and treatment protocols. J. Chronic Fatigue Syndr. 2003, 11, 7–115. [Google Scholar] [CrossRef]
- Clayton, E.W. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: An IOM report on redefining an illness. JAMA 2015, 313, 1101–1102. [Google Scholar] [CrossRef]
- de Kleijn, W.P.; De Vries, J.; Wijnen, P.A.; Drent, M. Minimal (clinically) important differences for the Fatigue Assessment Scale in sarcoidosis. Respir. Med. 2011, 105, 1388–1395. [Google Scholar] [CrossRef] [Green Version]
- Drent, M.; Lower, E.E.; De Vries, J. Sarcoidosis-associated fatigue. Eur. Respir. J. 2012, 40, 255–263. [Google Scholar] [CrossRef] [Green Version]
- Rabin, R.; de Charro, F. EQ-5D: A measure of health status from the EuroQol Group. Ann. Med. 2001, 33, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Kawada, T.; Suzuki, S.; Kubota, F.; Ohnishi, N.; Satoh, K. Content and cross validity of the Todai Health Index depression scale in relation to the Center for Epidemiologic Studies Depression Scale and the Zung Self-rating Depression Scale. J. Occup. Health 1999, 41, 154–159. [Google Scholar] [CrossRef] [Green Version]
- Zung, W.W.; Richards, C.B.; Short, M.J. Self-rating depression scale in an outpatient clinic. Further validation of the SDS. Arch. Gen. Psychiatry 1965, 13, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Kusano, M.; Shimoyama, Y.; Sugimoto, S.; Kawamura, O.; Maeda, M.; Minashi, K.; Kuribayashi, S.; Higuchi, T.; Zai, H.; Ino, K.; et al. Development and evaluation of FSSG: Frequency scale for the symptoms of GERD. J. Gastroenterol. 2004, 39, 888–891. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Kernan, K.F.; Carcillo, J.A. Hyperferritinemia and inflammation. Int. Immunol. 2017, 29, 401–409. [Google Scholar] [CrossRef] [Green Version]
- Senjo, H.; Higuchi, T.; Okada, S.; Takahashi, O. Hyperferritinemia: Causes and significance in a general hospital. Hematology 2018, 23, 817–822. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Knovich, M.A.; Coffman, L.G.; Torti, F.M.; Torti, S.V. Serum ferritin: Past, present and future. Biochim. Biophys. Acta 2010, 1800, 760–769. [Google Scholar] [CrossRef] [Green Version]
- Cortes Rivera, M.; Mastronardi, C.; Silva-Aldana, C.T.; Arcos-Burgos, M.; Lidbury, B.A. Myalgic encephalomyelitis/chronic fatigue syndrome: A comprehensive review. Diagnostics 2019, 9, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, G.; Anderson, G.; Maes, M. Hypothalamic-pituitary-adrenal hypofunction in myalgic encephalomyelitis (ME)/chronic fatigue syndrome (CFS) as a consequence of activated immune-inflammatory and oxidative and nitrosative pathways. Mol. Neurobiol. 2017, 54, 6806–6819. [Google Scholar] [CrossRef] [PubMed]
