The Impact of Multiparametric Magnetic Resonance Imaging on Treatment Strategies for Incidental Prostate Cancer after Holmium Laser Enucleation of the Prostate
Abstract
1. Introduction
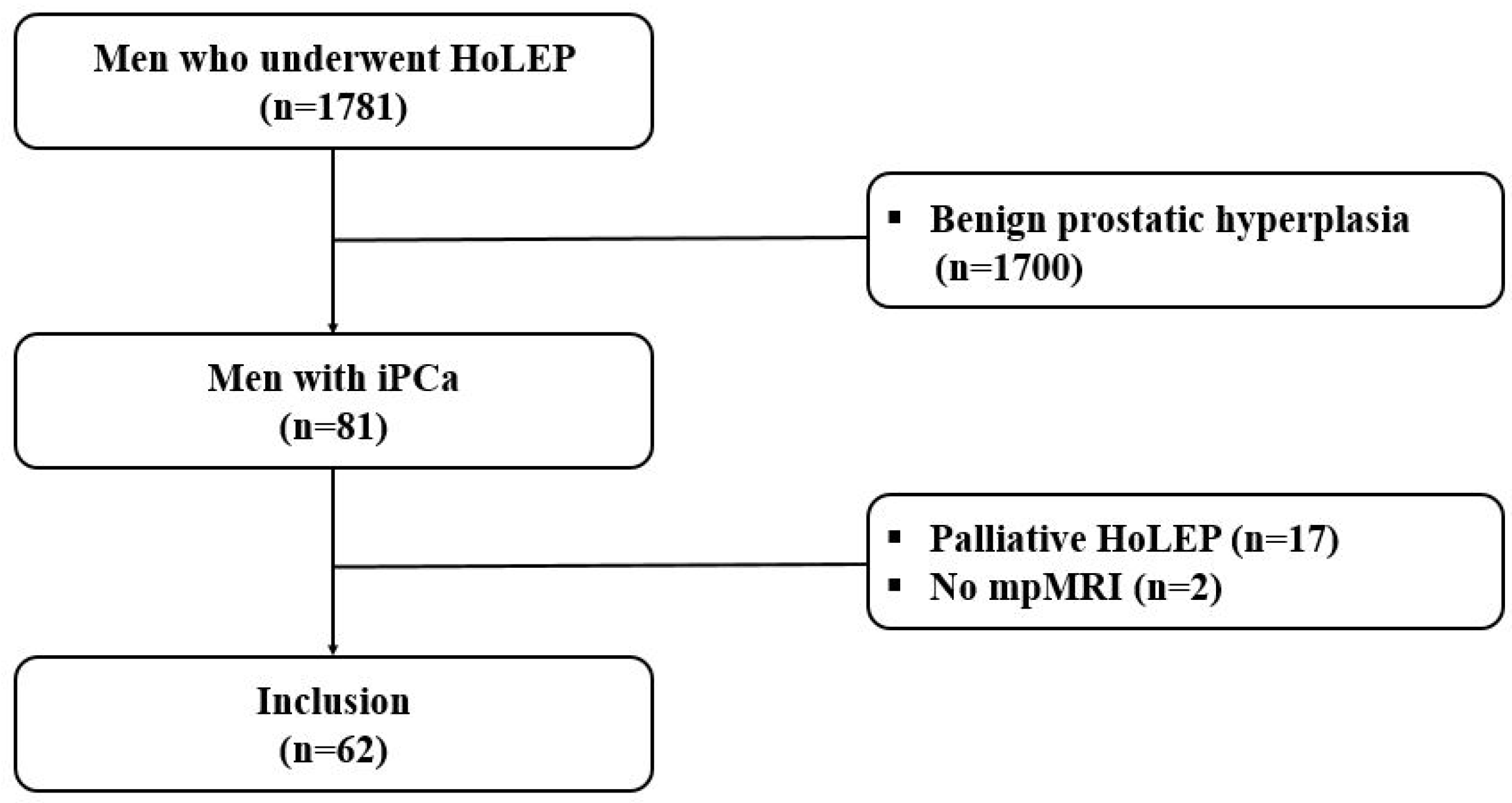
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Statistical Analysis
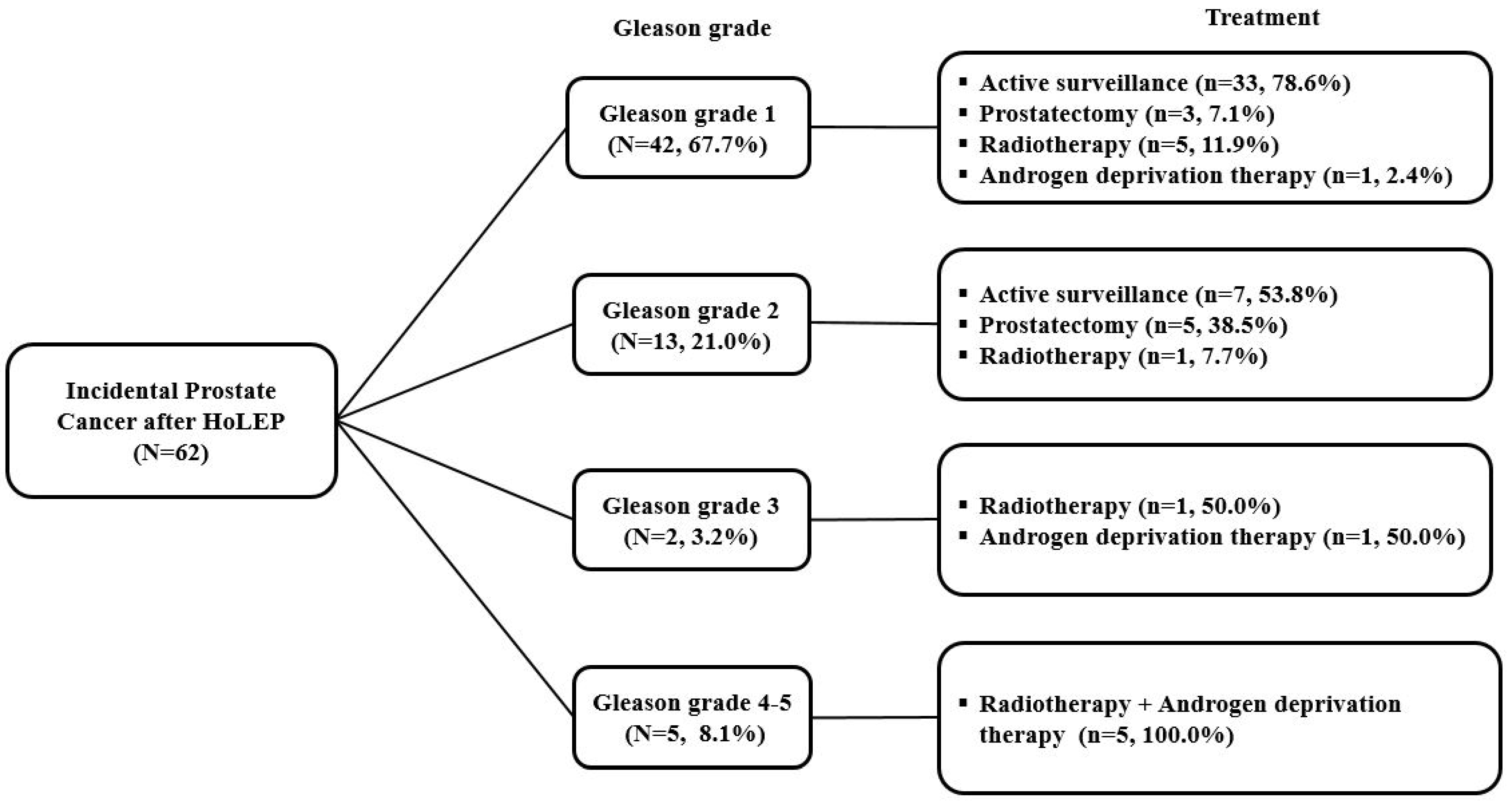
3. Results
3.1. Baseline Demographic and Clinicopathologic Characteristics
3.2. Treatment Strategy for iPCa
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gratzke, C.; Bachmann, A.; Descazeaud, A.; Drake, M.J.; Madersbacher, S.; Mamoulakis, C.; Oelke, M.; Tikkinen, K.A.O.; Gravas, S. EAU Guidelines on the Assessment of Non-neurogenic Male Lower Urinary Tract Symptoms including Benign Prostatic Obstruction. Eur. Urol. 2015, 67, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Bhojani, N.; Boris, R.S.; Monn, M.F.; Mandeville, J.A.; Lingeman, J.E. Coexisting prostate cancer found at the time of holmium laser enucleation of the prostate for benign prostatic hyperplasia: Predicting its presence and grade in analyzed tissue. J. Endourol. 2015, 29, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, M.; Welte, M.N.; Grossmann, L.; Preisser, F.; Theissen, L.H.; Humke, C.; Deuker, M.; Bernatz, S.; Gild, P.; Ahyai, S.; et al. Multiparametric MRI may Help to Identify Patients with Prostate Cancer in a Contemporary Cohort of Patients with Clinical Bladder Outlet Obstruction Scheduled for Holmium Laser Enucleation of the Prostate (HoLEP). Front. Surg. 2021, 8, 633196. [Google Scholar] [CrossRef] [PubMed]
- Pandolfo, S.D.; Crauso, F.; Aveta, A.; Cilio, S.; Barone, B.; Napolitano, L.; Scarpato, A.; Mirto, B.F.; Serino, F.; Del Giudice, F.; et al. A Novel Low-Cost Uroflowmetry for Patient Telemonitoring. Int. J. Environ. Res. Public Health 2023, 20, 3287. [Google Scholar] [CrossRef] [PubMed]
- Krambeck, A.E.; Handa, S.E.; Lingeman, J.E. Experience with more than 1,000 holmium laser prostate enucleations for benign prostatic hyperplasia. J. Urol. 2013, 189, S141–S145. [Google Scholar] [CrossRef] [PubMed]
