Cardiometabolic Profile, Physical Activity, and Quality of Life in Breast Cancer Survivors after Different Physical Exercise Protocols: A 34-Month Follow-Up Study
Abstract
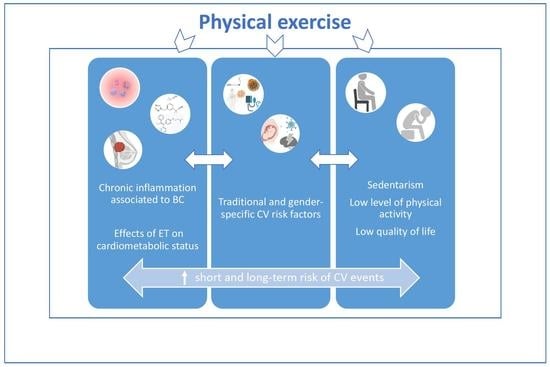
:1. Introduction
2. Materials and Methods
2.1. Anthropometric Assessment
2.2. CV Eligibility and Complete CV Evaluation
2.3. Quality-of-Life Assessment
2.4. Daily Physical Activity Measurements
2.5. Salivary Samples
2.6. Follow-Up Evaluation
2.7. Physical Exercise Protocols
2.7.1. Walking (W) Group
2.7.2. Nordic Walking (NW) Group
2.7.3. Resistance Exercise Training (RET) Group
2.8. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef]
- Lima, S.M.; Kehm, R.D.; Terry, M.B. Global breast cancer incidence and mortality trends by region, age-groups, and fertility patterns. EClinicalMedicine 2021, 38, 100985. [Google Scholar] [CrossRef] [PubMed]
- Mehta, L.S.; Watson, K.E.; Barac, A.; Beckie, T.M.; Bittner, V.; Cruz-Flores, S.; Dent, S.; Kondapalli, L.; Ky, B.; Okwuosa, T.; et al. Cardiovascular Disease and Breast Cancer: Where These Entities Intersect: A Scientific Statement From the American Heart Association. Circulation 2018, 137, e30–e66. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; He, Y.; Hu, X. Cardio-Oncology: Mechanisms, Drug Combinations, and Reverse Cardio-Oncology. Int. J. Mol. Sci. 2022, 23, 10617. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Z.; Liang, B. Comparison of American and European guidelines for cardio-oncology of heart failure. Heart Fail. Rev. 2023. [Google Scholar] [CrossRef]
- Cheung, Y.M.; Ramchand, S.K.; Yeo, B.; Grossmann, M. Cardiometabolic Effects of Endocrine Treatment of Estrogen Receptor-Positive Early Breast Cancer. J. Endocr. Soc. 2019, 3, 1283–1301. [Google Scholar] [CrossRef] [Green Version]
- Salerni, S.; Di Francescomarino, S.; Cadeddu, C.; Acquistapace, F.; Maffei, S.; Gallina, S. The different role of sex hormones on female cardiovascular physiology and function: Not only oestrogens. Eur. J. Clin. Investig. 2015, 45, 634–645. [Google Scholar] [CrossRef]
- Lyon, A.R.; Lopez-Fernandez, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Lahart, I.M.; Metsios, G.S.; Nevill, A.M.; Carmichael, A.R. Physical activity, risk of death and recurrence in breast cancer survivors: A systematic review and meta-analysis of epidemiological studies. Acta Oncol. 2015, 54, 635–654. [Google Scholar] [CrossRef]
- Sturgeon, K.M.; Ky, B.; Libonati, J.R.; Schmitz, K.H. The effects of exercise on cardiovascular outcomes before, during, and after treatment for breast cancer. Breast Cancer Res. Treat. 2014, 143, 219–226. [Google Scholar] [CrossRef] [Green Version]
- Di Blasio, A.; Morano, T.; Cianchetti, E.; Gallina, S.; Bucci, I.; Di Santo, S.; Tinari, C.; Di Donato, F.; Izzicupo, P.; Di Baldassarre, A.; et al. Psychophysical health status of breast cancer survivors and effects of 12 weeks of aerobic training. Complement. Ther. Clin. Pract. 2017, 27, 19–26. [Google Scholar] [CrossRef]
- Di Blasio, A.; Morano, T.; Bucci, I.; Di Santo, S.; D’Arielli, A.; Castro, C.G.; Cugusi, L.; Cianchetti, E.; Napolitano, G. Physical exercises for breast cancer survivors: Effects of 10 weeks of training on upper limb circumferences. J. Phys. Ther. Sci. 2016, 28, 2778–2784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulo, T.R.S.; Rossi, F.E.; Viezel, J.; Tosello, G.T.; Seidinger, S.C.; Simoes, R.R.; de Freitas, R., Jr.; Freitas, I.F., Jr. The impact of an exercise program on quality of life in older breast cancer survivors undergoing aromatase inhibitor therapy: A randomized controlled trial. Health Qual. Life Outcomes 2019, 17, 17. [Google Scholar] [CrossRef]
- Schutz, S.; Aidar, F.J.; Souza, R.L.M.; Dos Santos, J.L.; Voltarelli, F.A.; Vieira Junior, R.C.; Soares, N.M.M.; Marcal, A.C. Different Methods of Physical Training Applied to Women Breast Cancer Survivors: A Systematic Review. Front. Physiol. 2021, 12, 639406. [Google Scholar] [CrossRef] [PubMed]
- Hartman, S.J.; Marinac, C.R.; Bellettiere, J.; Godbole, S.; Natarajan, L.; Patterson, R.E.; Kerr, J. Objectively measured sedentary behavior and quality of life among survivors of early stage breast cancer. Support Care Cancer 2017, 25, 2495–2503. [Google Scholar] [CrossRef]
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.I.; Scherer, R.W.; Geigle, P.M.; Berlanstein, D.R.; Topaloglu, O.; Gotay, C.C.; Snyder, C. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst. Rev. 2012, 8, CD007566. [Google Scholar] [CrossRef]
- Fassier, P.; Zelek, L.; Partula, V.; Srour, B.; Bachmann, P.; Touillaud, M.; Druesne-Pecollo, N.; Galan, P.; Cohen, P.; Hoarau, H.; et al. Variations of physical activity and sedentary behavior between before and after cancer diagnosis: Results from the prospective population-based NutriNet-Sante cohort. Medicine 2016, 95, e4629. [Google Scholar] [CrossRef]
- Furmaniak, A.C.; Menig, M.; Markes, M.H. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst. Rev. 2016, 9, CD005001. [Google Scholar] [CrossRef]
- Ibrahim, E.M.; Al-Homaidh, A. Physical activity and survival after breast cancer diagnosis: Meta-analysis of published studies. Med. Oncol. 2011, 28, 753–765. [Google Scholar] [CrossRef]
- Basen-Engquist, K.; Hughes, D.; Perkins, H.; Shinn, E.; Taylor, C.C. Dimensions of physical activity and their relationship to physical and emotional symptoms in breast cancer survivors. J. Cancer Surviv. 2008, 2, 253–261. [Google Scholar] [CrossRef] [Green Version]
- Zhu, G.; Zhang, X.; Wang, Y.; Xiong, H.; Zhao, Y.; Sun, F. Effects of exercise intervention in breast cancer survivors: A meta-analysis of 33 randomized controlled trails. OncoTargets Ther. 2016, 9, 2153–2168. [Google Scholar] [CrossRef] [Green Version]
- Segar, M.; Katch, V.; Roth, R.; Garcia, A.; Portner, T.; Glickman, S.; Haslanger, S.; Wilkins, E. The effect of aerobic exercise on self-esteem and depressive and anxiety symptoms among breast cancer survivors. Oncol. Nurs. Forum. 1998, 25, 107–113. [Google Scholar] [PubMed]
