Clinical Characteristics and Surgical Outcomes of Metastatic Spine Tumors in the Very Elderly: A Prospective Cohort Study in a Super-Aged Society
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Patients and Procedures
2.3. Evaluation
2.4. Statistical Analysis
3. Results
3.1. Patient Demographics and Clinical Characteristics at Surgery
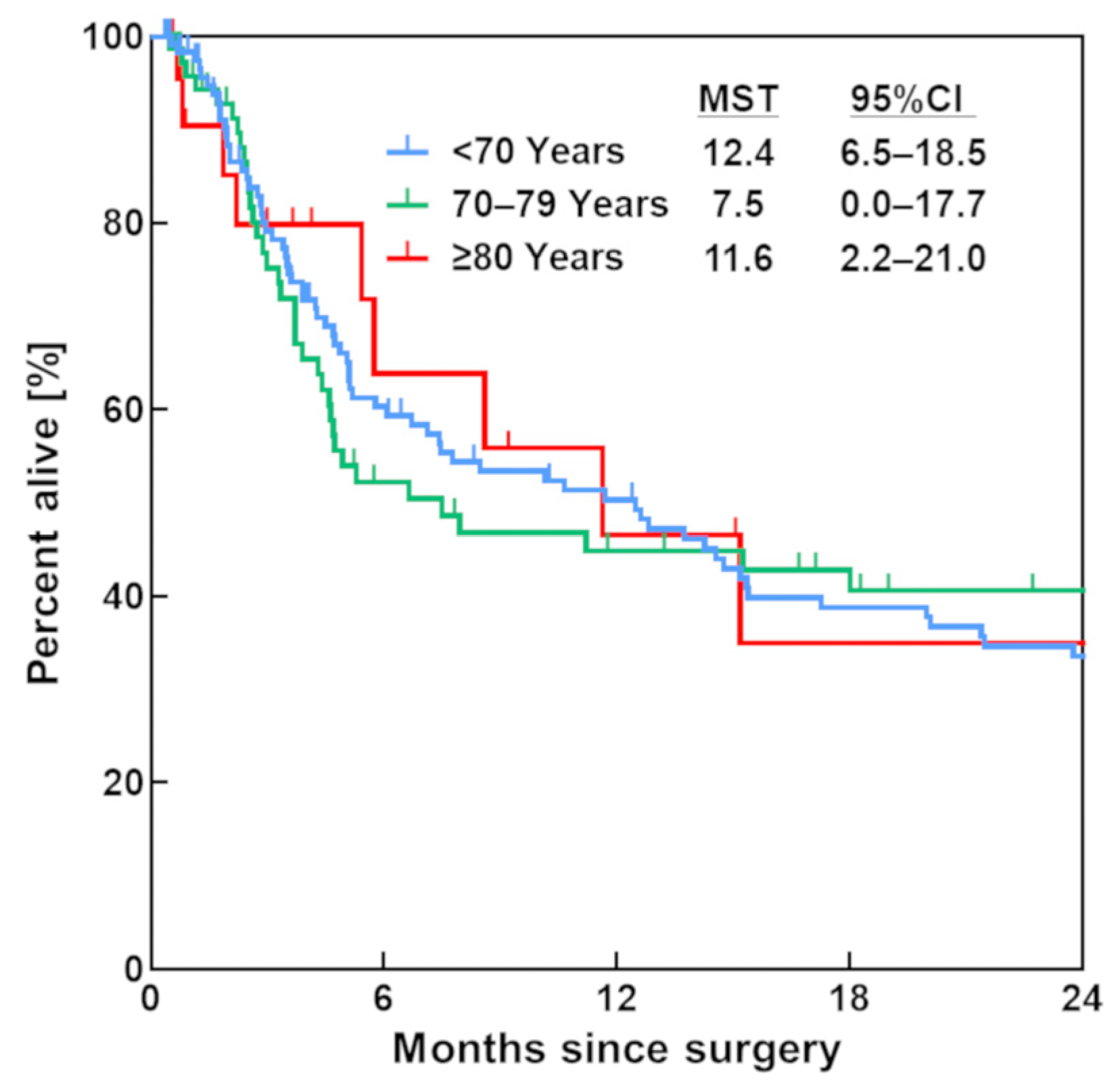
3.2. Survival Rate
3.3. Complications
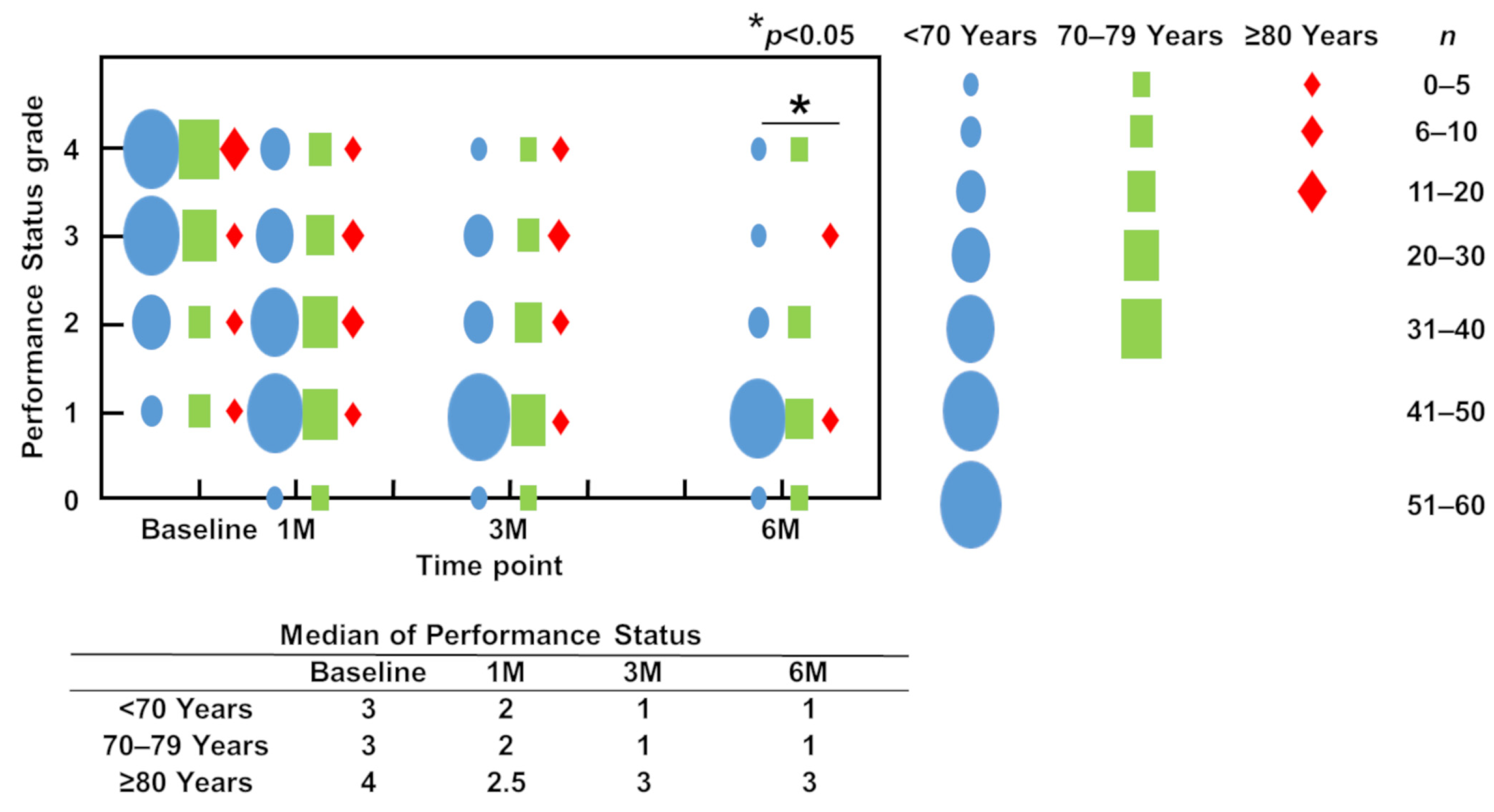
3.4. ECOGPS (PS)
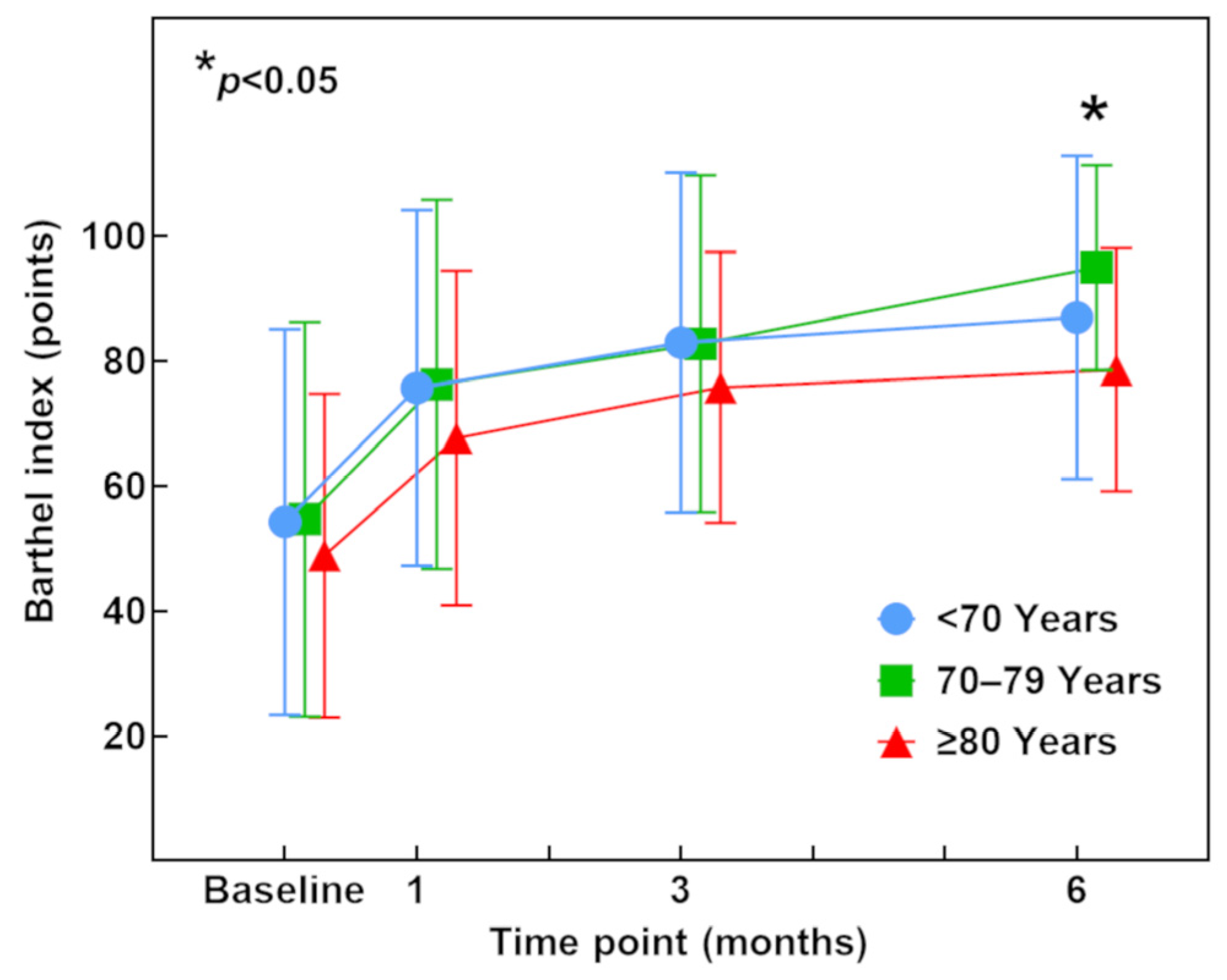
3.5. Barthel Index (ADL)
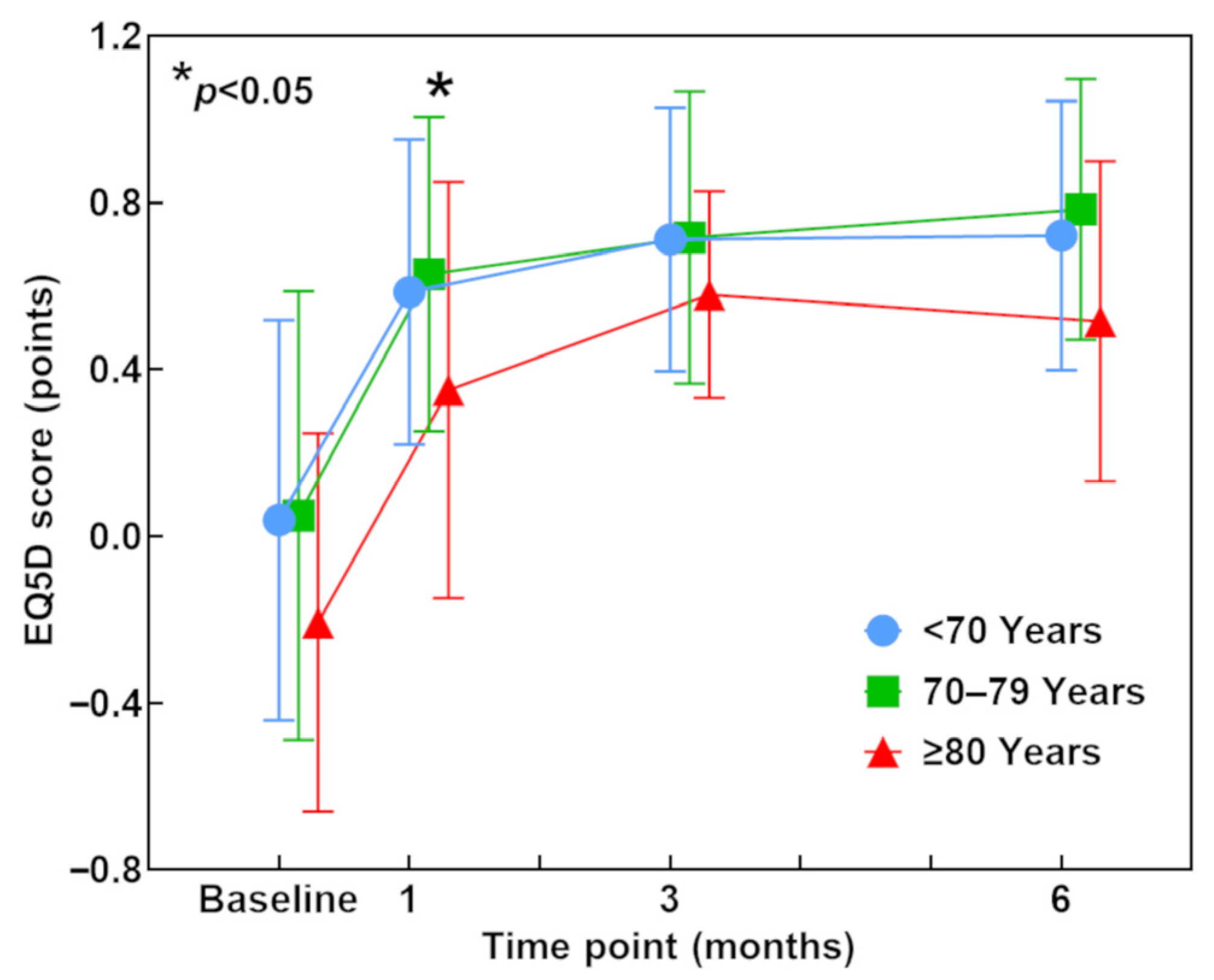
3.6. EQ-5D Score (QOL)
3.7. Individual Chronological Changes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harel, R.; Angelov, L. Spine metastases: Current treatments and future directions. Eur. J. Cancer 2010, 46, 2696–2707. [Google Scholar] [CrossRef]
- Klimo, P., Jr.; Schmidt, M.H. Surgical management of spinal metastases. Oncologist 2004, 9, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Kakutani, K.; Sakai, Y.; Maeno, K.; Takada, T.; Yurube, T.; Kurakawa, T.; Miyazaki, S.; Terashima, Y.; Ito, M.; Hara, H.; et al. Prospective Cohort Study of Performance Status and Activities of Daily Living After Surgery for Spinal Metastasis. Clin. Spine Surg. 2017, 30, E1026–E1032. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y.; Kakutani, K.; Sakai, Y.; Yurube, T.; Miyazaki, S.; Takada, T.; Hoshino, Y.; Kuroda, R. Prospective cohort study of surgical outcome for spinal metastases in patients aged 70 years or older. Bone Jt. J. 2020, 102, 1709–1716. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y.; Kakutani, K.; Sakai, Y.; Zhang, Z.; Yurube, T.; Miyazaki, S.; Kakiuchi, Y.; Takeoka, Y.; Tsujimoto, R.; Miyazaki, K.; et al. Surgical outcomes and risk factors for poor outcomes in patients with cervical spine metastasis: A prospective study. J. Orthop. Surg. Res. 2021, 16, 423. [Google Scholar] [CrossRef]