- Lerner, L.B.; McVary, K.T.; Barry, M.J.; Bixler, B.R.; Dahm, P.; Das, A.K.; Gandhi, M.C.; Kaplan, S.A.; Kohler, T.S.; Martin, L.; et al. Management of Lower Urinary Tract Symptoms Attributed to Benign Prostatic Hyperplasia: AUA GUIDELINE PART II—Surgical Evaluation and Treatment. J. Urol. 2021, 206, 818–826. [Google Scholar] [CrossRef]
- Yilmaz, M.; Toprak, T.; Suarez-Ibarrola, R.; Sigle, A.; Gratzke, C.; Miernik, A. Incidental prostate cancer after holmium laser enucleation of the prostate—A narrative review. Andrologia 2022, 54, e14332. [Google Scholar] [CrossRef]
- Magistro, G.; Keller, P.; Westhofen, T.; Schott, M.; Tamalunas, A.; Weinhold, P.; Stief, C.G. The significance of a high preoperative PSA level for the detection of incidental prostate cancer in LUTS patients with large prostates. World J. Urol. 2021, 39, 1481–1487. [Google Scholar] [CrossRef]
- van der Leest, M.; Cornel, E.; Israel, B.; Hendriks, R.; Padhani, A.R.; Hoogenboom, M.; Zamecnik, P.; Bakker, D.; Setiasti, A.Y.; Veltman, J.; et al. Head-to-head Comparison of Transrectal Ultrasound-guided Prostate Biopsy Versus Multiparametric Prostate Resonance Imaging with Subsequent Magnetic Resonance-guided Biopsy in Biopsy-naive Men with Elevated Prostate-specific Antigen: A Large Prospective Multicenter Clinical Study. Eur. Urol. 2019, 75, 570–578. [Google Scholar] [CrossRef]
- Sathianathen, N.J.; Omer, A.; Harriss, E.; Davies, L.; Kasivisvanathan, V.; Punwani, S.; Moore, C.M.; Kastner, C.; Barrett, T.; Van Den Bergh, R.C.; et al. Negative Predictive Value of Multiparametric Magnetic Resonance Imaging in the Detection of Clinically Significant Prostate Cancer in the Prostate Imaging Reporting and Data System Era: A Systematic Review and Meta-analysis. Eur. Urol. 2020, 78, 402–414. [Google Scholar] [CrossRef]