- Kirkham, A.A.; Davis, M.K. Exercise Prevention of Cardiovascular Disease in Breast Cancer Survivors. J. Oncol. 2015, 2015, 917606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, J.M.; Nilsen, T.S.; Gupta, D.; Jones, L.W. Exercise Therapy and Cardiovascular Toxicity in Cancer. Circulation 2018, 137, 1176–1191. [Google Scholar] [CrossRef] [PubMed]
- Gasbarro, V.; Michelini, S.; Antignani, P.L.; Tsolaki, E.; Ricci, M.; Allegra, C. The CEAP-L classification for lymphedemas of the limbs: The Italian experience. Int. Angiol. 2009, 28, 315–324. [Google Scholar] [PubMed]
- Marfell-Jones, M.; Olds, T.; Stewart, A.; Carter, L. International Standards for Anthropometric Assessment; International Society for the Advancement of Kinanthropometry: Potchefstroom, South Africa, 2006. [Google Scholar]
- Du Bois, D.; Du Bois, E.F. A formula to estimate the approximate surface area if height and weight be known. Nutrition 1989, 5, 303–311. [Google Scholar]
- Sampaio, L.R.; Simoes, E.J.; Assis, A.M.; Ramos, L.R. Validity and reliability of the sagittal abdominal diameter as a predictor of visceral abdominal fat. Arq. Bras. Endocrinol. Metabol. 2007, 51, 980–986. [Google Scholar] [CrossRef] [Green Version]
- Comitato Organizzativo Cardiologico per l’idoneità Allo Sport. Protocolli Cardiologici Per il Giudizio di Idoneità Allo Sport Agonistico; Casa Editrice Scientifica Internazionale: Roma, Italy, 2017. [Google Scholar]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.H.; Fetics, B.; Nevo, E.; Rochitte, C.E.; Chiou, K.R.; Ding, P.A.; Kawaguchi, M.; Kass, D.A. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J. Am. Coll. Cardiol. 2001, 38, 2028–2034. [Google Scholar] [CrossRef] [Green Version]
- Iacobellis, G.; Willens, H.J. Echocardiographic epicardial fat: A review of research and clinical applications. J. Am. Soc. Echocardiogr. 2009, 22, 1311–1319, quiz 1417–1318. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flore, R.; Ponziani, F.R.; Tinelli, G.; Arena, V.; Fonnesu, C.; Nesci, A.; Santoro, L.; Tondi, P.; Santoliquido, A. New modalities of ultrasound-based intima-media thickness, arterial stiffness and non-coronary vascular calcifications detection to assess cardiovascular risk. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1430–1441. [Google Scholar] [PubMed]
- Stein, J.H.; Korcarz, C.E.; Hurst, R.T.; Lonn, E.; Kendall, C.B.; Mohler, E.R.; Najjar, S.S.; Rembold, C.M.; Post, W.S. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: A consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J. Am. Soc. Echocardiogr. 2008, 21, 93–111, quiz 189–190. [Google Scholar] [CrossRef] [PubMed]
- Zardi, E.M.; Di Geso, L.; Afeltra, A.; Zardi, D.M.; Giorgi, C.; Salaffi, F.; Carotti, M.; Gutierrez, M.; Filippucci, E.; Grassi, W. An ultrasound automated method for non-invasive assessment of carotid artery pulse wave velocity. J. Investig. Med. 2018, 66, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Chirinos, J.A.; Segers, P.; Hughes, T.; Townsend, R. Large-Artery Stiffness in Health and Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 74, 1237–1263. [Google Scholar] [CrossRef]
- Beutner, F.; Teren, A.; Gielen, S.; Schuler, G.; Wirkner, K.; Tiller, D.; Loeffler, M.; Scholz, M. Automated photoplethysmography-based determination of ankle-brachial index: A validation study against Doppler sonography. Clin. Res. Cardiol. 2012, 101, 875–883. [Google Scholar] [CrossRef]
- Treanor, C.; Donnelly, M. A methodological review of the Short Form Health Survey 36 (SF-36) and its derivatives among breast cancer survivors. Qual. Life Res. 2015, 24, 339–362. [Google Scholar] [CrossRef]
- Apolone, G.; Mosconi, P. The Italian SF-36 Health Survey: Translation, validation and norming. J. Clin. Epidemiol. 1998, 51, 1025–1036. [Google Scholar] [CrossRef]
- Scheers, T.; Philippaerts, R.; Lefevre, J. SenseWear-determined physical activity and sedentary behavior and metabolic syndrome. Med. Sci. Sports Exerc. 2013, 45, 481–489. [Google Scholar] [CrossRef]
- Rich, C.; Geraci, M.; Griffiths, L.; Sera, F.; Dezateux, C.; Cortina-Borja, M. Quality control methods in accelerometer data processing: Defining minimum wear time. PLoS ONE 2013, 8, e67206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Holland, B.J.; Frings-Dresen, M.H.; Sluiter, J.K. Measuring short-term and long-term physiological stress effects by cortisol reactivity in saliva and hair. Int. Arch. Occup. Environ. Health 2012, 85, 849–852. [Google Scholar] [CrossRef] [Green Version]
- Patel, K.K.; Arnold, S.V.; Chan, P.S.; Tang, Y.; Jones, P.G.; Guo, J.; Buchanan, D.M.; Qintar, M.; Decker, C.; Morrow, D.A.; et al. Validation of the Seattle angina questionnaire in women with ischemic heart disease. Am. Heart J. 2018, 201, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Chan, P.; Gosch, K.; Li, Y.; Reid, K.; Tang, F.; Spertus, J. Abstract 54: The SAQ-7: A Short Version of the Seattle Angina Questionnaire. Circ. Cardiovasc. Qual. Outcomes 2013, 6, A54. [Google Scholar] [CrossRef]
- Chan, P.S.; Jones, P.G.; Arnold, S.A.; Spertus, J.A. Development and validation of a short version of the Seattle angina questionnaire. Circ. Cardiovasc. Qual. Outcomes 2014, 7, 640–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.H.; Stewart, S.M. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): A systematic review. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craig, C.L.; Marshall, A.L.; Sjostrom, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [Green Version]
- Natalucci, V.; Villarini, M.; Emili, R.; Acito, M.; Vallorani, L.; Barbieri, E.; Villarini, A. Special Attention to Physical Activity in Breast Cancer Patients during the First Wave of COVID-19 Pandemic in Italy: The DianaWeb Cohort. J. Pers. Med. 2021, 11, 381. [Google Scholar] [CrossRef]
- Scoring Protocol for the International Physical Activity Questionnaire (IPAQ). Available online: https://sites.google.com/view/ipaq/score (accessed on 10 October 2022).
- Borg, G. Borg’s Perceived Exertion and Pain Scales; Human Kinetics: Champaign, IL, USA, 1998. [Google Scholar]
- Persinger, R.; Foster, C.; Gibson, M.; Fater, D.; Porcari, J. Consistency of the talk test for exercise prescription. Med. Sci. Sports Exerc. 2004, 36, 1632–1636. [Google Scholar]
- Pouliot, M.C.; Despres, J.P.; Lemieux, S.; Moorjani, S.; Bouchard, C.; Tremblay, A.; Nadeau, A.; Lupien, P.J. Waist circumference and abdominal sagittal diameter: Best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am. J. Cardiol. 1994, 73, 460–468. [Google Scholar] [CrossRef]
- Ohrvall, M.; Berglund, L.; Vessby, B. Sagittal abdominal diameter compared with other anthropometric measurements in relation to cardiovascular risk. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 497–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huai, P.; Liu, J.; Ye, X.; Li, W.Q. Association of Central Obesity with All Cause and Cause-Specific Mortality in US Adults: A Prospective Cohort Study. Front. Cardiovasc. Med. 2022, 9, 816144. [Google Scholar] [CrossRef] [PubMed]
- An, K.Y.; Kim, S.; Oh, M.; Lee, H.S.; Yang, H.I.; Park, H.; Lee, J.W.; Jeon, J.Y. Cardiopulmonary fitness but not muscular fitness associated with visceral adipose tissue mass. Arch. Physiol. Biochem. 2021, 127, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Pischon, T.; Boeing, H.; Hoffmann, K.; Bergmann, M.; Schulze, M.B.; Overvad, K.; van der Schouw, Y.T.; Spencer, E.; Moons, K.G.; Tjonneland, A.; et al. General and abdominal adiposity and risk of death in Europe. N. Engl. J. Med. 2008, 359, 2105–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyrstad, S.M.; Edvardsen, E.; Hansen, B.H.; Anderssen, S.A. Waist circumference thresholds and cardiorespiratory fitness. J. Sport Health Sci. 2019, 8, 17–22. [Google Scholar] [CrossRef]
- Jones, L.M.; Stoner, L.; Brown, C.; Baldi, J.C.; McLaren, B. Cardiorespiratory fitness predicts cardiovascular health in breast cancer survivors, independent of body composition, age and time post-treatment completion. Breast Cancer 2019, 26, 729–737. [Google Scholar] [CrossRef]
- Moller, G.; Ritz, C.; Kjolbaek, L.; Vuholm, S.; Korndal, S.K.; Larsen, T.M.; Pedersen, O.; Saris, W.; Astrup, A.; Lauritzen, L.; et al. Sagittal abdominal diameter and waist circumference appear to be equally good as identifiers of cardiometabolic risk. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 518–527. [Google Scholar] [CrossRef]
- Saad, M.A.N.; Jorge, A.J.L.; de Avila, D.X.; Martins, W.A.; Dos Santos, M.M.S.; Tedeschi, L.T.; Cavalcanti, I.L.; Rosa, M.L.G.; Filho, R. Sagittal abdominal diameter as a marker of visceral obesity in older primary care patients. J. Geriatr. Cardiol. 2020, 17, 279–283. [Google Scholar] [CrossRef]
- Vasques, A.C.; Souza, J.R.; Yamanaka, A.; de Oliveira Mda, S.; Novaes, F.S.; Pareja, J.C.; Geloneze, B. Sagittal abdominal diameter as a marker for epicardial adipose tissue in premenopausal women. Metabolism 2013, 62, 1032–1036. [Google Scholar] [CrossRef]
- Thomas, G.A.; Cartmel, B.; Harrigan, M.; Fiellin, M.; Capozza, S.; Zhou, Y.; Ercolano, E.; Gross, C.P.; Hershman, D.; Ligibel, J.; et al. The effect of exercise on body composition and bone mineral density in breast cancer survivors taking aromatase inhibitors. Obesity 2017, 25, 346–351. [Google Scholar] [CrossRef] [Green Version]
- Skrypnik, D.; Bogdanski, P.; Madry, E.; Karolkiewicz, J.; Ratajczak, M.; Krysciak, J.; Pupek-Musialik, D.; Walkowiak, J. Effects of Endurance and Endurance Strength Training on Body Composition and Physical Capacity in Women with Abdominal Obesity. Obes. Facts 2015, 8, 175–187. [Google Scholar] [CrossRef] [PubMed]
- de Paulo, T.R.S.; Winters-Stone, K.M.; Viezel, J.; Rossi, F.E.; Aro, B.L.; Trindade, A.; Codogno, J.S.; Freitas Junior, I.F. Comparing exercise responses to aerobic plus resistance training between postmenopausal breast cancer survivors undergoing aromatase inhibitor therapy and healthy women. Disabil. Rehabil. 2019, 41, 2175–2182. [Google Scholar] [CrossRef] [PubMed]
- Ennour-Idrissi, K.; Maunsell, E.; Diorio, C. Effect of physical activity on sex hormones in women: A systematic review and meta-analysis of randomized controlled trials. Breast Cancer Res. 2015, 17, 139. [Google Scholar] [CrossRef]
- Swain, C.T.V.; Drummond, A.E.; Boing, L.; Milne, R.L.; English, D.R.; Brown, K.A.; van Roekel, E.H.; Dixon-Suen, S.C.; Lynch, M.J.; Moore, M.M.; et al. Linking Physical Activity to Breast Cancer via Sex Hormones, Part 1: The Effect of Physical Activity on Sex Steroid Hormones. Cancer Epidemiol. Biomark. Prev. 2022, 31, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Tosti, K.P.; Hackney, A.C.; Battaglini, C.L.; Evans, E.S.; Groff, D. Exercise in patients with breast cancer and healthy controls: Energy substrate oxidation and blood lactate responses. Integr. Cancer Ther. 2011, 10, 6–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adraskela, K.; Veisaki, E.; Koutsilieris, M.; Philippou, A. Physical Exercise Positively Influences Breast Cancer Evolution. Clin. Breast Cancer 2017, 17, 408–417. [Google Scholar] [CrossRef]
- Lambert, M.; Brunet, J.; Couture-Lalande, M.E.; Bielajew, C. Aerobic physical activity and salivary cortisol levels among women with a history of breast cancer. Complement. Ther. Med. 2019, 42, 12–18. [Google Scholar] [CrossRef]
- Di Blasio, A.; Morano, T.; Lancia, F.; Viscioni, G.; Bucci, I.; Grossi, S.; Cimini, A.; Cianchetti, E.; Verrocchio, S.; Izzcupo, P.; et al. The Role of the Environment and Type of Exercise on Acute Adrenal Modulation and Perceived Distress of Breast Cancer Survivors Practising Light-Intensity Physical Exercise. Arch. Breast Cancer 2022, 9, 152–161. [Google Scholar] [CrossRef]
- Evans, E.S.; Hackney, A.C.; Pebole, M.M.; McMurray, R.G.; Muss, H.B.; Deal, A.M.; Battaglini, C.L. Adrenal Hormone and Metabolic Biomarker Responses to 30 min of Intermittent Cycling Exercise in Breast Cancer Survivors. Int. J. Sports Med. 2016, 37, 921–929. [Google Scholar] [CrossRef] [Green Version]
- Giovannelli, P.; Di Donato, M.; Galasso, G.; Di Zazzo, E.; Bilancio, A.; Migliaccio, A. The Androgen Receptor in Breast Cancer. Front. Endocrinol. 2018, 9, 492. [Google Scholar] [CrossRef] [Green Version]
- Upmanyu, N.; Bulldan, A.; Failing, K.; Scheiner-Bobis, G. DHEAS prevents pro-metastatic and proliferative effects of 17ss-estradiol on MCF-7 breast cancer cells. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118600. [Google Scholar] [CrossRef] [PubMed]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corra, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef]
- de Sousa, E.C.; Abrahin, O.; Ferreira, A.L.L.; Rodrigues, R.P.; Alves, E.A.C.; Vieira, R.P. Resistance training alone reduces systolic and diastolic blood pressure in prehypertensive and hypertensive individuals: Meta-analysis. Hypertens. Res. 2017, 40, 927–931. [Google Scholar] [CrossRef]
- Nascimento, D.D.C.; da Silva, C.R.; Valduga, R.; Saraiva, B.; de Sousa Neto, I.V.; Vieira, A.; Funghetto, S.S.; Silva, A.O.; Oliveira, S.D.C.; Pereira, G.B.; et al. Blood pressure response to resistance training in hypertensive and normotensive older women. Clin. Interv. Aging 2018, 13, 541–553. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.; Mortimer, J.; Spicer, D.; Tripathy, D.; Dieli-Conwright, C. Ps 17-28 the Effect of a Combined Aerobic and Resistance Exercise Training on Blood Pressure in Breast Cancer Survivors with Hypertension. J. Hypertens. 2016, 34, e481. [Google Scholar] [CrossRef]
- Umpierre, D.; Stein, R. Hemodynamic and vascular effects of resistance training: Implications for cardiovascular disease. Arq. Bras. Cardiol. 2007, 89, 256–262. [Google Scholar] [CrossRef]
- Lee, K.; Tripathy, D.; Demark-Wahnefried, W.; Courneya, K.S.; Sami, N.; Bernstein, L.; Spicer, D.; Buchanan, T.A.; Mortimer, J.E.; Dieli-Conwright, C.M. Effect of Aerobic and Resistance Exercise Intervention on Cardiovascular Disease Risk in Women with Early-Stage Breast Cancer: A Randomized Clinical Trial. JAMA Oncol. 2019, 5, 710–714. [Google Scholar] [CrossRef]
- Wang, S.; Yang, T.; Qiang, W.; Shen, A.; Zhao, Z.; Chen, X.; Xi, C.; Liu, H.; Guo, F. Effectiveness of physical exercise on the cardiovascular system in breast cancer patients: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Clin. Pract. 2021, 44, 101426. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Aboyans, V.; Blacher, J.; Brodmann, M.; Brutsaert, D.L.; Chirinos, J.A.; De Carlo, M.; Delgado, V.; Lancellotti, P.; Lekakis, J.; et al. The role of ventricular-arterial coupling in cardiac disease and heart failure: Assessment, clinical implications and therapeutic interventions. A consensus document of the European Society of Cardiology Working Group on Aorta & Peripheral Vascular Diseases, European Association of Cardiovascular Imaging, and Heart Failure Association. Eur. J. Heart Fail. 2019, 21, 402–424. [Google Scholar] [CrossRef] [Green Version]
- Wohlfahrt, P.; Redfield, M.M.; Lopez-Jimenez, F.; Melenovsky, V.; Kane, G.C.; Rodeheffer, R.J.; Borlaug, B.A. Impact of general and central adiposity on ventricular-arterial aging in women and men. JACC Heart Fail. 2014, 2, 489–499. [Google Scholar] [CrossRef]
- Coutinho, T.; Borlaug, B.A.; Pellikka, P.A.; Turner, S.T.; Kullo, I.J. Sex differences in arterial stiffness and ventricular-arterial interactions. J. Am. Coll. Cardiol. 2013, 61, 96–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chantler, P.D. Arterial Ventricular Uncoupling with Age and Disease and Recoupling with Exercise. Exerc. Sport Sci. Rev. 2017, 45, 70–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hieda, M.; Howden, E.; Shibata, S.; Fujimoto, N.; Bhella, P.S.; Hastings, J.L.; Tarumi, T.; Sarma, S.; Fu, Q.; Zhang, R.; et al. Impact of Lifelong Exercise Training Dose on Ventricular-Arterial Coupling. Circulation 2018, 138, 2638–2647. [Google Scholar] [CrossRef] [PubMed]
- Lane, A.D.; Yan, H.; Ranadive, S.M.; Kappus, R.M.; Sun, P.; Cook, M.D.; Harvey, I.; Woods, J.; Wilund, K.; Fernhall, B. Sex differences in ventricular-vascular coupling following endurance training. Eur. J. Appl. Physiol. 2014, 114, 2597–2606. [Google Scholar] [CrossRef] [Green Version]
- Lekavich, C.L.; Allen, J.D.; Bensimhon, D.R.; Bateman, L.A.; Slentz, C.A.; Samsa, G.P.; Kenjale, A.A.; Duscha, B.D.; Douglas, P.S.; Kraus, W.E. Aerobic Versus Resistance Training Effects on Ventricular-Arterial Coupling and Vascular Function in the STRRIDE-AT/RT Trial. Front. Cardiovasc. Med. 2021, 8, 638929. [Google Scholar] [CrossRef]
- Narayan, H.K.; Finkelman, B.; French, B.; Plappert, T.; Hyman, D.; Smith, A.M.; Margulies, K.B.; Ky, B. Detailed Echocardiographic Phenotyping in Breast Cancer Patients: Associations with Ejection Fraction Decline, Recovery, and Heart Failure Symptoms over 3 Years of Follow-up. Circulation 2017, 135, 1397–1412. [Google Scholar] [CrossRef]
- Koelwyn, G.J.; Lewis, N.C.; Ellard, S.L.; Jones, L.W.; Gelinas, J.C.; Rolf, J.D.; Melzer, B.; Thomas, S.M.; Douglas, P.S.; Khouri, M.G.; et al. Ventricular-Arterial Coupling in Breast Cancer Patients After Treatment with Anthracycline-Containing Adjuvant Chemotherapy. Oncologist 2016, 21, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Narayan, H.K.; French, B.; Khan, A.M.; Plappert, T.; Hyman, D.; Bajulaiye, A.; Domchek, S.; DeMichele, A.; Clark, A.; Matro, J.; et al. Noninvasive Measures of Ventricular-Arterial Coupling and Circumferential Strain Predict Cancer Therapeutics-Related Cardiac Dysfunction. JACC Cardiovasc. Imaging 2016, 9, 1131–1141. [Google Scholar] [CrossRef]
- Cavalcante, J.L.; Lima, J.A.; Redheuil, A.; Al-Mallah, M.H. Aortic stiffness: Current understanding and future directions. J. Am. Coll. Cardiol. 2011, 57, 1511–1522. [Google Scholar] [CrossRef] [Green Version]
- Vlachopoulos, C.; Terentes-Printzios, D.; Laurent, S.; Nilsson, P.M.; Protogerou, A.D.; Aznaouridis, K.; Xaplanteris, P.; Koutagiar, I.; Tomiyama, H.; Yamashina, A.; et al. Association of Estimated Pulse Wave Velocity with Survival: A Secondary Analysis of SPRINT. JAMA Netw. Open 2019, 2, e1912831. [Google Scholar] [CrossRef]
- DuPont, J.J.; Kenney, R.M.; Patel, A.R.; Jaffe, I.Z. Sex differences in mechanisms of arterial stiffness. Br. J. Pharmacol. 2019, 176, 4208–4225. [Google Scholar] [CrossRef]
- Tanaka, H. Antiaging Effects of Aerobic Exercise on Systemic Arteries. Hypertension 2019, 74, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Ashor, A.W.; Lara, J.; Siervo, M.; Celis-Morales, C.; Mathers, J.C. Effects of exercise modalities on arterial stiffness and wave reflection: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2014, 9, e110034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, D.J.; Maiorana, A.; O’Driscoll, G.; Taylor, R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J. Physiol. 2004, 561 Pt 1, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Molisz, A.; Schmederer, Z.; Siebert, J.; Kadamani, T.; Glasner, P.; Roslonkiewicz, K.; Nowicka-Sauer, K.; Gutknecht, P.; Trzeciak, B.; Suchanowski, A. Haemodynamic parameters in postmenopausal women—Beneficial effect of moderate continuous exercise training. Ann. Agric. Environ. Med. 2019, 26, 425–428. [Google Scholar] [CrossRef]