- Ma, Y.; He, S.; Liu, T.; Yang, X.; Zhao, J.; Yu, H.; Feng, J.; Xu, W.; Xiao, J. Quality of Life of Patients with Spinal Metastasis from Cancer of Unknown Primary Origin: A Longitudinal Study of Surgical Management Combined with Postoperative Radiation Therapy. J. Bone Jt. Surg. Am. 2017, 99, 1629–1639. [Google Scholar] [CrossRef]
- Miyazaki, S.; Kakutani, K.; Sakai, Y.; Ejima, Y.; Maeno, K.; Takada, T.; Yurube, T.; Terashima, Y.; Ito, M.; Kakiuchi, Y.; et al. Quality of life and cost-utility of surgical treatment for patients with spinal metastases: Prospective cohort study. Int. Orthop. 2017, 41, 1265–1271. [Google Scholar] [CrossRef]
- Kakutani, K.; Sakai, Y.; Zhang, Z.; Yurube, T.; Takeoka, Y.; Kanda, Y.; Miyazaki, K.; Ohnishi, H.; Matsuo, T.; Ryu, M.; et al. Survival Rate after Palliative Surgery Alone for Symptomatic Spinal Metastases: A Prospective Cohort Study. J. Clin. Med. 2022, 11, 6227. [Google Scholar] [CrossRef] [PubMed]
- Patchell, R.A.; Tibbs, P.A.; Regine, W.F.; Payne, R.; Saris, S.; Kryscio, R.J.; Mohiuddin, M.; Young, B. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: A randomised trial. Lancet 2005, 366, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, A.J.; Losina, E.; Ferrone, M.L.; Schwab, J.H.; Chi, J.H.; Blucher, J.A.; Silva, G.S.; Chen, A.T.; Harris, M.B.; Kang, J.D.; et al. Ambulatory status after surgical and nonsurgical treatment for spinal metastasis. Cancer 2019, 125, 2631–2637. [Google Scholar]
- Rades, D.; Huttenlocher, S.; Dunst, J.; Bajrovic, A.; Karstens, J.H.; Rudat, V.; Schild, S.E. Matched pair analysis comparing surgery followed by radiotherapy and radiotherapy alone for metastatic spinal cord compression. J. Clin. Oncol. 2010, 28, 3597–3604. [Google Scholar] [CrossRef] [PubMed]
- Rades, D.; Küchler, J.; Graumüller, L.; Abusamha, A.; Schild, S.E.; Gliemroth, J. Radiotherapy with or without Decompressive Surgery for Metastatic Spinal Cord Compression: A Retrospective Matched-Pair Study Including Data from Prospectively Evaluated Patients. Cancers 2022, 14, 1260. [Google Scholar] [CrossRef]
- Carreon, L.Y.; Puno, R.M.; Dimar, J.R., 2nd; Glassman, S.D.; Johnson, J.R. Perioperative complications of posterior lumbar decompression and arthrodesis in older adults. J. Bone Jt. Surg. Am. 2003, 85, 2089–2092. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Imagama, S.; Ando, K.; Ishiguro, N.; Yamashita, M.; Eguchi, Y.; Matsumoto, M.; Ishii, K.; Hikata, T.; Seki, S.; et al. Complications Associated with Spine Surgery in Patients Aged 80 Years or Older: Japan Association of Spine Surgeons with Ambition (JASA) Multicenter Study. Global Spine J. 2017, 7, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Fujita, N.; Okada, E.; Tsuji, O.; Nagoshi, N.; Asazuma, T.; Nakamura, M.; Matsumoto, M.; Watanabe, K. Clinical Outcomes, Complications, and Cost-effectiveness in Surgically Treated Adult Spinal Deformity Over 70 Years: A Propensity score-Matched Analysis. Clin. Spine Surg. 2020, 33, E14–E20. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.G.; DiPaola, C.P.; Ryken, T.C.; Bilsky, M.H.; Shaffrey, C.I.; Berven, S.H.; Harrop, J.S.; Fehlings, M.G.; Boriani, S.; Chou, D.; et al. A novel classification system for spinal instability in neoplastic disease: An evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine 2010, 35, E1221–E1229. [Google Scholar] [CrossRef]
- Quraishi, N.A.; Ramoutar, D.; Sureshkumar, D.; Manoharan, S.R.; Spencer, A.; Arealis, G.; Edwards, K.L.; Boszczyk, B.M. Metastatic spinal cord compression as a result of the unknown primary tumour. Eur. Spine J. 2014, 23, 1502–1507. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, H.; Okada, R.; Takagi, T.; Takahashi, M.; Murata, H.; Harada, H.; Nishimura, T.; Asakura, H.; Ogawa, H. New prognostic factors and scoring system for patients with skeletal metastasis. Cancer Med. 2014, 3, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Tokuhashi, Y.; Matsuzaki, H.; Oda, H.; Oshima, M.; Ryu, J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine 2005, 30, 2186–2191. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The barthel index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar] [PubMed]
- EuroQol—A new facility for the measurement of health-related quality of life. Health Policy 1990, 16, 199–208. [CrossRef]
- Frankel, H.L.; Hancock, D.O.; Hyslop, G.; Melzak, J.; Michaelis, L.S.; Ungar, G.H.; Vernon, J.D.; Walsh, J.J. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia 1969, 7, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Wan, Y.; Zou, X.; Peng, X.; Liu, S. Is surgery for spine metastasis reasonable in patients older than 60 years? Clin. Orthop. Relat. Res. 2013, 471, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Nanjo, Y.; Nagashima, H.; Dokai, T.; Hamamoto, Y.; Hashiguchi, H.; Ishii, H.; Kameyama, Y.; Morio, Y.; Murata, M.; Tanida, A.; et al. Clinical features and surgical outcomes of lumbar spinal stenosis in patients aged 80 years or older: A multi-center retrospective study. Arch. Orthop. Trauma Surg. 2013, 133, 1243–1248. [Google Scholar] [CrossRef]
- Rihn, J.A.; Hilibrand, A.S.; Zhao, W.; Lurie, J.D.; Vaccaro, A.R.; Albert, T.J.; Weinstein, J. Effectiveness of surgery for lumbar stenosis and degenerative spondylolisthesis in the octogenarian population: Analysis of the Spine Patient Outcomes Research Trial (SPORT) data. J. Bone Jt. Surg. Am. 2015, 97, 177–185. [Google Scholar] [CrossRef]