- Park, K.J.; Choi, S.H.; Lee, J.S.; Kim, J.K.; Kim, M.H.; Jeong, I.G. Risk Stratification of Prostate Cancer According to PI-RADS(R) Version 2 Categories: Meta-Analysis for Prospective Studies. J. Urol. 2020, 204, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Di Mauro, E.; Di Bello, F.; Califano, G.; Morra, S.; Creta, M.; Celentano, G.; Abate, M.; Fraia, A.; Pezone, G.; Marino, C.; et al. Incidence and Predicting Factors of Histopathological Features at Robot-Assisted Radical Prostatectomy in the mpMRI Era: Results of a Single Tertiary Referral Center. Medicina 2023, 59, 625. [Google Scholar] [CrossRef]
- Hutchison, D.; Peabody, H.; Kuperus, J.M.; Humphrey, J.E.; Ryan, M.; Moriarity, A.; Brede, C.M.; Lane, B.R. Management of prostate cancer after holmium laser enucleation of the prostate. Urol. Oncol. 2021, 39, 297.e1–297.e8. [Google Scholar] [CrossRef]
- Ploussard, G.; Rouviere, O.; Roupret, M.; van den Bergh, R.; Renard-Penna, R. The current role of MRI for guiding active surveillance in prostate cancer. Nat. Rev. Urol. 2022, 19, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Kruger-Stokke, B.; Bertilsson, H.; Langorgen, S.; Sjobakk, T.A.E.; Bathen, T.F.; Selnaes, K.M. Multiparametric Prostate MRI in Biopsy-Naive Men: A Prospective Evaluation of Performance and Biopsy Strategies. Front. Oncol. 2021, 11, 745657. [Google Scholar] [CrossRef] [PubMed]
- Barentsz, J.O.; Richenberg, J.; Clements, R.; Choyke, P.; Verma, S.; Villeirs, G.; Rouviere, O.; Logager, V.; Futterer, J.J. European Society of Urogenital Radiology. ESUR prostate MR guidelines 2012. Eur. Radiol. 2012, 22, 746–757. [Google Scholar] [CrossRef]
- Park, S.Y.; Jung, D.C.; Oh, Y.T.; Cho, N.H.; Choi, Y.D.; Rha, K.H.; Hong, S.J.; Han, K. Prostate Cancer: PI-RADS Version 2 Helps Preoperatively Predict Clinically Significant Cancers. Radiology 2016, 280, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A.; Grading, C. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef]
- Epstein, J.I.; Amin, M.B.; Reuter, V.E.; Humphrey, P.A. Contemporary Gleason Grading of Prostatic Carcinoma: An Update with Discussion on Practical Issues to Implement the 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am. J. Surg. Pathol. 2017, 41, e1–e7. [Google Scholar] [CrossRef]