- Yersal, O.; Eryilmaz, U.; Akdam, H.; Meydan, N.; Barutca, S. Arterial Stiffness in Breast Cancer Patients Treated with Anthracycline and Trastuzumab-Based Regimens. Cardiol. Res. Pract. 2018, 2018, 5352914. [Google Scholar] [CrossRef] [Green Version]
- Vallerio, P.; Sarno, L.; Stucchi, M.; Musca, F.; Casadei, F.; Maloberti, A.; Lestuzzi, C.; Mancia, G.; Moreo, A.; Palazzi, M.; et al. Long-Term Effects of Radiotherapy on Arterial Stiffness in Breast Cancer Women. Am. J. Cardiol. 2016, 118, 771–776. [Google Scholar] [CrossRef]
- Joaquim, A.; Leao, I.; Antunes, P.; Capela, A.; Viamonte, S.; Alves, A.J.; Helguero, L.A.; Macedo, A. Impact of physical exercise programs in breast cancer survivors on health-related quality of life, physical fitness, and body composition: Evidence from systematic reviews and meta-analyses. Front. Oncol. 2022, 12, 955505. [Google Scholar] [CrossRef]
- Hong, F.; Ye, W.; Kuo, C.H.; Zhang, Y.; Qian, Y.; Korivi, M. Exercise Intervention Improves Clinical Outcomes, but the “Time of Session” is Crucial for Better Quality of Life in Breast Cancer Survivors: A Systematic Review and Meta-Analysis. Cancers 2019, 11, 706. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.; Spence, R.R.; Steele, M.L.; Sandler, C.X.; Peake, J.M.; Hayes, S.C. A Systematic Review and Meta-Analysis of the Safety, Feasibility, and Effect of Exercise in Women with Stage II+ Breast Cancer. Arch. Phys. Med. Rehabil. 2018, 99, 2621–2636. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Li, Y.; Liu, D. Effects of exercise on the quality of life in breast cancer patients: A systematic review of randomized controlled trials. Support Care Cancer 2019, 27, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Lahart, I.M.; Metsios, G.S.; Nevill, A.M.; Carmichael, A.R. Physical activity for women with breast cancer after adjuvant therapy. Cochrane Database Syst. Rev. 2018, 1, CD011292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frikkel, J.; Gotte, M.; Beckmann, M.; Kasper, S.; Hense, J.; Teufel, M.; Schuler, M.; Tewes, M. Fatigue, barriers to physical activity and predictors for motivation to exercise in advanced Cancer patients. BMC Palliat. Care 2020, 19, 43. [Google Scholar] [CrossRef]
- D’Ascenzi, F.; Anselmi, F.; Fiorentini, C.; Mannucci, R.; Bonifazi, M.; Mondillo, S. The benefits of exercise in cancer patients and the criteria for exercise prescription in cardio-oncology. Eur. J. Prev. Cardiol. 2021, 28, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.M.; Zabor, E.C.; Schwitzer, E.; Koelwyn, G.J.; Adams, S.C.; Nilsen, T.S.; Moskowitz, C.S.; Matsoukas, K.; Iyengar, N.M.; Dang, C.T.; et al. Efficacy of Exercise Therapy on Cardiorespiratory Fitness in Patients with Cancer: A Systematic Review and Meta-Analysis. J. Clin. Oncol. 2018, 36, 2297–2305. [Google Scholar] [CrossRef]
- Sasso, J.P.; Eves, N.D.; Christensen, J.F.; Koelwyn, G.J.; Scott, J.; Jones, L.W. A framework for prescription in exercise-oncology research. J. Cachexia Sarcopenia Muscle 2015, 6, 115–124. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |

| General Population | AET | RET | p-Value | |
|---|---|---|---|---|
| (n = 57) | (n = 31) | (n = 26) | ||
| Age, years | 53.1 ± 6.8 | |||
| TA, % | 72.14 ± 24.5 | 66.0 ± 29.9 | 79.3 ± 13.8 | 0.042 |
| PE protocol, n (%) | 0.001 | |||
| AET | 31 (54.4%) | 14 (45.2%) | 0 (0%) | |
| W | 17 (29.8%) | 0 (0%) | 26 (100%) | |
| NW | 14 (24.6%) | 17 (54.8%) | 0 (0%) | |
| RET | 26 (45.6%) | |||
| BC recurrence, n (%) | 2 (3.6%) | 2 (6.5%) | 0 (0%) | 0.19 |
| RT, n (%) | 19 (33.3%) | 7 (22.6%) | 12 (46.2%) | 0.06 |
| HT, n (%) | 39 (68.4%) | 17 (54.8%) | 21 (80.8%) | 0.039 |
| AIs, n (%) | 24 (42.2%) | 8 (25.8%) | 16 (61.5%) | 0.039 |
| Tamoxifen, n (%) | 15 (27.3%) | 10 (32%) | 5 (19.2%) | 0.27 |
| CV therapy, n (%) | ||||
| Statins, n (%) | 1 (1.7%) | 1 (3.2%) | 0 (0%) | 0.36 |
| BB, n (%) | 6 (10.5%) | 1 (3.2%) | 5 (19.2%) | 0.05 |
| ACE-I, n (%) | 11 (19.3%) | 4 (12.9%) | 7 (26.9%) | 0.18 |
| APT, n (%) | 4 (1.7%) | 3 (9.7%) | 1 (3.9%) | 0.39 |
| CCB, n (%) | 1 (1.7%) | 1 (3.2%) | 0 (0%) | 0.36 |
| Diuretics, n (%) | 6 (10.5%) | 2 (6.5%) | 4 (15.4%) | 0.27 |
| SF-36 | ||||
| PF | 80 [65, 85] | 80 [60, 85] | 80 [70, 90] | 0.22 |
| SC | 75 [62, 87] | 75 [50, 100] | 75 [62, 87] | 0.25 |
| MH | 64 [52, 76] | 64 [48, 76] | 64 [52, 80] | 0.45 |
| P | 52 [41, 74] | 52 [41, 74] | 52 [41, 84] | 0.59 |
| RLP | 75 [25, 100] | 50 [25, 100] | 75 [10, 100] | 0.95 |
| RLM | 66 [33, 100] | 66 [0, 100] | 66 [33, 100] | 0.67 |
| EV | 55 [45, 65] | 55 [40, 65] | 55 [45, 65] | 0.44 |
| HP | 56 [42, 72] | 55 [42, 82] | 56 [40, 72] | 0.72 |
| AET | RET | |||||
|---|---|---|---|---|---|---|
| n = 31 | n = 26 | |||||
| T0 | T1 | p-Value | T0 | T1 | p-Value | |
| Metabolic parameters | ||||||
| Weight, kg | 68 ± 11.7 | 67.3 ± 12.1 | 0.2 | 65.4 ± 13.4 | 66.1 ± 13.6 | 0.11 |
| BMI | 26.2 ± 5 | - | - | 25.2 ± 5.08 | - | - |
| HC, cm | 100.6 ± 20.9 | 99.5 ± 21.01 | 0.13 | 103.3 ± 9.5 | 101.4 ± 9 | 0.06 |
| WC, cm | 81.1 ± 18.3 | 80.3 ± 18.3 | 0.05 | 89.6 ± 11.2 | 82.4 ± 11.06 | <0.001 |
| SAD, cm | 25.4 ± 6.2 | 25.1 ± 6.1 | 0.28 | 25.6 ± 3.5 | 25.4 ± 3.9 | 0.15 |
| DHEAS, ug/dL | 0.29 ± 0.22 | 0.20 ± 0.15 | 0.002 | 0.47 ± 0.6 | 0.33 ± 0.47 | 0.002 |
| Cortisol, ug/dL | 0.10 ± 0.06 | 0.10 ± 0.08 | 0.79 | 0.15 ± 0.1 | 0.07 ± 0.06 | 0.002 |
| Cortisol/DHEAS | 0.67 ± 1.1 | 0.57 ± 0.5 | 0.6 | 0.78 ± 0.8 | 0.001 ±0.006 | <0.001 |
| IMT, mm | 0.67 ± 0.12 | 0.65 ± 0.08 | 0.05 | 0.69 ± 0.1 | 0.61 ± 0.14 | 0.005 |
| Epicardial fat, mm | 8.1 ± 2.6 | 6.5 ± 1.4 | <0.001 | 8.4 ± 2.3 | 6.7 ± 1.3 | 0.003 |
| CV parameters | ||||||
| SBP, mmHg | 130.3 ± 16.9 | 130 ± 13.8 | 0.9 | 132.6 ± 20.9 | 124 ± 17.8 | 0.004 |
| DBP, mmHg | 79.7 ± 11.2 | 78.9 ± 11.3 | 0.67 | 80 ± 12.8 | 74.3 ± 12.7 | 0.003 |
| MBP, mmHg | 96.6 ± 12.3 | 95.9 ± 11.5 | 0.9 | 97.5 ± 14.9 | 90.9 ± 13.8 | 0.001 |
| PWV, m/sec | 9.5 ± 2.9 | 6.9 ± 3.2 | <0.001 | 8.6 ± 2.2 | 7.8 ± 2.2 | 0.19 |
| HR, bpm | 74.2 ± 10.2 | 71.7 ± 9.6 | 0.05 | 73.6 ± 9.2 | 71.1 ± 10.2 | 0.12 |
| RWT | 0.4 ± 0.04 | 0.38 ± 0.05 | 0.15 | 0.35 ± 0.06 | 0.37 ± 0.04 | 0.02 |
| E/E’ | 7.6 ± 3.9 | 6.2 ± 2.7 | 0.3 | 6.6 ± 2.1 | 5.9 ± 1.8 | 0.3 |
| TAPSE, mm | 22.9 ± 3.9 | 23.7 ± 3.8 | 0.5 | 23.4 ± 3.3 | 23.1 ± 3.7 | 0.7 |
| MAPSE, mm | 14.9 ± 1.9 | 15.5 ± 2.2 | 0.33 | 15.7 ± 2.2 | 17.4 ± 2.6 | 0.003 |
| GLS, % | 22.2 ± 3.9 | 26.3 ± 3.3 | <0.001 | 22.1 ± 2.6 | 25.7 ± 3.2 | 0.01 |
| PAPs, mmHg | 27.6 ± 5.4 | 28.5 ± 2.9 | 0.5 | 26.6 ± 2 | 24.4 ± 3.1 | 0.45 |
| LA EF, mL | 53.8 ± 9.2 | 55.2 ± 7.4 | 0.5 | 58.3 ± 9.05 | 66.7 ± 9.6 | 0.005 |
| LVEF, % | 60.2 ± 5.2 | 59.8 ± 4.8 | 0.9 | 64 ± 3.9 | 63 ± 4 | 0.4 |
| FAC, % | 0.45 ± 0.07 | 0.45 ± 0.08 | 0.7 | 0.47 ± 0.06 | 0.5 ± 0.06 | 0.18 |
| SV, mL | 48.9 ± 9.3 | 55.4 ± 10.1 | 0.006 | 51 ± 10.6 | 60 ± 12.5 | 0.002 |
| EA | 2.5 ± 0.7 | 2.2 ± 0.5 | 0.15 | 2.3 ± 0.5 | 1.8 ± 0.8 | 0.006 |
| EES | 2.3 ± 0.8 | 2 ± 0.6 | 0.18 | 1.93 ± 0.5 | 1.1 ± 0.3 | <0.001 |
| V/A | 1.14 ± 0.3 | 1.2 ± 0.3 | 0.6 | 2.4 ± 0.9 | 1.2 ± 0.4 | <0.001 |
| SF-36 | ||||||
| PF | 80 [60, 85] | 85 [75, 95] | <0.001 | 80 [70, 90] | 87.5 [80, 95] | 0.05 |
| SC | 75 [50, 100] | 75 [50, 100] | 0.9 | 75 [62, 87] | 87 [75, 100] | 0.03 |
| MH | 64 [48, 76] | 64 [52, 80] | 0.05 | 64 [52, 80] | 74 [68, 84] | 0.003 |
| P | 52 [41, 74] | 74 [52, 84] | 0.001 | 52 [41, 84] | 72 [61, 84] | 0.26 |
| RLP | 50 [25, 100] | 100 [0, 100] | 0.1 | 75 [10, 100] | 100 [54, 100] | 0.13 |
| RLM | 66 [0, 100] | 100 [66, 100] | 0.06 | 66 [33, 100] | 100 [66, 100] | 0.13 |
| EV | 55 [40, 65] | 60 [45, 70] | 0.04 | 55 [45, 65] | 55 [50, 75] | 0.17 |
| HP | 55 [42, 82] | 67 [52, 86] | <0.001 | 56 [40, 72] | 67 [52, 76] | 0.13 |
| Daily PA assessment | ||||||
| METs, avg | 1.37 ± 0.23 | 1.46 ± 0.25 | 0.008 | 1.32 ± 0.17 | 1.38 ± 0.25 | 0.18 |
| STEPS (no.) | 10,003 [8084, 11,401] | 10,057 [8431, 13,212] | 0.8 | 8339 [7077, 9611] | 10,348 [8185, 13,050] | 0.004 |
| LIPAT (min) | 275 [154, 385] | 334.5 [192, 402] | 0.29 | 250 [171, 329] | 290 [170, 426] | 0.8 |
| MIPAT (min) | 45.5 [25, 87] | 64 [37, 119] | 0.07 | 47.5 [23, 81] | 49.5 [39, 103] | 0.23 |
| VIPAT (min) | 0 [0, 7] | 2,5 [0, 13] | 0.04 | 0 [0, 4] | 0 [0, 8] | 0.58 |
| ST (min) | 1036.5 [913, 1207] | 977 [862, 1125] | 0.05 | 1065 [1003, 1220] | 1041 [974, 1199] | 0.68 |
| AET | RET | p-Value | |
|---|---|---|---|
| n = 31 | n = 26 | ||
| Metabolic parameters | |||
| WC, cm | (-)0.01 ± 0.01 | (-)0.05 ± 0.02 | 0.014 |
| SAD, cm | (-)0.01 ± 0.01 | 0.08 ± 0.03 | 0.019 |
| DHEAS, ug/dL | 0.05 ± 0.09 | (-)0.32 ± 0.10 | 0.013 |
| Cortisol/DHEAS | 0.25 ± 0.12 | (-)0.98 ± 0.13 | 0.001 |
| CV parameters | |||
| SBP, mmHg | 0.001 ± 0.02 | (-)0.06 ± 0.02 | 0.021 |
| MBP, mmHg | (-)0.01 ± 0.02 | (-)0.06 ± 0.02 | 0.038 |
| PWV, cm/sec | (-)0.31 ± 0.08 | (-)0.03 ± 0.08 | 0.015 |
| EES | (-)0.04 ± 0.07 | (-)0.40 ± 0.08 | 0.001 |
| EA | (-)0.05 ± 0.05 | (-)0.22 ± 0.06 | 0.026 |
| V/A | 0.12 ± 0.08 | (-)0.41 ± 0.09 | 0.001 |
| SF-36 | |||
| PF | 0.2 ± 0.4 | 0.01 ± 0.1 | 0.03 |
| NW | W | RET | p-Value | |
|---|---|---|---|---|
| n = 14 | n = 17 | n = 26 | ||
| Metabolic parameters | ||||
| WC, cm | (-)0.01 ± 0.01 | (-)0.01 ± 0.01 | (-)0.05 ± 0.02 | 0.004 |
| DHEAS, ug/dL | 0.14 ± 0.12 | (-)0.10 ± 0.16 | (-)0.30 ± 0.10 | 0.026 |
| Cortisol/DHEAS | 0.28 ± 0.16 | 0.19 ± 0.22 | (-)0.9 ± 0.13 | 0.001 |
| CV parameters | ||||
| SBP, mmHg | (-)0.03 ± 0.03 | 0.03 ± 0.02 | (-)0.06 ± 0.02 | 0.017 |
| PWV, cm/sec | (-)0.49 ± 0.11 | (-)0.15 ± 0.10 | (-)0.04 ± 0.07 | 0.005 |
| EES | (-)0.15 ± 0.10 | 0.08 ± 0.10 | (-)0.41 ± 0.07 | 0.001 |
| EA | (-)0.09 ± 0.07 | (-)0.01 ± 0.07 | (-)0.23 ± 0.06 | 0.07 |
| V/A | 0.12 ± 0.12 | 0.12 ± 0.12 | (-)0.41 ± 0.09 | 0.001 |
| Follow-Up | ||||
|---|---|---|---|---|
| General Population | AET | RET | p-Value | |
| n = 36 | n = 16 | n = 20 | ||
| Follow-up, months | 34 ± 3.6 | 38.2 ± 1 | 31 | <0.001 |
| BC recurrency at FU, n (%) | 2 (5.6%) | 1 (6.2%) | 1 (5%) | 0.99 |
| Hospitalization for CV issues, n (%) | 1 (2.8%) | 1 (6.2%) | 0 (0%) | <0.001 |
| Novel CV medications, n (%) | 2 (5.6%) | 2 (13.4%) | 0 (0%) | <0.001 |
| IPAQ | 2160 [600–3990] | 2160 [990.0, 3400.0] | 2212.5 [540.0, 5730.0] | 0.90 |
| High level | 15 (41.7%) | 6 (37.5%) | 11 (55%) | 0.39 |
| Low level | 9 (25%) | 2 (13.4%) | 7 (35%) | 0.012 |
| Moderate level | 11 (30.5%) | 8 (50%) | 1 (5%) | 0.10 |
| SAQ-7 | 100.0 [86.1, 100] | 100 [100, 100] | 88.9 [86.1, 100.0] | 0.13 |
| SAQ-PL | 100.0 [58.3, 100] | 101 [100, 100] | 75 [58.3, 100] | 0.26 |
| SAQ-AF | 100.0 [100, 100] | 100 [100, 100] | 100 [100, 100] | 0.41 |
| SAQ-QL | 100.0 [100, 100] | 100 [100, 100] | 100 [100, 100] | 0.41 |
| SF-36 | ||||
| PF | 75 [65, 80] | 75 [70, 85] | 70 [65, 80] | 0.56 |
| SC | 67 [44, 89] | 56 [44, 100] | 67 [56, 89] | 0.64 |
| MH | 68 [52, 84] | 72 [52, 84] | 62 [52, 84] | 0.51 |
| P | 67 [44, 89] | 67 [44, 100] | 61.5 [44, 89] | 0.97 |
| RLP | 50 [25, 100] | 75 [0, 100] | 50 [25, 100] | 0.88 |
| RLM | 67 [33, 100] | 67 [33, 100] | 67 [33, 100] | 0.87 |
| EV | 50 [35, 70] | 55 [35, 70] | 47.5 [40, 75] | 0.63 |
| HP | 55 [35, 80] | 55 [40, 85] | 50 [35, 80] | 0.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bucciarelli, V.; Bianco, F.; Di Blasio, A.; Morano, T.; Tuosto, D.; Mucedola, F.; Di Santo, S.; Cimini, A.; Napolitano, G.; Bucci, I.; et al. Cardiometabolic Profile, Physical Activity, and Quality of Life in Breast Cancer Survivors after Different Physical Exercise Protocols: A 34-Month Follow-Up Study. J. Clin. Med. 2023, 12, 4795. https://doi.org/10.3390/jcm12144795
Bucciarelli V, Bianco F, Di Blasio A, Morano T, Tuosto D, Mucedola F, Di Santo S, Cimini A, Napolitano G, Bucci I, et al. Cardiometabolic Profile, Physical Activity, and Quality of Life in Breast Cancer Survivors after Different Physical Exercise Protocols: A 34-Month Follow-Up Study. Journal of Clinical Medicine. 2023; 12(14):4795. https://doi.org/10.3390/jcm12144795
Chicago/Turabian StyleBucciarelli, Valentina, Francesco Bianco, Andrea Di Blasio, Teresa Morano, Desiree Tuosto, Francesco Mucedola, Serena Di Santo, Alessandra Cimini, Giorgio Napolitano, Ines Bucci, and et al. 2023. "Cardiometabolic Profile, Physical Activity, and Quality of Life in Breast Cancer Survivors after Different Physical Exercise Protocols: A 34-Month Follow-Up Study" Journal of Clinical Medicine 12, no. 14: 4795. https://doi.org/10.3390/jcm12144795
APA StyleBucciarelli, V., Bianco, F., Di Blasio, A., Morano, T., Tuosto, D., Mucedola, F., Di Santo, S., Cimini, A., Napolitano, G., Bucci, I., Di Baldassarre, A., Cianchetti, E., & Gallina, S. (2023). Cardiometabolic Profile, Physical Activity, and Quality of Life in Breast Cancer Survivors after Different Physical Exercise Protocols: A 34-Month Follow-Up Study. Journal of Clinical Medicine, 12(14), 4795. https://doi.org/10.3390/jcm12144795