- Rasa, S.; Nora-Krukle, Z.; Henning, N.; Eliassen, E.; Shikova, E.; Harrer, T.; Scheibenbogen, C.; Murovska, M.; Prusty, B.K.; European Network on ME/CFS. Chronic viral infections in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J. Transl. Med. 2018, 16, 268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bansal, R.; Gubbi, S.; Koch, C.A. COVID-19 and chronic fatigue syndrome: An endocrine perspective. J. Clin. Transl. Endocrinol. 2022, 27, 100284. [Google Scholar] [CrossRef]
- Desai, A.D.; Lavelle, M.; Boursiquot, B.C.; Wan, E.Y. Long-term complications of COVID-19. Am. J. Physiol. Cell Physiol. 2022, 322, C1–C11. [Google Scholar] [CrossRef]
- Tana, C.; Bentivegna, E.; Cho, S.J.; Harriott, A.M.; García-Azorín, D.; Labastida-Ramirez, A.; Ornello, R.; Raffaelli, B.; Beltrán, E.R.; Ruscheweyh, R.; et al. Long COVID headache. J. Headache Pain. 2022, 23, 93. [Google Scholar] [CrossRef]
- Tana, C.; Giamberardino, M.A.; Martelletti, P. Long COVID and especially headache syndromes. Curr. Opin. Neurol. 2023, 36, 168–174. [Google Scholar] [CrossRef]
- Fukuda, S.; Nojima, J.; Motoki, Y.; Yamaguti, K.; Nakatomi, Y.; Okawa, N.; Fujiwara, K.; Watanabe, Y.; Kuratsune, H. A potential biomarker for fatigue: Oxidative stress and anti-oxidative activity. Biol. Psychol. 2016, 118, 88–93. [Google Scholar] [CrossRef]
- Loebel, M.; Grabowski, P.; Heidecke, H.; Bauer, S.; Hanitsch, L.G.; Wittke, K.; Meisel, C.; Reinke, P.; Volk, H.D.; Fluge, O.; et al. Antibodies to beta adrenergic and muscarinic cholinergic receptors in patients with Chronic Fatigue Syndrome. Brain Behav. Immun. 2016, 52, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Baklund, I.H.; Dammen, T.; Moum, T.A.; Kristiansen, W.; Duarte, D.S.; Castro-Marrero, J.; Helland, I.B.; Strand, E.B. Evaluating routine blood tests according to clinical symptoms and diagnostic criteria in individuals with myalgic encephalomyelitis/chronic fatigue syndrome. J. Clin. Med. 2021, 10, 3105. [Google Scholar] [CrossRef]
- Bateman, L.; Bested, A.C.; Bonilla, H.F.; Chheda, B.V.; Chu, L.; Curtin, J.M.; Dempsey, T.T.; Dimmock, M.E.; Dowell, T.G.; Felsenstein, D.; et al. Myalgic encephalomyelitis/chronic fatigue syndrome: Essentials of diagnosis and management. Mayo Clin. Proc. 2021, 96, 2861–2878. [Google Scholar] [CrossRef]
- Nacul, L.; Authier, F.J.; Scheibenbogen, C.; Lorusso, L.; Helland, I.B.; Martin, J.A.; Sirbu, C.A.; Mengshoel, A.M.; Polo, O.; Behrends, U.; et al. European Network on Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (EUROMENE): Expert consensus on the diagnosis, service provision, and care of people with ME/CFS in Europe. Medicina 2021, 57, 510. [Google Scholar] [CrossRef]
- Mahroum, N.; Alghory, A.; Kiyak, Z.; Alwani, A.; Seida, R.; Alrais, M.; Shoenfeld, Y. Ferritin—From iron, through inflammation and autoimmunity, to COVID-19. J. Autoimmun. 2022, 126, 102778. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Li, H.; Li, L.; Liu, C.; Yan, S.; Chen, H.; Li, Y. Ferritin in the coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. J. Clin. Lab. Anal. 2020, 34, e23618. [Google Scholar] [CrossRef]
- Kaushal, K.; Kaur, H.; Sarma, P.; Bhattacharyya, A.; Sharma, D.J.; Prajapat, M.; Pathak, M.; Kothari, A.; Kumar, S.; Rana, S.; et al. Serum ferritin as a predictive biomarker in COVID-19. a systematic review, meta-analysis and meta-regression analysis. J. Crit. Care 2022, 67, 172–181. [Google Scholar] [CrossRef]
- Clemente, I.; Sinatti, G.; Cirella, A.; Santini, S.J.; Balsano, C. Alteration of inflammatory parameters and psychological post-traumatic syndrome in long-COVID patients. Int. J. Environ. Res. Public Health 2022, 19, 7103. [Google Scholar] [CrossRef] [PubMed]
- Elanwar, R.; Hussein, M.; Magdy, R.; Eid, R.A.; Yassien, A.; Abdelsattar, A.S.; Alsharaway, L.A.; Fathy, W.; Hassan, A.; Kamal, Y.S. Physical and mental fatigue in subjects recovered from COVID-19 infection: A case-control study. Neuropsychiatr. Dis. Treat. 2021, 17, 2063–2071. [Google Scholar] [CrossRef] [PubMed]
- Pasini, E.; Corsetti, G.; Romano, C.; Scarabelli, T.M.; Chen-Scarabelli, C.; Saravolatz, L.; Dioguardi, F.S. Serum metabolic profile in patients with long-covid (PASC) syndrome: Clinical implications. Front. Med. 2021, 8, 714426. [Google Scholar] [CrossRef]
- Sonnweber, T.; Grubwieser, P.; Sahanic, S.; Böhm, A.K.; Pizzini, A.; Luger, A.; Schwabl, C.; Koppelstätter, S.; Kurz, K.; Puchner, B.; et al. The Impact of Iron Dyshomeostasis and Anaemia on Long-Term Pulmonary Recovery and Persisting Symptom Burden after COVID-19: A Prospective Observational Cohort Study. Metabolites 2022, 12, 546. [Google Scholar] [CrossRef]
- Ehsani, S. COVID-19 and iron dysregulation: Distant sequence similarity between hepcidin and the novel coronavirus spike glycoprotein. Biol. Direct 2020, 15, 19. [Google Scholar] [CrossRef]
- Kedor, C.; Freitag, H.; Meyer-Arndt, L.; Wittke, K.; Hanitsch, L.G.; Zoller, T.; Steinbeis, F.; Haffke, M.; Rudolf, G.; Heidecker, B.; et al. A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nat. Commun. 2022, 13, 5104. [Google Scholar] [CrossRef]
- Ruiz-Nunez, B.; Tarasse, R.; Vogelaar, E.F.; Janneke Dijck-Brouwer, D.A.; Muskiet, F.A.J. Higher prevalence of “Low T3 Syndrome” in patients with chronic fatigue syndrome: A case-control study. Front. Endocrinol. 2018, 9, 97. [Google Scholar] [CrossRef] [Green Version]
- Ackerman, D.; Gems, D. Insulin/IGF-1 and hypoxia signaling act in concert to regulate iron homeostasis in Caenorhabditis elegans. PLoS Genet. 2012, 8, e1002498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torti, F.M.; Torti, S.V. Regulation of ferritin genes and protein. Blood 2002, 99, 3505–3516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estivariz, C.F.; Ziegler, T.R. Nutrition and the insulin-like growth factor system. Endocrine 1997, 7, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Maggio, M.; De Vita, F.; Lauretani, F.; Butto, V.; Bondi, G.; Cattabiani, C.; Nouvenne, A.; Meschi, T.; Dall’Aglio, E.; Ceda, G.P. IGF-1, the cross road of the nutritional, inflammatory and hormonal pathways to frailty. Nutrients 2013, 5, 4184–4205. [Google Scholar] [CrossRef] [Green Version]


| ME/CFS (n = 50) | Non-ME/CFS (n = 89) | No Fatigue (n = 95) | p-Value | |
|---|---|---|---|---|
| Characteristics | ||||
| Sex: male/female | 24/26 | 37/52 | 38/57 | 0.482 (a) |
| Age (years), median (IQR) | 42 (30.3–51.8) | 39 (24.0–50.0) | 43 (29.5–51.0) | 0.519 (b) |
| BMI (kg/m2), median (IQR) | 24.7 (21.3–27.4) | 23.4 (20.7–25.7) | 22.7 (19.8–27.0) | 0.326 (b) |
| Days from the onset to the first visit, median (IQR) | 128 (72.3–205.5) ☨ | 73 (55.0–114.0) ☨ | 106 (73.0–152.0) | <0.01 (b) |
| Acute COVID-19 treatments | ||||
| At home (%) | 17 (34.0) | 35 (39.3) | 45 (47.4) | 0.587 (a) |
| At accommodation facilities (%) | 15 (30.0) | 35 (39.3) | 27 (28.4) | 0.357 (a) |
| Hospitalized (%) | 22 (44.0) | 27 (30.3) | 30 (31.6) | 0.139 (a) |
| Oxygen therapy (%) | 10 (20.0) | 12 (13.5) | 16 (16.8) | 0.339 (a) |
| Steroid (%) | 12 (24.0) | 13 (14.6) | 13 (14.5) | 0.176 (a) |
| Vital signs | ||||
| Systolic blood pressure (mmHg), median (IQR) | 129 (112–140) | 120 (107–130) | 120 (108–137) | 0.068 (b) |
| Diastolic blood pressure (mmHg), median (IQR) | 74 (66–82) | 73 (61–81) | 69 (63–80) | 0.581 (b) |
| Heart rate (bpm), median (IQR) | 82 (75–89) | 81 (73–91) | 79 (73–89) | 0.627 (b) |
| ME/CFS (n = 48) | Non-ME/CFS (n = 87) | No Fatigue (n = 89) | p-Value | |
|---|---|---|---|---|
| Blood cell counts | ||||
| WBC (×103/μL) | 6.62 (5.32–7.44) | 5.89 (4.77–7.09) | 6.01 (4.75–7.58) | 0.560 |
| RBC (×106/μL) | 4.72 (4.42–5.00) | 4.54 (4.23–4.94) | 4.51 (4.14–4.91) | 0.187 |
| Hb (g/dL) | 14.70(13.9–15.7) | 14.3 (13.1–15.3) | 14.1 (13.0–15.2) | 0.091 |
| Plt (×103/μL) | 265.0 (215.3–308.3) | 262.0 (236.0–314.5) | 253.5 (228.5–291.8) | 0.611 |
| Biochemistry | ||||
| TP (g/dL) | 7.2 (7.0–7.5) | 7.1 (6.9–7.5) | 7.1 (6.9–7.4) | 0.284 |
| Alb (g/dL) | 4.5 (4.3–4.7) | 4.4 (4.2–4.6) | 4.4 (4.1–4.6) | 0.083 |
| T-Bil (mg/dL) | 0.72 (0.48–0.89) | 0.60 (0.49–0.83) | 0.60 (0.47–0.82) | 0.368 |
| AST (U/L) | 19.5 (15.0–25.0) | 19.0 (15.0–24.0) | 19.0 (16.0–23.0) | 0.952 |
| ALT (U/L) | 17.0 (12.8–33.3) | 16.0 (11.5–29.5) | 17.0 (11.0–27.3) | 0.505 |
| ALP (U/L) | 68.0 (58.3–84.0) | 65.0 (59.5–82.5) | 69.0 (58.0–94.3) | 0.632 |
| CK (U/L) | 75.0 (58.8–111.0) | 72.0 (56.5–97.5) | 77.0 (59.0–112.0) | 0.522 |
| UN (mg/dL) | 12.7 (10.5–14.7) | 11.5 (10.0–13.8) | 12.9 (11.0–15.5) | 0.086 |
| Cr (mg/dL) | 0.73 (0.60–0.83) | 0.69 (0.59–0.80) | 0.67 (0.59–0.77) | 0.487 |