- Capogrosso, P.; Capitanio, U.; Vertosick, E.A.; Ventimiglia, E.; Chierigo, F.; Oreggia, D.; Moretti, D.; Briganti, A.; Vickers, A.J.; Montorsi, F.; et al. Temporal Trend in Incidental Prostate Cancer Detection at Surgery for Benign Prostatic Hyperplasia. Urology 2018, 122, 152–157. [Google Scholar] [CrossRef]
- Han, J.H.; Chung, D.H.; Cho, M.C.; Ku, J.H.; Jeong, C.W.; Kwak, C.; Paick, J.S.; Oh, S.J. Natural history of incidentally diagnosed prostate cancer after holmium laser enucleation of the prostate. PLoS ONE 2023, 18, e0278931. [Google Scholar] [CrossRef] [PubMed]
- Elkoushy, M.A.; Elshal, A.M.; Elhilali, M.M. Incidental Prostate Cancer Diagnosis During Holmium Laser Enucleation: Assessment of Predictors, Survival, and Disease Progression. Urology 2015, 86, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.M.; Donovan, J.L.; Turner, E.L.; Metcalfe, C.; Young, G.J.; Walsh, E.I.; Lane, J.A.; Noble, S.; Oliver, S.E.; Evans, S.; et al. Effect of a Low-Intensity PSA-Based Screening Intervention on Prostate Cancer Mortality: The CAP Randomized Clinical Trial. JAMA 2018, 319, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Futterer, J.J.; Briganti, A.; De Visschere, P.; Emberton, M.; Giannarini, G.; Kirkham, A.; Taneja, S.S.; Thoeny, H.; Villeirs, G.; Villers, A. Can Clinically Significant Prostate Cancer Be Detected with Multiparametric Magnetic Resonance Imaging? A Systematic Review of the Literature. Eur. Urol. 2015, 68, 1045–1053. [Google Scholar] [CrossRef]
- Tay, K.J.; Gupta, R.T.; Holtz, J.; Silverman, R.K.; Tsivian, E.; Schulman, A.; Moul, J.W.; Polascik, T.J. Does mpMRI improve clinical criteria in selecting men with prostate cancer for active surveillance? Prostate Cancer Prostatic Dis. 2017, 20, 323–327. [Google Scholar] [CrossRef]
- Chung, D.Y.; Goh, H.J.; Koh, D.H.; Kim, M.S.; Lee, J.S.; Jang, W.S.; Choi, Y.D. Clinical significance of multiparametric MRI and PSA density as predictors of residual tumor (pT0) following radical prostatectomy for T1a-T1b (incidental) prostate cancer. PLoS ONE 2018, 13, e0210037. [Google Scholar] [CrossRef]
- Klein, C.; Marquette, T.; