Non-Hormonal Contraception
Abstract
1. Introduction
1.1. History of Contraceptives
1.2. Overview of Human Reproduction
1.3. Unintended Pregnancy
2. Current Solutions
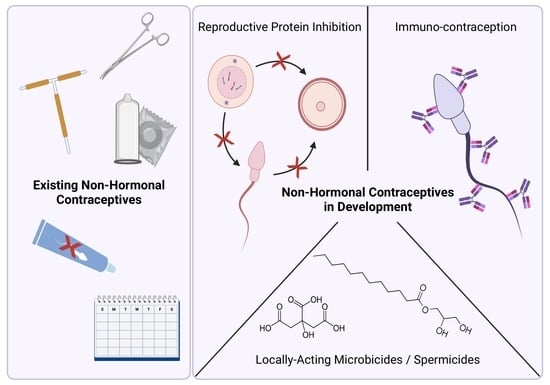
2.1. Existing Non-Hormonal Contraceptives
2.1.1. Permanent Non-Hormonal Contraceptives
- Female Sterilization
- 2.
- Male Sterilization
2.1.2. Reversible Non-Hormonal Contraceptives
- Copper Intrauterine Device
- 2.
- Chemical and Physical Barriers
- 3.
- Traditional Family Planning
3. Non-Hormonal Contraceptives in Development
3.1. Locally Acting Microbials and Spermicides
3.2. Small Molecule Reproductive Protein Inhibition
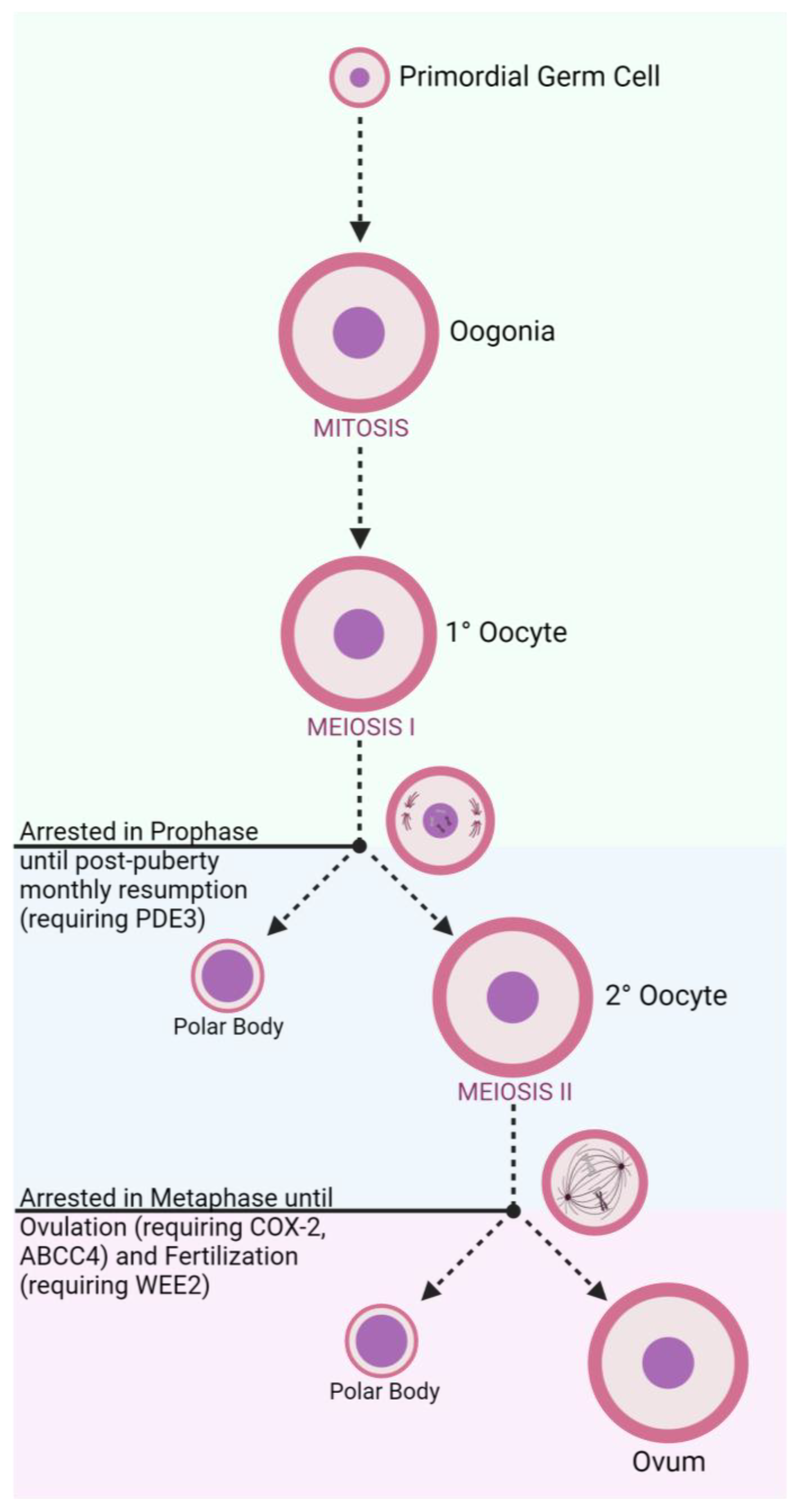
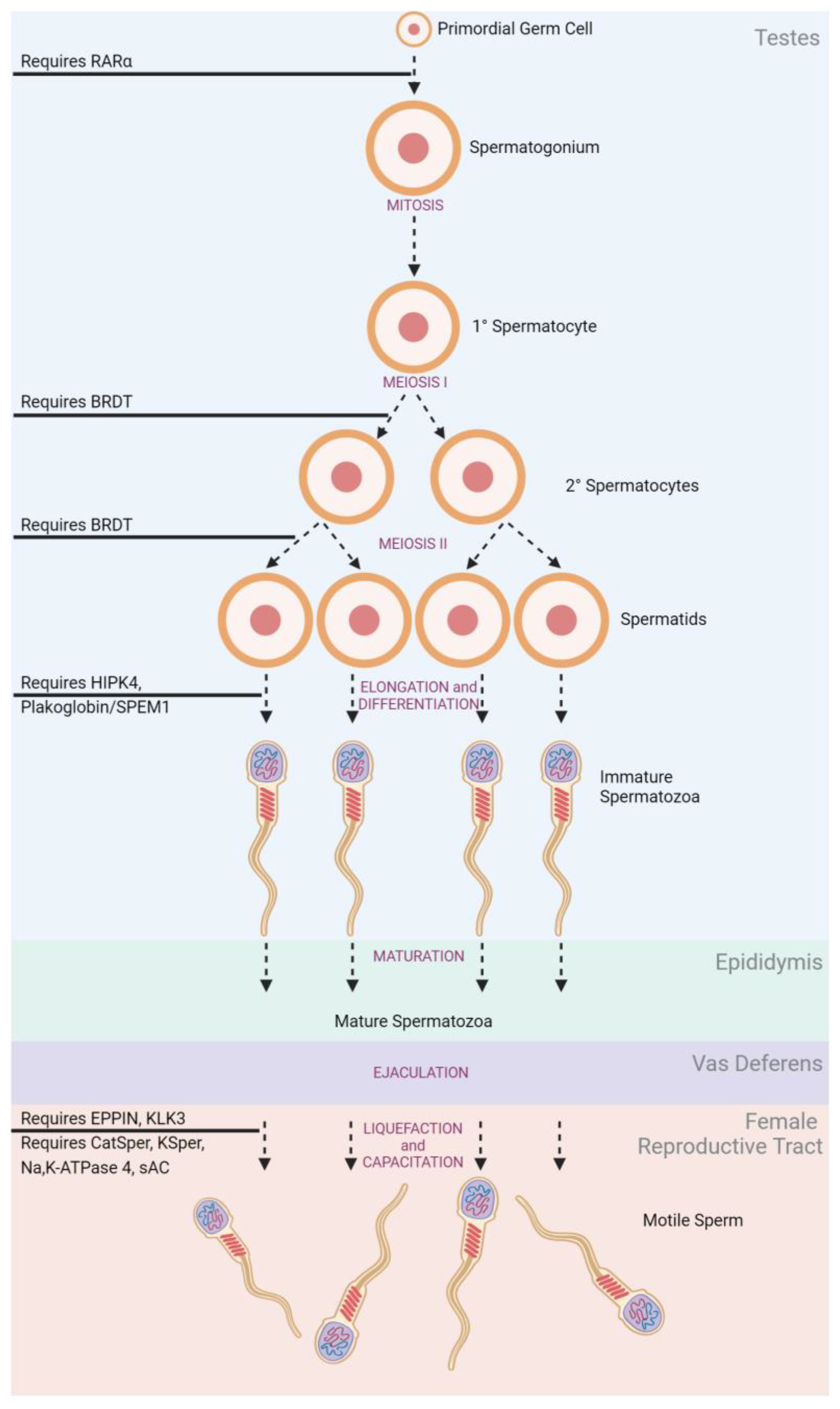
3.2.1. Disruption of Gamete Production
3.2.2. Disruption of Sperm Transit
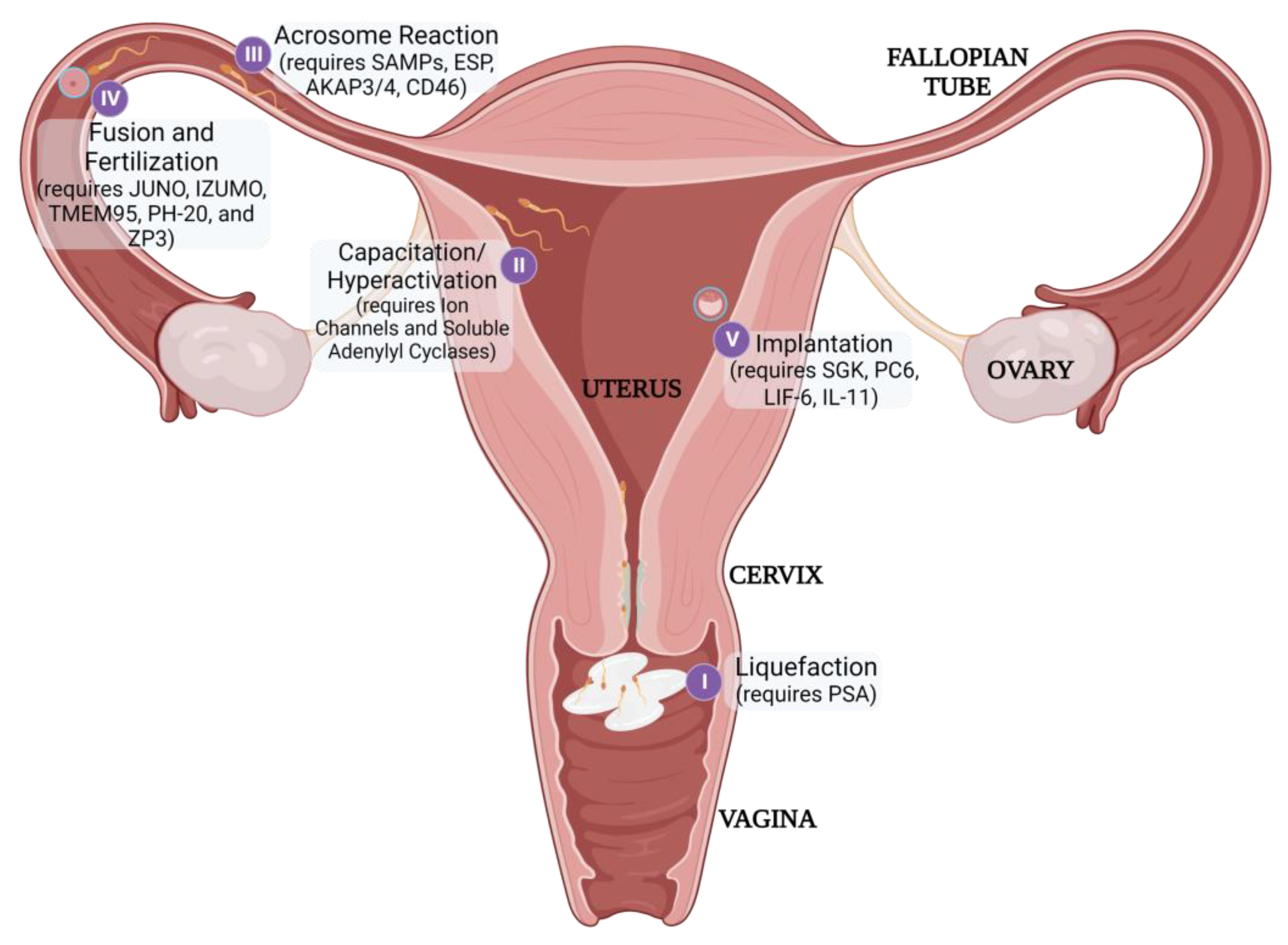
3.2.3. Disruption of Fertilization and Implantation
3.3. Immunocontraception
3.4. Non-Surgical Alternatives for Permanent Sterilization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haimov-Kochman, R.; Sciaky-Tamir, Y.; Hurwitz, A. Reproduction concepts and practices in ancient Egypt mirrored by modern medicine. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 123, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Bujalkova, M. Birth control in antiquity. Bratisl. Lek. Listy 2007, 108, 163–166. [Google Scholar]
- Gradvohl, E. Doghearted women: The origins of the counting method. Orvostorteneti Kozlemenyek 2001, 46, 135–140. [Google Scholar] [PubMed]
- Khan, F.; Mukhtar, S.; Sriprasad, S.; Dickinson, I.K. The story of the condom. Indian J. Urol. 2013, 29, 12–15. [Google Scholar] [CrossRef]
- Amy, J.J.; Thiery, M. The condom: A turbulent history. Eur. J. Contracept. Reprod. Health Care 2015, 20, 387–402. [Google Scholar] [CrossRef]
- Sharma, R.S.; Saxena, R.; Singh, R. Infertility assisted reproduction: A historical modern scientific perspective. Indian J. Med. Res. 2018, 148, S10–S14. [Google Scholar] [CrossRef] [PubMed]
- Gordon, L. The politics of population: Birth control and the eugenics movement. Radic. Am. 1974, 8, 61–98. [Google Scholar]
- Christin-Maitre, S. History of oral contraceptive drugs and their use worldwide. Best Pr. Res. Clin. Endocrinol. Metab. 2013, 27, 3–12. [Google Scholar] [CrossRef]
- Burkman, R.; Bell, C.; Serfaty, D. The evolution of combined oral contraception: Improving the risk-to-benefit ratio. Contraception 2011, 84, 19–34. [Google Scholar] [CrossRef]
- Chan, L.M.; Westhoff, C.L. Tubal sterilization trends in the United States. Fertil. Steril. 2010, 94, 1–6. [Google Scholar] [CrossRef]
- Abbe, C.R.; Page, S.T.; Thirumalai, A. Focus: Sex Reproduction: Male Contraception. Yale J. Biol. Med. 2020, 93, 603. [Google Scholar]
- Long, E.J.; Lee, M.S.; Blithe, D.L. Update on Novel Hormonal and Nonhormonal Male Contraceptive Development. J. Clin. Endocrinol. Metab. 2021, 106, e2381–e2392. [Google Scholar] [CrossRef] [PubMed]
- Cohen, B.L.; Katz, M. Pituitary and ovarian function in women receiving hormonal contraception. Contraception 1979, 20, 475–487. [Google Scholar] [CrossRef]
- Nieschlag, E. The struggle for male hormonal contraception. Best Pr. Res. Clin. Endocrinol. Metab. 2011, 25, 369–375. [Google Scholar] [CrossRef]
- Rivera, R.; Yacobson, I.; Grimes, D. The mechanism of action of hormonal contraceptives and intrauterine contraceptive devices. Am. J. Obstet. Gynecol. 1999, 181, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Larose, H.; Shami, A.N.; Abbott, H.; Manske, G.; Lei, L.; Hammoud, S.S. Gametogenesis: A journey from inception to conception. Curr. Top Dev. Biol. 2019, 132, 257–310. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Caiazza, F. Oocyte Maturation A story of arrest and release. Front. Biosci. 2013, S5, 451–477. [Google Scholar] [CrossRef]
- Wassarman, P.M.; Litscher, E.S. Female fertility and the zona pellucida. Elife 2022, 11, e76106. [Google Scholar] [CrossRef]
- Hess, R.A.; De Franca, L.R. Spermatogenesis and cycle of the seminiferous epithelium. Mol. Mech. Spermatogenes. 2008, 363, 1–15. [Google Scholar]
- Griswold, M.D. The central role of Sertoli cells in spermatogenesis. Semin. Cell Dev. Biol. 1998, 9, 411–416. [Google Scholar] [CrossRef]