| LDL-C (mg/dL) | 118.5 (102.0–146.3) | 117.0 (99.5–149.5) | 123.0 (88.0–138.0) | 0.625 |
| BS (mg/dL) | 99.0 (93.8–108.3) | 100.0 (90.0–110.0) | 101.0 (91.3–115.0) | 0.767 |
| CRP (mg/dL) | 0.06 (0.03–0.10) | 0.06 (0.03–0.14) | 0.07 (0.02–0.16) | 0.770 |
| ESR (mm/hr) | 7.0 (3.5–11.3) | 9.0 (5.0–17.0) | 8.0 (5.0–14.0) | 0.086 |
| Ferritin (μg/L) | 193.0 (58.8–353.8) ☨ | 98.2 (40.4–251.5) | 86.7 (37.5–209.0) ☨ | <0.05 |
| IgG (mg/dL) | 1193.9 (1075.2–1283.1) | 1188.8 (1061.2–1353.6) | 1258.3 (1055.6–1391.2) | 0.533 |
| Fibrinogen (mg/dL) | 282 (256–308) | 298 (257–348) | 288 (253–348) | 0.194 |
| D-dimer (μg/L) | 0.5 (0.5–0.5) | 0.5 (0.5–0.5) | 0.5 (0.5–0.5) | 0.353 |
| ME/CFS (n = 48) | Non-ME/CFS (n = 87) | No Fatigue (n = 89) | p-Value | |
|---|---|---|---|---|
| Endocrine data | ||||
| Cortisol (μg/dL) | 7.1 (5.0–9.3) | 7.8 (5.4–10.2) | 6.5 (4.2–8.6) | 0.102 |
| ACTH (pg/mL) | 22.2 (14.1–29.6) | 20.5 (14.9–28.5) | 19.6 (13.9–25.6) | 0.598 |
| FT4 (ng/dL) | 1.28 (1.16–1.36) | 1.26 (1.17–1.41) | 1.24 (1.14–1.35) | 0.426 |
| TSH (μIU/mL) | 1.37 (0.99–2.01) ☨ | 1.06 (0.80–1.56) ☨ | 1.41 (1.01–2.08) | <0.05 |
| GH (ng/mL) | 0.22 (0.05–0.67) ☨ | 0.19 (0.07–0.66) | 0.37 (0.17–1.11) ☨ | <0.01 |
| IGF-I (ng/mL) | 161 (132–211) | 145 (122–191) | 139 (104.0–199.0) | 0.121 |
| ACTH/Cortisol | 3.32 (2.09–4.57) | 2.86 (2.03–4.02) | 3.30 (2.43–4.59) | 0.348 |
| FT4/TSH | 0.87 (0.64–1.22) ☨ | 1.15 (0.83–1.69) ☨ | 0.85 (0.56–1.34) | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamamoto, Y.; Otsuka, Y.; Tokumasu, K.; Sunada, N.; Nakano, Y.; Honda, H.; Sakurada, Y.; Hasegawa, T.; Hagiya, H.; Otsuka, F. Utility of Serum Ferritin for Predicting Myalgic Encephalomyelitis/Chronic Fatigue Syndrome in Patients with Long COVID. J. Clin. Med. 2023, 12, 4737. https://doi.org/10.3390/jcm12144737
Yamamoto Y, Otsuka Y, Tokumasu K, Sunada N, Nakano Y, Honda H, Sakurada Y, Hasegawa T, Hagiya H, Otsuka F. Utility of Serum Ferritin for Predicting Myalgic Encephalomyelitis/Chronic Fatigue Syndrome in Patients with Long COVID. Journal of Clinical Medicine. 2023; 12(14):4737. https://doi.org/10.3390/jcm12144737
Chicago/Turabian StyleYamamoto, Yukichika, Yuki Otsuka, Kazuki Tokumasu, Naruhiko Sunada, Yasuhiro Nakano, Hiroyuki Honda, Yasue Sakurada, Toru Hasegawa, Hideharu Hagiya, and Fumio Otsuka. 2023. "Utility of Serum Ferritin for Predicting Myalgic Encephalomyelitis/Chronic Fatigue Syndrome in Patients with Long COVID" Journal of Clinical Medicine 12, no. 14: 4737. https://doi.org/10.3390/jcm12144737
APA StyleYamamoto, Y., Otsuka, Y., Tokumasu, K., Sunada, N., Nakano, Y., Honda, H., Sakurada, Y., Hasegawa, T., Hagiya, H., & Otsuka, F. (2023). Utility of Serum Ferritin for Predicting Myalgic Encephalomyelitis/Chronic Fatigue Syndrome in Patients with Long COVID. Journal of Clinical Medicine, 12(14), 4737. https://doi.org/10.3390/jcm12144737