- Isogai, N.; Nagoshi, N.; Iwanami, A.; Kono, H.; Kobayashi, Y.; Tsuji, T.; Fujita, N.; Yagi, M.; Watanabe, K.; Kitamura, K.; et al. Surgical Treatment of Cervical Spondylotic Myelopathy in the Elderly: Outcomes in Patients Aged 80 Years or Older. Spine 2018, 43, E1430–E1436. [Google Scholar] [CrossRef] [PubMed]
- Lau, D.; Leach, M.R.; Than, K.D.; Ziewacz, J.; La Marca, F.; Park, P. Independent predictors of complication following surgery for spinal metastasis. Eur. Spine J. 2013, 22, 1402–1407. [Google Scholar] [CrossRef]
- Luksanapruksa, P.; Buchowski, J.M.; Zebala, L.P.; Kepler, C.K.; Singhatanadgige, W.; Bumpass, D.B. Perioperative Complications of Spinal Metastases Surgery. Clin. Spine Surg. 2017, 30, 4–13. [Google Scholar] [CrossRef]
- Paulino Pereira, N.R.; Ogink, P.T.; Groot, O.Q.; Ferrone, M.L.; Hornicek, F.J.; van Dijk, C.N.; Bramer, J.A.M.; Schwab, J.H. Complications and reoperations after surgery for 647 patients with spine metastatic disease. Spine J. 2019, 19, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Amelot, A.; Balabaud, L.; Choi, D.; Fox, Z.; Crockard, H.A.; Albert, T.; Arts, C.M.; Buchowski, J.M.; Bunger, C.; Chung, C.K.; et al. Surgery for metastatic spine tumors in the elderly. Advanced age is not a contraindication to surgery! Spine J. 2017, 17, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Takaki, Y.; Yamakawa, K.; Goto, T.; Kondo, T. Optimal schedule of preoperative embolization for spinal metastasis surgery. Spine 2013, 38, 1964–1969. [Google Scholar] [CrossRef] [PubMed]

| <70 Years | 70–79 Years | ≥80 Years | ||
|---|---|---|---|---|
| n = 119 | n = 73 | n = 24 | p | |
| Age (mean (range)) | 58.2 (24–69) | 73.8 (70–79) | 84.1 (80–92) | <0.001 |
| Sex (n) | 0.945 * | |||
| Male | 71 | 45 | 15 | |
| Female | 48 | 28 | 9 | |
| Malignancy of primary cancer | 0.157 * | |||
| Slow growth | 25 | 22 | 4 | |
| Moderate growth | 56 | 24 | 8 | |
| Rapid growth | 38 | 27 | 12 | |
| New Katagiri score (mean (range)) | 5.3 (1–9) | 5.1 (1–8) | 5.0 (1–8) | 0.725 |
| Revised Tokuhashi score (mean (range)) | 5.8 (1–13) | 6.3 (1–11) | 6.3 (1–12) | 0.366 |
| SINS (mean (range)) | 10.5 (3–18) | 10.4 (4–16) | 11.3 (2–17) | 0.399 |
| Lesion location | 0.539 * | |||
| Cervical spine | 19 | 14 | 3 | |
| Thoracic spine | 73 | 44 | 11 | |
| Lumbar spine | 24 | 13 | 9 | |
| Sacral spine | 3 | 2 | 1 | |
| Preoperative chemotherapy (n) | 0.438 * | |||
| Yes | 51 | 38 | 12 | |
| No | 68 | 35 | 12 | |
| Preoperative radiotherapy (n) | 0.407 * | |||
| Yes | 35 | 22 | 4 | |
| No | 84 | 51 | 20 | |
| ECOGPS grade (n) | 0.206 * | |||
| PS 1 | 8 | 8 | 1 | |
| PS 2 | 23 | 9 | 2 | |
| PS 3 | 41 | 25 | 5 | |
| PS 4 | 47 | 31 | 16 | |
| Barthel index (mean (range)) | 54.3 (0–100) | 54.7 (0–100) | 48.9 (0–100) | 0.711 |
| EQ-5D (mean (range)) | 0.004 (−0.594–1.000) | 0.049 (−0.594–1.000) | −0.207 (−0.594–0.691) | 0.092 |
| Frankel classification (n) | 0.496 * | |||
| Garde A, B, and C | 47 | 35 | 11 | |
| Garde D and E | 72 | 38 | 13 | |
| Surgical method | 0.725 * | |||
| Instrumentation and decompression | 94 | 60 | 18 | |
| Instrumentation alone | 25 | 13 | 6 | |
| Surgery type (n) | 0.174 * | |||
| Emergency surgery | 43 | 23 | 4 | |
| Non-emergency surgery | 76 | 50 | 20 | |
| Screw technique (n) | 0.166 * | |||
| Open technique | 87 | 57 | 14 | |
| Percutaneous technique | 32 | 16 | 10 | |
| Operative time (mean (range)) (min) | 190.9 (59–440) | 196.2 (30–370) | 176.1 (73–370) | 0.464 |
| Blood loss (mean (range)) (g) | 317.3 (0–2500) | 371.3 (0–1800) | 269.9 (0–820) | 0.441 |
| Number of fused vertebrae (mean (range)) | 6.3 (3–12) | 6.5 (3–12) | 6.0 (3–9) | 0.927 |
| Total | <70 Years | 70–79 Years | ≥80 Years | |
|---|---|---|---|---|
| n = 216 | n = 119 | n = 73 | n = 24 | |