- O’donnell, L. Mechanisms of spermiogenesis and spermiation and how they are disturbed. Spermatogenesis 2014, 4, e979623. [Google Scholar] [CrossRef]
- Guzick, D.S.; Overstreet, J.W.; Factor-Litvak, P.; Brazil, C.K.; Nakajima, S.T.; Coutifaris, C.; Carson, S.A.; Cisneros, P.; Steinkampf, M.P.; Hill, J.A.; et al. Sperm Morphology, Motility, and Concentration in Fertile and Infertile Men. N. Engl. J. Med. 2001, 345, 1388–1393. [Google Scholar] [CrossRef]
- Sullivan, R.; Mieusset, R. The human epididymis: Its function in sperm maturation. Hum. Reprod. Updat. 2016, 22, 574–587. [Google Scholar] [CrossRef]
- Suarez, S.; Pacey, A.A. Sperm transport in the female reproductive tract. Hum. Reprod. Updat. 2005, 12, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Okabe, M. Sperm–egg interaction and fertilization: Past, present, and future. Biol. Reprod. 2018, 99, 134–146. [Google Scholar] [CrossRef]
- Anamthathmakula, P.; Winuthayanon, W. Mechanism of semen liquefaction and its potential for a novel non-hormonal contraception. Biol. Reprod. 2020, 103, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Suarez, S.S. Control of hyperactivation in sperm. Hum. Reprod. Updat. 2008, 14, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Tulsiani, D.R.; Abou-Haila, A.; Loeser, C.R.; Pereira, B.M. The Biological and Functional Significance of the Sperm Acrosome and Acrosomal Enzymes in Mammalian Fertilization. Exp. Cell Res. 1998, 240, 151–164. [Google Scholar] [CrossRef]
- Nie, G.-Y.; Butt, A.R.; Salamonsen, L.A.; Findlay, J.K. Hormonal and non-hormonal agents at implantation as targets for contraception. Reprod. Fertil. Dev. 1997, 9, 65–76. [Google Scholar] [CrossRef]
- Bearak, J.; Popinchalk, A.; Ganatra, B.; Moller, A.B.; Tunçalp, Ö.; Beavin, C.; Alkema, L. Unintended pregnancy and abortion by income, region, and the legal status of abortion: Estimates from a comprehensive model for 1990–2019. Lancet Glob. Health 2020, 8, e1152–e1161. [Google Scholar] [CrossRef]
- Mokwena, K.E. Neglecting Maternal Depression Compromises Child Health and Development Outcomes, and Violates Children’s Rights in South Africa. Children 2021, 8, 609. [Google Scholar] [CrossRef] [PubMed]
- Mughal, M.K.; Giallo, R.; Arnold, P.D.; Kehler, H.; Bright, K.; Benzies, K.; Wajid, A.; Kingston, D. Trajectories of maternal distress and risk of child developmental delays: Findings from the All Our Families (AOF) pregnancy cohort. J. Affect. Disord. 2019, 248, 1–12. [Google Scholar] [CrossRef]
- Yazdkhasti, M.; Pourreza, A.; Pirak, A.; Abdi, F. Unintended Pregnancy and Its Adverse Social and Economic Consequences on Health System: A Narrative Review Article. Iran. J. Public Health 2015, 44, 12–21. [Google Scholar]
- Qiu, X.; Zhang, S.; Sun, X.; Li, H.; Wang, D. Unintended pregnancy and postpartum depression: A meta-analysis of cohort and case-control studies. J. Psychosom. Res. 2020, 138, 110259. [Google Scholar] [CrossRef] [PubMed]
- Ganatra, B.; Gerdts, C.; Rossier, C.; Johnson, B.R.; Tunçalp, Ö.; Assifi, A.; Alkema, L. Global, regional, and subregional classification of abortions by safety, 2010–2014: Estimates from a Bayesian hierarchical model. Lancet 2017, 390, 2372–2381. [Google Scholar] [CrossRef]
- Singh, S.; Remez, L.; Sedgh, G.; Kwok, L.; Onda, T. Abortion Worldwide 2017: Uneven Progress and Unequal Access; Guttmacher Institute: New York, NY, USA, 2018. [Google Scholar]
- Singh, S.; Maddow-Zimet, I. Facility-based treatment for medical complications resulting from unsafe pregnancy termination in the developing world, 2012: A review of evidence from 26 countries. BJOG Int. J. Obstet. Gynaecol. 2016, 123, 1489–1498. [Google Scholar] [CrossRef]
- Haddad, L.B.; Nour, N.M. Unsafe abortion: Unnecessary maternal mortality. Rev. Obstet. Gynecol. 2009, 2, 122–126. [Google Scholar] [PubMed]
- Teal, S.; Edelman, A. Contraception selection, effectiveness, and adverse effects: A review. JAMA 2021, 326, 2507–2518. [Google Scholar] [CrossRef]
- United Nations, Department of Economic and Social Affairs, Population Division. Contraceptive use by method. Data Booklet 2019, 1–28. [Google Scholar]
- Mody, S.K.; Cansino, C.; Rible, R.; Farala, J.P.; Steinauer, J.; Harken, T. Contraceptive use among women with medical conditions: Factors that influence method choice. Semin. Perinatol. 2020, 44, 151310. [Google Scholar] [CrossRef]
- Solanke, B.L. Factors influencing contraceptive use and non-use among women of advanced reproductive age in Nigeria. J. Health Popul. Nutr. 2017, 36, 1. [Google Scholar] [CrossRef] [PubMed]
- McCoy, J.A. The Politics of Reproductive Policy Restrictions: Family Planning Policy in the United States; University of California, Riverside: Riverside, CA, USA, 2020. [Google Scholar]
- Khoramrooz, M.; Rezapour, A.; Shirinbakhsh, S.; Khosravi, A. Investigating changes in unintended pregnancies before and after the changes in the family planning policies in Iran: A multivariate decomposition analysis. Med. J. Islam. Repub. Iran 2019, 33, 134. [Google Scholar] [CrossRef] [PubMed]
- Korachais, C.; Macouillard, E.; Meessen, B. How user fees influence contraception in low and middle income countries: A systematic review. Stud. Fam. Plan. 2016, 47, 341–356. [Google Scholar] [CrossRef]
- Rice, W.S.; Turan, B.; White, K.; Turan, J.M. Norms and stigma around unintended pregnancy in Alabama: Associations with recent contraceptive use and dual method use among young women. Women Health 2018, 58, 1151–1166. [Google Scholar] [CrossRef] [PubMed]
- Zemlak, J.L.; Bryant, A.P.; Jeffers, N.K. Systematic Review of Contraceptive Use Among Sex Workers in North America. J. Obstet. Gynecol. Neonatal Nurs. 2020, 49, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Gueye, A.; Speizer, I.S.; Corroon, M.; Okigbo, C.C. Belief in family planning myths at the individual and community levels and modern contraceptive use in urban Africa. Int. Perspect. Sex. Reprod. Health 2015, 41, 191. [Google Scholar] [CrossRef]
- Cheung, E.; Free, C. Factors influencing young women’s decision making regarding hormonal contraceptives: A qualitative study. Contraception 2005, 71, 426–431. [Google Scholar] [CrossRef]
- Gizzo, S.; Bertocco, A.; Saccardi, C.; Di Gangi, S.; Litta, P.S.; D’Antona, D.; Nardelli, G.B. Female sterilization: Update on clinical efficacy, side effects and contraindications. Minim. Invasive Ther. Allied Technol. 2014, 23, 261–270. [Google Scholar] [CrossRef]
- Schwingl, P.J.; Guess, H. Safety and effectiveness of vasectomy. Fertil. Steril. 2000, 73, 923–936. [Google Scholar] [CrossRef]
- Kaneshiro, B.; Aeby, T. Long-term safety, efficacy, and patient acceptability of the intrauterine Copper T-380A contraceptive device. Int. J. Women’s Health 2010, 2, 211–220. [Google Scholar] [CrossRef]
- Goldstuck, N.D.; Cheung, T.S. The efficacy of intrauterine devices for emergency contraception and beyond: A systematic review update. Int. J. Women’s Health 2019, 11, 471–479. [Google Scholar] [CrossRef]
- Raymond, E.G.; Chen, P.L.; Luoto, J.; Spermicide Trial Group. Contraceptive effectiveness and safety of five nonoxynol-9 spermicides: A randomized trial. Obstet. Gynecol. 2004, 103, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.A.; Chappell, B.T.; Maximos, B.; Culwell, K.R.; Dart, C.; Howard, B. A novel vaginal pH regulator: Results from the phase 3 AMPOWER contraception clinical trial. Contraception X 2020, 2, 100031. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.K.; Lindberg, L.D.; Higgins, J.A. Pull and pray or extra protection? Contraceptive strategies involving withdrawal among US adult women. Contraception 2014, 90, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Mansour, D.; Inki, P.; Gemzell-Danielsson, K. Efficacy of contraceptive methods: A review of the literature. Eur. J. Contracept. Reprod. Health Care 2010, 15, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Daniels, K.; Abma, J. Current Contraceptive Status Among Women Aged 15–49: United States, 2015–2017. National Center for Health Statistics; 2018 Dec. Report No.: 327. Available online: https://www.cdc.gov/nchs/products/databriefs/db327.htm#:~:text=%2C%202015%E2%80%932017.-,Current%20use%20of%20female%20sterilization%2C%20the%20pill%2C%20the%20condom%2C,on%20female%20sterilization%20for%20contraception (accessed on 3 May 2023).