| Lung | 42 (19.4) | 26 (21.8) | 13 (17.8) | 3 (12.5) |
| Kidney | 21 (9.7) | 9 (7.6) | 9 (12.3) | 3 (12.5) |
| Liver | 20 (9.3) | 11 (9.2) | 7 (9.6)) | 2 (8.3) |
| Breast | 19 (8.8) | 12 (10.1) | 4 (5.5) | 3 (12.5) |
| Myeloma | 15 (6.9) | 10 (8.4) | 4 (5.5) | 1 (4.2) |
| Thyroid | 13 (6.0) | 3 (2.5) | 9 (12.3) | 1 (4.2) |
| Lymphoma | 13 (6.0) | 8 (6.7) | 4 (5.5) | 1 (4.2) |
| Colon | 13 (6.0) | 4 (3.4) | 4 (5.5) | 5 (20.8) |
| Prostate | 9 (4.2) | 5 (4.2) | 3 (4.1) | 1 (4.2) |
| Bladder | 4 (1.9) | 0 (0.0) | 3 (4.1) | 1 (4.2) |
| Esophagus | 4 (1.9) | 3 (2.5) | 1 (1.4) | 0 (0.0) |
| Unknown | 6 (2.8) | 2 (1.7) | 4 (5.5) | 0 (0.0) |
| Others | 39 (18.1) | 26 (21.8) | 8 (11.0) | 3 (12.5) |
| Total | <70 Years | 70–79 Years | ≥80 Years | |
|---|---|---|---|---|
| n = 216 | n = 119 | n = 73 | n = 24 | |
| Number of patients (%) | 40 (18.5) | 18 (15.1) | 13 (17.8) | 9 (37.5) |
| Wound infection/dehiscence | 13 | 9 | 3 | 1 |
| Pneumonia | 7 | 2 | 1 | 4 |
| Urinary tract infection | 5 | 3 | 0 | 2 |
| Implant failure | 5 | 2 | 2 | 1 |
| Adjacent segment fracture | 4 | 3 | 1 | 0 |
| Deep vein thrombosis | 3 | 1 | 2 | 0 |
| Others | 10 | 4 | 4 | 2 |
| Number of complications | 47 | 24 | 13 | 10 |
| <70 Years | 70–79 Years | ≥80 Years | ||
|---|---|---|---|---|
| n = 119 | n = 73 | n = 24 | p | |
| ECOGPS | ||||
| Improvement | 89 (74.8) | 55 (75.3) | 18 (75.0) | 0.928 |
| Unchanged | 27 (22.7) | 17 (23.3) | 6 (25.0) | |
| Deterioration | 3 (2.5) | 1 (1.4) | 0 (0.0) | |
| Re-deterioration | 16 (13.8) | 8 (14.3) | 5 (20.8) | 0.486 |
| Barthel index | ||||
| Improvement | 87 (73.1) | 50 (68.5) | 15 (62.5) | 0.175 |
| Unchanged | 28 (23.5) | 23 (31.5) | 7 (29.2) | |
| Deterioration | 4 (3.4) | 0 (0.0) | 2 (8.3) | |
| Re-deterioration | 11 (14.3) | 10 (10.3) | 6 (21.2) | 0.073 |
| EQ-5D | ||||
| Improvement | 105 (88.2) | 62 (84.9) | 18 (75.0) | 0.464 |
| Unchanged | 12 (10.1) | 8 (11.0) | 5 (20.8) | |
| Deterioration | 2 (1.7) | 3 (4.1) | 1 (4.2) | |
| Re-deterioration | 16 (13.7) | 12 (17.1) | 8 (34.8) | 0.049 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanda, Y.; Kakutani, K.; Sakai, Y.; Miyazaki, K.; Matsuo, T.; Yurube, T.; Takeoka, Y.; Ohnishi, H.; Ryu, M.; Kumagai, N.; et al. Clinical Characteristics and Surgical Outcomes of Metastatic Spine Tumors in the Very Elderly: A Prospective Cohort Study in a Super-Aged Society. J. Clin. Med. 2023, 12, 4747. https://doi.org/10.3390/jcm12144747
Kanda Y, Kakutani K, Sakai Y, Miyazaki K, Matsuo T, Yurube T, Takeoka Y, Ohnishi H, Ryu M, Kumagai N, et al. Clinical Characteristics and Surgical Outcomes of Metastatic Spine Tumors in the Very Elderly: A Prospective Cohort Study in a Super-Aged Society. Journal of Clinical Medicine. 2023; 12(14):4747. https://doi.org/10.3390/jcm12144747
Chicago/Turabian StyleKanda, Yutaro, Kenichiro Kakutani, Yoshitada Sakai, Kunihiko Miyazaki, Tomoya Matsuo, Takashi Yurube, Yoshiki Takeoka, Hiroki Ohnishi, Masao Ryu, Naotoshi Kumagai, and et al. 2023. "Clinical Characteristics and Surgical Outcomes of Metastatic Spine Tumors in the Very Elderly: A Prospective Cohort Study in a Super-Aged Society" Journal of Clinical Medicine 12, no. 14: 4747. https://doi.org/10.3390/jcm12144747
APA StyleKanda, Y., Kakutani, K., Sakai, Y., Miyazaki, K., Matsuo, T., Yurube, T., Takeoka, Y., Ohnishi, H., Ryu, M., Kumagai, N., Kuroshima, K., Hiranaka, Y., Kawamoto, T., Hara, H., Hoshino, Y., Hayashi, S., Akisue, T., & Kuroda, R. (2023). Clinical Characteristics and Surgical Outcomes of Metastatic Spine Tumors in the Very Elderly: A Prospective Cohort Study in a Super-Aged Society. Journal of Clinical Medicine, 12(14), 4747. https://doi.org/10.3390/jcm12144747