- Siemons, S.E.; Vleugels, M.P.; van Balken, M.R.; Braat, D.D.; Nieboer, T.E. Male or female sterilization—The decision making process: Counselling and regret. Sex. Reprod. Health 2022, 33, 100767. [Google Scholar] [CrossRef]
- Bajwa, S.S.; Kulshrestha, A. Anaesthesia for laparoscopic surgery: General vs regional anaesthesia. J. Minimal Access Surg. 2016, 12, 4–9. [Google Scholar] [CrossRef]
- Huppelschoten, A.G.; Bijleveld, K.; Braams, L.; Schoot, B.C.; van Vliet, H.A. Laparoscopic Sterilization Under Local Anesthesia with Conscious Sedation Versus General Anesthesia: Systematic Review of the Literature. J. Minim. Invasive Gynecol. 2018, 25, 393–401. [Google Scholar] [CrossRef]
- Greenberg, J.A. Hysteroscopic sterilization: History and current methods. Rev. Obstet. Gynecol. 2008, 1, 113–121. [Google Scholar]
- Boardman, L.A.; DeSimone, M.; Allen, R.H. Barriers to completion of desired postpartum sterilization. Rhode Isl. Med. J. 2013, 2013, 96. [Google Scholar]
- Bullington, B.W.; Arora, K.S. Fulfillment of Desired Postpartum Permanent Contraception: A Health Disparities Issue. Reprod. Sci. 2022, 29, 2620–2624. [Google Scholar] [CrossRef] [PubMed]
- Richie, C. Voluntary sterilization for childfree women: Understanding patient profiles, evaluating accessibility, examining legislation. Hastings Cent. Rep. 2013, 43, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Curtis, K.M.; Mohllajee, A.P.; Peterson, H.B. Regret following female sterilization at a young age: A systematic review. Contraception 2006, 73, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Danvers, A.A.; Evans, T.A. Risk of sterilization regret and age: An analysis of the National Survey of Family Growth, 2015–2019. Obstet. Gynecol. 2022, 139, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Dockray, J.; Jarvi, K.; Grober, E.D.; Lo, K.C. Mini-incision Vasectomy Reversal Using the No-Scalpel Vasectomy Instruments and Principles. In Male Infertility: Contemporary Clinical Approaches, Andrology, ART and Antioxidants; Parekattil, S.J., Esteves, S.C., Agarwal, A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 215–221. [Google Scholar]
- Smith, R.; Patel, A. Vasectomy reversal: A clinical update. Asian J. Androl. 2016, 18, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Akintomide, H.; Brima, N.; Mansour, D.J.; Shawe, J. Copper IUD continuation, unwanted effects and cost consequences at 1 year in users aged under 30–a secondary analysis of the EURAS-IUD study. Eur. J. Contracept. Reprod. Health Care 2021, 26, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.J.; Hofler, L.G.; Modest, A.M.; Harvey, L.F.; Wu, L.H.; Hacker, M.R. Continuation of copper and levonorgestrel intrauterine devices: A retrospective cohort study. Am. J. Obstet. Gynecol. 2017, 217, 57.e1–57.e6. [Google Scholar] [CrossRef] [PubMed]
- Akintomide, H.; Barnes, P.; Brima, N.; Mansour, D. Higher discontinuation rate with a standard-sized compared to a small-sized ‘gold standard’ copper intrauterine device: A case-control review. BMJ Sex. Reprod. Health 2019, 45, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Baram, I.; Weinstein, A.; Trussell, J. The IUB, a newly invented IUD: A brief report. Contraception 2014, 89, 139–141. [Google Scholar] [CrossRef]
- Festin, M.P.R.; Peregoudov, A.; Seuc, A.; Kiarie, J.; Temmerman, M. Effect of BMI and body weight on pregnancy rates with LNG as emergency contraception: Analysis of four WHO HRP studies. Contraception 2017, 95, 50–54. [Google Scholar] [CrossRef]
- Grimes, D.A.; Lopez, L.M.; Raymond, E.G.; Halpern, V.; Nanda, K.; Schulz, K.F. Spermicide used alone for contraception. Cochrane Database Syst. Rev. 2013, 5, CD005218. [Google Scholar]
- Xu, M.; Zhao, M.; Li, R.H.W.; Lin, Z.; Chung, J.P.W.; Li, T.C.; Lee, T.-L.; Chan, D.Y.L. Effects of nonoxynol-9 (N-9) on sperm functions: Systematic review and meta-analysis. Reprod. Fertil. 2022, 3, R19–R33. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.C.; Chen, M.J. New Contraception Update—Annovera, Phexxi, Slynd, and Twirla. Curr. Obstet. Gynecol. Rep. 2022, 11, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Beksinska, M.; Wong, R.; Smit, J. Male and female condoms: Their key role in pregnancy and STI/HIV prevention. Best Pr. Res. Clin. Obstet. Gynaecol. 2019, 66, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Harsin, J.D.; Gelino, B.W.; Strickland, J.C.; Johnson, M.W.; Berry, M.S.; Reed, D.D. Behavioral economics and safe sex: Examining condom use decisions from a reinforcer pathology framework. J. Exp. Anal. Behav. 2021, 116, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Kestelman, P.; Trussell, J. Efficacy of the Simultaneous Use of Condoms and Spermicides. Fam. Plan. Perspect. 1991, 23, 226. [Google Scholar] [CrossRef]
- Sanders, S.A.; Yarber, W.L.; Kaufman, E.L.; Crosby, R.A.; Graham, C.A.; Milhausen, R.R. Condom use errors and problems: A global view. Sex. Health 2012, 9, 81–95. [Google Scholar] [CrossRef]
- Simmons, R.G.; Jennings, V. Fertility awareness-based methods of family planning. Best Prac. Res. Clin. Obstet. Gynaecol. 2020, 66, 68–82. [Google Scholar] [CrossRef]
- Freundl, G.; Sivin, I.; Batár, I. State-of-the-art of non-hormonal methods of contraception: IV. Natural family planning. Eur. J. Contracept. Reprod. Health Care 2010, 15, 113–123. [Google Scholar] [CrossRef]
- Kennedy, C.E.; Yeh, P.T.; Gaffield, M.E. Contraception values and preferences: Protocol and methods for a global systematic review. Contraception 2020, 101, 69–73. [Google Scholar] [CrossRef]
- Stafford, M.K.; Ward, H.; Flanagan, A.; Rosenstein, I.J.; Taylor-Robinson, D.; Smith, J.R.; Weber, J.; Kitchen, V.S. Safety Study of Nonoxynol-9 as a Vaginal Microbicide: Evidence of Adverse Effects. Am. J. Ther. 1998, 17, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, L.; Ramjee, G.; Alary, M.; Vuylsteke, B.; Chandeying, V.; Rees, H.; Sirivongrangson, P.; Tshibaka, L.M.; Ettiègne-Traoré, V.; Uaheowitchai, C.; et al. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: A randomised controlled trial. Lancet 2002, 360, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Tanphaichitr, N.; Srakaew, N.; Alonzi, R.; Kiattiburut, W.; Kongmanas, K.; Zhi, R.; Li, W.; Baker, M.; Wang, G.; Hickling, D. Potential Use of Antimicrobial Peptides as Vaginal Spermicides/Microbicides. Pharmaceuticals 2016, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Del Priore, G.; Malanowska-Stega, J.; Shalaby, S.W.; Richman, S. A pilot safety and tolerability study of a nonhormonal vaginal contraceptive ring. J. Reprod. Med. 2010, 54, 685–690. [Google Scholar]
- Coudray, M.S.; Madhivanan, P. Bacterial vaginosis—A brief synopsis of the literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 245, 143–148. [Google Scholar] [CrossRef]
- Jael, O.; Kariuki, W.K.; Gichuhi, M.P. Safety Study of Three Concentrations of UniPron Vaginal Contraceptive Microbicidal Gel. Am. J. Biomed. Res. 2018, 6, 33–39. [Google Scholar] [CrossRef]
- Mburu, N.; Obiero, J.O.; Waititu, K.; Mwaura, B.N.; Orawo, J.O.; Farah, I.O.; Mwethera, P.G. Safety studies of a recently developed microbicidal contraceptive gel (UniPron) in female baboons (Papio anubis). Afr. J. Reprod. Health 2009, 13, 95–104. [Google Scholar]
- Obiero, J.A.; Mburu, M.N.; Ndung’u, B.M.; Waititu, K.K.; Mulei, I.; Farah, I.O.; Mwethera, P.G. UniPron is a fully effective non-hormonal reversible contraceptive in baboon model (Papio anubis). J. Reprod. Contracept. 2008, 19, 107–118. [Google Scholar] [CrossRef]
- Khilwani, B.; Badar, A.; Ansari, A.S.; Lohiya, N.K. RISUG® as a male contraceptive: Journey from bench to bedside. Basic Clin. Androl. 2020, 30, 2–12. [Google Scholar] [CrossRef]
- Chaube, S.; Misro, M. Effect of styrene maleic anhydride (SMA) on viability and integrity of isolated rat oocytes in vitro. Contraception 2002, 66, 469–472. [Google Scholar] [CrossRef]
- Lohiya, N.; Alam, I.; Hussain, M.; Khan, S.; Ansari, A. RISUG: An intravasal injectable male contraceptive. Indian J. Med. Res. 2014, 140, S63–S72. [Google Scholar] [PubMed]
- Jha, R.K.; Jha, P.K.; Guha, S.K. Smart RISUG: A potential new contraceptive and its magnetic field-mediated sperm interaction. Int. J. Nanomed. 2009, 4, 55–64. [Google Scholar] [CrossRef]
- Buck, K.A.; Stadick, J.L.; Frazier, M.L. Preparing for sperm-targeted contraception: College students’ perceptions and intentions related to non-hormonal intravas injectable gel. Public Health Nurs. 2020, 37, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, B.; Rameshbabu, A.P.; Ghosh, K.; Jha, P.; Jha, R.; Murugesan, S.; Chattopadhyay, S.; Dhara, S.; Mondal, K.C.; Basak, P.; et al. Impact of styrene maleic anhydride (SMA) based hydrogel on rat fallopian tube as contraceptive implant with selective antimicrobial property. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 94, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Mauck, C.K.; Weiner, D.H.; Ballagh, S.A.; Creinin, M.D.; Archer, D.F.; Schwartz, J.L.; Pymar, H.C.; Lai, J.-J.; Rencher, W.F.; Callahan, M.M. Single and multiple exposure tolerance study of polystyrene sulfonate gel: A phase I safety and colposcopy study. Contraception 2004, 70, 77–83. [Google Scholar] [CrossRef]
- Anderson, R.A.; Feathergill, K.; Diao, X.; Cooper, M.; Kirkpatrick, R.; Spear, P.; Waller, D.P.; Chany, C.; Doncel, G.F.; Herold, B.; et al. Evaluation of poly(styrene-4-sulfonate) as a preventive agent for conception and sexually transmitted diseases. J. Androl. 2000, 21, 862–875. [Google Scholar]
- Garg, S.; Vermani, K.; Garg, A.; Anderson, R.A.; Rencher, W.B.; Zaneveld, L.J.D. Development and Characterization of Bioadhesive Vaginal Films of Sodium Polystyrene Sulfonate (PSS), a Novel Contraceptive Antimicrobial Agent. Pharm. Res. 2005, 22, 584–595. [Google Scholar] [CrossRef]
- Naz, R.K.; Lough, M.L.; Barthelmess, E.K. Curcumin: A novel non-steroidal contraceptive with antimicrobial properties. Front. Biosci.-Elite 2016, 8, 113–128. [Google Scholar] [CrossRef]
- Naz, R.K. Can curcumin provide an ideal contraceptive? Mol. Reprod. Dev. 2011, 78, 116–123. [Google Scholar] [CrossRef]
- Ball, C.; Krogstad, E.; Chaowanachan, T.; Woodrow, K.A. Drug-Eluting Fibers for HIV-1 Inhibition and Contraception. PLoS ONE 2012, 7, e49792. [Google Scholar] [CrossRef]
- Sharma, N.; Palia, P.; Chaudhary, A.; Verma, K.; Kumar, I. A Review on Pharmacological Activities of Lupeol and its Triterpene Derivatives. J. Drug Deliv. Ther. 2020, 10, 325–332. [Google Scholar] [CrossRef]
- Mannowetz, N.; Miller, M.R.; Lishko, P.V. Regulation of the sperm calcium channel CatSper by endogenous steroids and plant triterpenoids. Proc. Natl. Acad. Sci. USA 2017, 114, 5743–5748. [Google Scholar] [CrossRef] [PubMed]
- Rehfeld, A.; Pedersen, C.M. Lupeol and pristimerin do not inhibit activation of the human sperm CatSper Ca (2+)-channel. F1000Research 2022, 11, 222. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, A.; Georg, G.I. BET proteins: Investigating BRDT as a potential target for male contraception. Bioorganic Med. Chem. Lett. 2020, 30, 126958. [Google Scholar] [CrossRef]
- Matzuk, M.M.; McKeown, M.R.; Filippakopoulos, P.; Li, Q.; Ma, L.; Agno, J.E.; Lemieux, M.E.; Picaud, S.; Yu, R.N.; Qi, J.; et al. Small-Molecule Inhibition of BRDT for Male Contraception. Cell 2012, 150, 673–684. [Google Scholar] [CrossRef]
- Li, F.; MacKenzie, K.R.; Jain, P.; Santini, C.; Young, D.W.; Matzuk, M.M. Metabolism of JQ1, an inhibitor of bromodomain and extra terminal bromodomain proteins, in human and mouse liver microsomes. Biol. Reprod. 2020, 103, 427–436. [Google Scholar] [CrossRef]
- Al Noman, A.; Kyzer, J.L.; Chung, S.S.W.; Wolgemuth, D.J.; Georg, G.I. Retinoic acid receptor antagonists for male contraception: Current status. Biol. Reprod. 2020, 103, 390–399. [Google Scholar] [CrossRef]
- Crapster, J.A.; Rack, P.G.; Hellmann, Z.J.; Le, A.D.; Adams, C.M.; Leib, R.D.; Chen, J.K. HIPK4 is essential for murine spermiogenesis. Elife 2020, 9, e50209. [Google Scholar] [CrossRef]
- Salicioni, A.M.; Gervasi, M.G.; Sosnik, J.; Tourzani, D.A.; Nayyab, S.; Caraballo, D.A.; Visconti, P.E. Testis-specific serine kinase protein family in male fertility and as targets for non-hormonal male contraception. Biol. Reprod. 2020, 103, 264–274. [Google Scholar] [CrossRef]
- Mdhluli, M.C.; van der Horst, G. The effect of oleanolic acid on sperm motion characteristics and fertility of male Wistar rats. Lab. Anim. 2002, 36, 432–437. [Google Scholar] [CrossRef]
- Das, N.; Chandran, P.; Chakraborty, S. Potent spermicidal effect of oleanolic acid 3-beta-d-glucuronide, an active principle isolated from the plant Sesbania sesban Merrill. Contraception 2011, 83, 167–175. [Google Scholar] [CrossRef]
- Rajasekaran, M.; Bapna, J.; Lakshmanan, S.; Nair, A.R.; Veliath, A.; Panchanadam, M. Antifertility effect in male rats of oleanolic acid, a triterpene from Eugenia jambolana flowers. J. Ethnopharmacol. 1988, 24, 115–121. [Google Scholar] [CrossRef]
- Fisher, D.; Mosaval, F.; Tharp, D.L.; Bowles, D.K.; Henkel, R. Oleanolic acid causes reversible contraception in male mice by increasing the permeability of the germinal epithelium. Reprod. Fertil. Dev. 2019, 31, 1589–1596. [Google Scholar] [CrossRef]
- Al-Alami, Z.M.; Shraideh, Z.A.; Taha, M.O. β-Caryophyllene as putative male contraceptive: Enhances spermatogenesis but not spermiogenesis in albino rats. Med. Chem. Res. 2015, 24, 3876–3884. [Google Scholar] [CrossRef]
- Chang, Z.; Qin, W.; Zheng, H.; Schegg, K.; Han, L.; Liu, X.; Wang, Y.; Wang, Z.; McSwiggin, H.; Peng, H.; et al. Triptonide is a reversible non-hormonal male contraceptive agent in mice and non-human primates. Nat. Commun. 2021, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Mruk, L.D.; Xia, W.; Bonanomi, M.; Silvestrini, B.; Cheng, C.-Y. Effective Delivery of Male Contraceptives Behind the Blood-Testis Barrier (BTB)—Lesson from Adjudin. Curr. Med. Chem. 2016, 23, 701–713. [Google Scholar] [CrossRef]
- Wang, L.; Yan, M.; Li, H.; Wu, S.; Ge, R.; Wong, C.K.; Cheng, C.Y. The non-hormonal male contraceptive adjudin exerts its effects via MAPs and signaling proteins mTORC1/rpS6 and FAK-Y407. Endocrinology 2021, 162, bqaa196. [Google Scholar] [CrossRef]
- Jensen, J.T.; Schwinof, K.M.; Zelinski-Wooten, M.B.; Conti, M.; DePaolo, L.V.; Stouffer, R.L. Phosphodiesterase 3 inhibitors selectively block the spontaneous resumption of meiosis by macaque oocytes in vitro. Hum. Reprod. 2002, 17, 2079–2084. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.T.; Stouffer, R.L.; Stanley, J.E.; Zelinski, M.B. Evaluation of the phosphodiesterase 3 inhibitor ORG 9935 as a contraceptive in female macaques: Initial trials. Contraception 2010, 81, 165–171. [Google Scholar] [CrossRef]
- O’rand, M.G.; Hamil, K.G.; Adevai, T.; Zelinski, M. Inhibition of sperm motility in male macaques with EP055, a potential non-hormonal male contraceptive. PLoS ONE 2018, 13, e0195953. [Google Scholar] [CrossRef]
- Barton, B.E.; Rock, J.K.; Willie, A.M.; Harris, E.A.; Finnerty, R.M.; Herrera, G.G.; Anamthathmakula, P.; Winuthayanon, W. Serine protease inhibitor disrupts sperm motility leading to reduced fertility in female mice†. Biol. Reprod. 2020, 103, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Syeda, S.S.; Sánchez, G.; Hong, K.H.; Hawkinson, J.E.; Georg, G.I.; Blanco, G. Design, Synthesis, and in Vitro and in Vivo Evaluation of Ouabain Analogues as Potent and Selective Na,K-ATPase α4 Isoform Inhibitors for Male Contraception. J. Med. Chem. 2018, 61, 1800–1820. [Google Scholar] [CrossRef] [PubMed]
- Carlson, A.E.; Burnett, L.A.; del Camino, D.; Quill, T.A.; Hille, B.; Chong, J.A.; Moran, M.M.; Babcock, D.F. Pharmacological Targeting of Native CatSper Channels Reveals a Required Role in Maintenance of Sperm Hyperactivation. PLoS ONE 2009, 4, e6844. [Google Scholar] [CrossRef] [PubMed]
- Curci, L.; Carvajal, G.; Sulzyk, V.; Gonzalez, S.N.; Cuasnicú, P.S. Pharmacological inactivation of CatSper blocks sperm fertilizing ability independently of the capacitation status of the cells: Implications for non-hormonal contraception. Front. Cell Dev. Biol. 2021, 9, 686461. [Google Scholar] [CrossRef]
- Balbach, M.; Ghanem, L.; Rossetti, T.; Kaur, N.; Ritagliati, C.; Ferreira, J.; Krapf, D.; Molina, L.C.P.; Santi, C.M.; Hansen, J.N.; et al. Soluble adenylyl cyclase inhibition prevents human sperm functions essential for fertilization. Mol. Hum. Reprod. 2021, 27, gaab054. [Google Scholar] [CrossRef]
- Hester, K.E.; Harper, M.J.; Duffy, D.M. Oral administration of the cyclooxygenase-2 (COX-2) inhibitor meloxicam blocks ovulation in non-human primates when administered to simulate emergency contraception. Hum. Reprod. 2010, 25, 360–367. [Google Scholar] [CrossRef]
- Peluffo, M.; Stanley, J.; Braeuer, N.; Rotgeri, A.; Fritzemeier, K.-H.; Fuhrmann, U.; Buchmann, B.; Adevai, T.; Murphy, M.; Zelinski, M.; et al. A prostaglandin E2 receptor antagonist prevents pregnancies during a preclinical contraceptive trial with female macaques. Hum. Reprod. 2014, 29, 1400–1412. [Google Scholar] [CrossRef]
- Duffy, D.M. Novel contraceptive targets to inhibit ovulation: The prostaglandin E2 pathway. Hum. Reprod. Updat. 2015, 21, 652–670. [Google Scholar] [CrossRef]
- Hanna, C.B.; Mudaliar, D.; John, K.; Allen, C.L.; Sun, L.; Hawkinson, J.E.; Schönbrunn, E.; Georg, G.I.; Jensen, J.T. Development of WEE2 kinase inhibitors as novel non-hormonal female contraceptives that target meiosis. Biol. Reprod. 2020, 103, 368–377. [Google Scholar] [CrossRef]
- Hanna, C.B.; Yao, S.; Martin, M.; Schönbrunn, E.; Georg, G.I.; Jensen, J.T.; Cuellar, R.A.D. Identification and Screening of Selective WEE2 Inhibitors to Develop Non-Hormonal Contraceptives that Specifically Target Meiosis. Chemistryselect 2019, 4, 13363–13369. [Google Scholar] [CrossRef]
- Kato, K.; Satouh, Y.; Nishimasu, H.; Kurabayashi, A.; Morita, J.; Fujihara, Y.; Oji, A.; Ishitani, R.; Ikawa, M.; Nureki, O. Structural and functional insights into IZUMO1 recognition by JUNO in mammalian fertilization. Nat. Commun. 2016, 7, 12198. [Google Scholar] [CrossRef]
- Wang, D.-G.; Huang, T.-H.; Xie, Q.-D.; An, G. ORIGINAL ARTICLE: Investigation of Recombinant Mouse Sperm Protein Izumo as a Potential Immunocontraceptive Antigen. Am. J. Reprod. Immunol. 2008, 59, 225–234. [Google Scholar] [CrossRef]
- Tang, S.; Lu, Y.; Skinner, W.M.; Sanyal, M.; Lishko, P.V.; Ikawa, M.; Kim, P.S. Human sperm TMEM95 binds eggs and facilitates membrane fusion. Proc. Natl. Acad. Sci. USA 2022, 119, e2207805119. [Google Scholar] [CrossRef]
- Cherr, G.N.; Yudin, A.I.; Overstreet, J.W. The dual functions of GPI-anchored PH-20: Hyaluronidase and intracellular signaling. Matrix Biol. 2001, 20, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Primakoff, P.; Woolman-Gamer, L.; Tung, K.S.; Myles, D.G. Reversible contraceptive effect of PH-20 immunization in male guinea pigs. Biol. Reprod. 1997, 56, 1142–1146. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Meyers, S.; Tollner, T.; Yudin, A.; Primakoff, P.; He, D.; Overstreet, J. Immunological response of female macaques to the PH-20 sperm protein following injection of recombinant proteins or synthesized peptides. J. Reprod. Immunol. 2002, 54, 93–115. [Google Scholar] [CrossRef]
- Cui, X.; Duckworth, J.A.; Molinia, F.C.; Cowan, P.E. Identification and evaluation of an infertility-associated ZP3 epitope from the marsupial brushtail possum (Trichosurus vulpecula). Vaccine 2010, 28, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Kitchener, A.L.; Harman, A.; Kay, D.J.; McCartney, C.A.; Mate, K.E.; Rodger, J.C. Immunocontraception of Eastern Grey kangaroos (Macropus giganteus) with recombinant brushtail possum (Trichosurus vulpecula) ZP3 protein. J. Reprod. Immunol. 2009, 79, 156–162. [Google Scholar] [CrossRef]
- Goldberg, E.; VandeBerg, J.L.; Mahony, M.C.; Doncel, G.F. Immune response of male baboons to testis-specific LDH-C4. Contraception 2001, 64, 93–98. [Google Scholar] [CrossRef]
- Shetty, J.; Wolkowicz, M.J.; Digilio, L.C.; Klotz, K.L.; Jayes, F.L.; Diekman, A.B.; Herr, J.C. SAMP14, a novel, acrosomal membrane-associated, glycosylphosphatidylinositol-anchored member of the Ly-6/urokinase-type plasminogen activator receptor superfamily with a role in sperm-egg interaction. J. Biol. Chem. 2003, 278, 30506–30515. [Google Scholar] [CrossRef]
- Hao, Z.; Wolkowicz, M.J.; Shetty, J.; Klotz, K.; Bolling, L.; Sen, B.; Westbrook, V.A.; Coonrod, S.; Flickinger, C.J.; Herr, J.C. SAMP32, a Testis-Specific, Isoantigenic Sperm Acrosomal Membrane-Associated Protein1. Biol. Reprod. 2002, 66, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Wolkowicz, M.J.; Digilio, L.; Klotz, K.; Shetty, J.; Flickinger, C.J.; Herr, J.C. Equatorial Segment Protein (ESP) Is a Human Alloantigen Involved in Sperm-Egg Binding and Fusion. J. Androl. 2008, 29, 272–282. [Google Scholar] [CrossRef]
- Miki, K.; Willis, W.D.; Brown, P.R.; Goulding, E.H.; Fulcher, K.D.; Eddy, E.M. Targeted Disruption of the Akap4 Gene Causes Defects in Sperm Flagellum and Motility. Dev. Biol. 2002, 248, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Inoue, N.; Ikawa, M.; Nakanishi, T.; Matsumoto, M.; Nomura, M.; Seya, T.; Okabe, M. Disruption of Mouse CD46 Causes an Accelerated Spontaneous Acrosome Reaction in Sperm. Mol. Cell. Biol. 2003, 23, 2614–2622. [Google Scholar] [CrossRef]
- Aljofan, M.; Singh, H.; Ho, H.; Xie, S.; Zhu, Y.; Sun, Z.; Nie, G. Inhibition of proprotein convertase 5/6 activity: Potential for nonhormonal women-centered contraception. Contraception 2012, 85, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.; Singh, H.; Heng, S.; Nero, T.L.; Paule, S.; Parker, M.W.; Johnson, A.T.; Jiao, G.-S.; Nie, G. Small Molecule Proprotein Convertase Inhibitors for Inhibition of Embryo Implantation. PLoS ONE 2013, 8, e81380. [Google Scholar] [CrossRef]
- Dolin, H. Leukemia Inhibitory Factor (LIF) Modulation: A Novel, Non-Hormonal Contraceptive Method. MSURJ 2020, 15, 72–77. [Google Scholar] [CrossRef]
- Dimitriadis, E.; Menkhorst, E. New generation contraceptives: Interleukin 11 family cytokines as non-steroidal contraceptive targets. J. Reprod. Immunol. 2011, 88, 233–239. [Google Scholar] [CrossRef]
- Griswold, M.D.; Meinsohn, M.-C.; Smith, O.E.; Bertolin, K.; Murphy, B.D. Spermatogenesis: The Commitment to Meiosis. Physiol. Rev. 2016, 96, 1–17. [Google Scholar] [CrossRef]
- Reynolds-Wright, J.J.; Anderson, R.A. Male contraception: Where are we going and where have we been? BMJ Sex. Reprod. Health 2019, 45, 236–242. [Google Scholar] [CrossRef]
- Yu, Z.; Ku, A.F.; Anglin, J.L.; Sharma, R.; Ucisik, M.N.; Faver, J.C.; Li, F.; Nyshadham, P.; Simmons, N.; Sharma, K.L.; et al. Discovery and characterization of bromodomain 2–specific inhibitors of BRDT. Proc. Natl. Acad. Sci. USA 2021, 118, e2021102118. [Google Scholar] [CrossRef] [PubMed]
- Vernet, N.; Dennefeld, C.; Rochette-Egly, C.; Oulad-Abdelghani, M.; Chambon, P.; Ghyselinck, N.B.; Mark, M. Retinoic Acid Metabolism and Signaling Pathways in the Adult and Developing Mouse Testis. Endocrinology 2006, 147, 96–110. [Google Scholar] [CrossRef]
- Mok, K.-W.; Mruk, D.D.; Lie, P.P.Y.; Lui, W.-Y.; Cheng, C.Y. Adjudin, a potential male contraceptive, exerts its effects locally in the seminiferous epithelium of mammalian testes. Reproduction 2011, 141, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yu, Y.; Yan, J.; Yan, L.-Y.; Zhao, Y.; Li, R.; Liu, P.; Hsueh, A.J.; Qiao, J. The Role of Cilostazol, a Phosphodiesterase 3 Inhibitor, on Oocyte Maturation and Subsequent Pregnancy in Mice. PLoS ONE 2012, 7, e30649. [Google Scholar] [CrossRef] [PubMed]
- Michael, G.O.; Silva, E.J.; Hamil, K.G. Non-hormonal male contraception: A review and development of an Eppin based contraceptive. Pharmacol. Ther. 2016, 157, 105–111. [Google Scholar]
- Bianchi, E.; Doe, B.; Goulding, D.; Wright, G.J. Juno is the egg Izumo receptor and is essential for mammalian fertilization. Nature 2014, 508, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Rajaxavier, J.; Singh, Y.; Brucker, S.Y.; Salker, M.S. The Enigmatic Role of Serum Glucocorticoid Inducible Kinase 1 in the Endometrium. Front. Cell Dev. Biol. 2020, 8, 556543. [Google Scholar] [CrossRef]
- AS, V.; Dhama, K.; Chakraborty, S.; Abdul Samad, H.; Latheef, S.; Sharun, K.; Chaicumpa, W. Role of antisperm antibodies in infertility, pregnancy, and potential for contraceptive and antifertility vaccine designs: Research progress and pioneering vision. Vaccines 2019, 7, 116. [Google Scholar]
- Kaumaya, P.T.; VanBuskirk, A.M.; Goldberg, E.; Pierce, S.K. Design and immunological properties of topographic immunogenic determinants of a protein antigen (LDH-C4) as vaccines. J. Biol. Chem. 1992, 267, 6338–6346. [Google Scholar] [CrossRef]
- Clark, S.; Naz, R.K. Presence and Incidence of Izumo Antibodies in Sera of Immunoinfertile Women and Men. Am. J. Reprod. Immunol. 2012, 69, 256–263. [Google Scholar] [CrossRef]
- Gupta, G.S. LDH-C4: A target with therapeutic potential for cancer and contraception. Mol. Cell. Biochem. 2012, 371, 115–127. [Google Scholar] [CrossRef]
- Joyce, C.; Freund, M. Peterson Contraceptive effects of intravaginal application of acrosin and hyaluronidase inhibitors in rabbit. Contraception 1979, 19, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Naz, R.K. Vaccine for human contraception targeting sperm Izumo protein and YLP12 dodecamer peptide. Protein Sci. 2014, 23, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Hardy, C.M.; Mobbs, K.J. Expression of recombinant mouse sperm protein sp56 and assessment of its potential for use as an antigen in an immunocontraceptive vaccine. Mol. Reprod. Dev. Inc. Gamete Res. 1999, 52, 216–224. [Google Scholar] [CrossRef]
- O’Rand, M.G.; Beavers, J.; Widgren, E.E.; Tung, K.S. Inhibition of fertility in female mice by immunization with a B-cell epitope, the synthetic sperm peptide, P10G. J. Reprod. Immunol. 1993, 25, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Holland, O.J.; Tong, M.; Shelling, A.N.; Chamley, L.W. The Role of SPRASA in Female Fertility. Reprod. Sci. 2015, 22, 452–461. [Google Scholar] [CrossRef]
- Kadam, A.L.; Fateh, M.; Naz, R.K. Fertilization antigen (FA-1) completely blocks human sperm binding to human zona pellucida: FA-1 antigen may be a sperm receptor for zona pellucida in humans. J. Reprod. Immunol. 1995, 29, 19–30. [Google Scholar] [CrossRef]
- Naz, R.K. Effect of fertilization antigen (FA-1) DNA vaccine on fertility of female mice. Mol. Reprod. Dev. 2006, 73, 1473–1479. [Google Scholar] [CrossRef]
- Trivedi, R.N.; Naz, R.K. Testis-Specific Antigen (TSA-1) is Expressed in Murine Sperm and its Antibodies inhibit Fertilization. Am. J. Reprod. Immunol. 2002, 47, 38–45. [Google Scholar] [CrossRef]
- Kurth, B.E.; Weston, C.; Prabhakara Reddi, P.; Bryant, D.; Bhattacharya, R.; Flickinger, C.J.; Herr, J.C. Oviductal antibody response to a defined recombinant sperm antigen in macaques. Biol. Reprod. 1997, 57, 981–989. [Google Scholar] [CrossRef]
- Khan, S.A.; Jadhav, S.V.; Suryawanshi, A.R.; Bhonde, G.S.; Gajbhiye, R.K.; Khole, V.V. Evaluation of Contraceptive Potential of a Novel Epididymal Sperm Protein SFP2 in a Mouse Model. Am. J. Reprod. Immunol. 2011, 66, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Khobarekar, B.G.; Vernekar, V.; Raghavan, V.; Kamada, M.; Maegawa, M.; Bandivdekar, A.H. Evaluation of the potential of synthetic peptides of 80kDa human sperm antigen (80kDaHSA) for the development of contraceptive vaccine for male. Vaccine 2008, 26, 3711–3718. [Google Scholar] [CrossRef] [PubMed]
- Lea, I.A.; Widgren, E.E.; O’Rand, M.G. Association of sperm protein 17 with A-kinase anchoring protein 3 in flagella. Reprod. Biol. Endocrinol. 2004, 2, 57. [Google Scholar] [CrossRef]
- Frolikova, M.; Sebkova, N.; Ded, L.; Dvorakova-Hortova, K. Characterization of CD46 and β1 integrin dynamics during sperm acrosome reaction. Sci. Rep. 2016, 6, srep33714. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K. Role of zona pellucida glycoproteins during fertilization in humans. J. Reprod. Immunol. 2015, 108, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Paterson, M.; Jennings, Z.A.; Wilson, M.R.; Aitken, R. The contraceptive potential of ZP3 and ZP3 peptides in a primate model. J. Reprod. Immunol. 2001, 53, 99–107. [Google Scholar] [CrossRef]
- Rajesh, K.N.; Naz, R.K.; Rajesh, C. Gene knockouts that cause female infertility: Search for novel contraceptive targets. Front. Biosci. 2005, 10, 2447–2459. [Google Scholar] [CrossRef]
- Mohd-Lila, M.A.; Yee, L.K.; Cen, L.S.; Bala, J.A.; Balakrishnan, K.N.; Allaudin, Z.N.; Abdullah, R. The application of naked DNA plasmid (DrZP3) and recombinant adenovirus (Ad-rZP3) in rat animal model to determine comparative efficacy of ZP3-Immunocontraceptive vaccines. Microb. Pathog. 2019, 134, 103572. [Google Scholar] [CrossRef]
- Hall, L.-L.H.; Yurewicz, E.C.; Sacco, A.G.; Moghissi, K.S. Generation and Characterization of Antibodies Against Synthetic Peptides of Porcine Zona Pellucida ZP3α. J. Soc. Gynecol. Investig. 1995, 2, 552–558. [Google Scholar] [CrossRef]
- Minhas, V.; Kumar, R.; Moitra, T.; Singh, R.; Panda, A.K.; Gupta, S.K. Immunogenicity and contraceptive efficacy of recombinant fusion protein encompassing Sp17 spermatozoa-specific protein and GnRH: Relevance of adjuvants and microparticles based delivery to minimize number of injections. Am. J. Reprod. Immunol. 2020, 83, e13218. [Google Scholar] [CrossRef]
- Minhas, V.; Shrestha, A.; Wadhwa, N.; Singh, R.; Gupta, S.K. Novel Sperm and Gonadotropin-releasing Hormone-based Recombinant Fusion Protein: Achievement of 100% Contraceptive Efficacy by Co-immunization of Male and Female Mice. Mol. Reprod. Dev. 2016, 83, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, B.; Fard, N.A.; Karkhane, A.A.; Shokrpoor, S.; Heidari, F. Evaluation of multi-epitope recombinant protein as a candidate for a contraceptive vaccine. J. Reprod. Immunol. 2021, 145, 103325. [Google Scholar] [CrossRef] [PubMed]
- Baldeon-Vaca, G.; Marathe, J.G.; Politch, J.A.; Mausser, E.; Pudney, J.; Doud, J.; Anderson, D.J. Production and characterization of a human antisperm monoclonal antibody against CD52g for topical contraception in women. EBioMedicine 2021, 69, 103478. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, B.; Schaefer, A.; Zhu, Y.; Saada, J.; Jacobs, T.M.; Chavez, E.C.; Lai, S.K. Engineering sperm-binding IgG antibodies for the development of an effective nonhormonal female contraception. Sci. Transl. Med. 2021, 13, eabd5219. [Google Scholar] [CrossRef] [PubMed]
- Lippes, J. Quinacrine sterilization (QS): Time for reconsideration. Contraception 2015, 92, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.X.; Lucchesi, L.; Gregory, K.W. Improvement of stability of polidocanol foam for nonsurgical permanent contraception. Contraception 2015, 92, 103–107. [Google Scholar] [CrossRef]
- Jensen, J.T.; Hanna, C.; Yao, S.; Thompson, E.; Bauer, C.; Slayden, O.D. Transcervical administration of polidocanol foam prevents pregnancy in female baboons. Contraception 2016, 94, 527–533. [Google Scholar] [CrossRef]
- Jensen, J.T.; Hanna, C.B.; Yao, S.; Mishler, E.; Chai, D.; Kiulia, N.M.; Nyachieo, A.; Slayden, O.D. Polidocanol/doxycycline foam for nonsurgical permanent female contraception: 6 month data baboon contraception study. Fertil. Steril. 2019, 112, e305–e306. [Google Scholar] [CrossRef]
| Method | Type | Duration | Efficacy 1 |
|---|---|---|---|
| Tubal Ligation | Surgical Sterilization | Permanent | 99.74% [50] |
| Vasectomy | Surgical Sterilization | Permanent | 99% [51] |
| Copper IUD | - | Long-Acting | 99.2%, 99.9% 2 [52,53] |
| Condom | Physical Barrier | Short-Acting | 87.0% [11] |
| N-9 | Chemical Barrier | Short-Acting | 78–90% [54] |
| Phexxi | Chemical Barrier | Short-Acting | 86% [55] |
| Withdrawal | Traditional | - | 80% [56] |
| Traditional Family Planning | Traditional | - | 79.6–96.2% [57] |
| Affected Process | Target | Drug Candidate |
|---|---|---|
| Spermatogenesis | BRDT | JQ1 [108,109,110] |
| RARα | YCT529 [111] | |
| HIPK4 | In Development [112] | |
| TSSK | In Development [113] | |
| Inter-Sertoli Junctions | Oleanolic Acid [114,115,116,117] | |
| CBR2 | β-caryophyllene [118] | |
| Sertoli–Germ Cell Junctions | Triptonide [119] | |
| Adjudin [120] | ||
| H2-Gamendazole [121] | ||
| Oogenesis | PDE3 | Milrinone [122] |
| ORG20864 [123] | ||
| Liquefaction | EPPIN | EP055 [124] |
| PSA | AEBSF [125] | |
| Capacitation | Na,K-ATPase 4 | In Development [126] |
| KSper | In Development [127] | |
| CatSper | HC-056456 [128] | |
| sAC | In Development [129] | |
| Ovulation | COX-2 | Celebrex [130] |
| Meloxicam [130] | ||
| BAY06 [131] | ||
| ABCC4 | In Development [132] | |
| Fertilization | WEE2 | In Development [133,134] |
| JUNO | In Development [135] | |
| IZUMO1 | Biologic [136] | |
| TMEM95 | In Development [137] | |
| PH-20 | Biologic [138,139,140] | |
| ZP3 | Biologic [141,142] | |
| LDH-C4 | Biologic [143] | |
| Acrosome Reaction | SAMP14 | Biologic [144] |
| SAMP32 | Biologic [145] | |
| ESP | In Development [146] | |
| AKAP3/4 | In Development [147] | |
| CD46 | In Development [148] | |
| Implantation | SGK | In Development [149] |
| PC6 | Poly-R [150] | |
| LIF-6 | In Development [151] | |
| IL-11 | In Development [152] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Howard, S.A.; Benhabbour, S.R. Non-Hormonal Contraception. J. Clin. Med. 2023, 12, 4791. https://doi.org/10.3390/jcm12144791
Howard SA, Benhabbour SR. Non-Hormonal Contraception. Journal of Clinical Medicine. 2023; 12(14):4791. https://doi.org/10.3390/jcm12144791
Chicago/Turabian StyleHoward, Sarah Anne, and Soumya Rahima Benhabbour. 2023. "Non-Hormonal Contraception" Journal of Clinical Medicine 12, no. 14: 4791. https://doi.org/10.3390/jcm12144791
APA StyleHoward, S. A., & Benhabbour, S. R. (2023). Non-Hormonal Contraception. Journal of Clinical Medicine, 12(14), 4791. https://doi.org/10.3390/jcm12144791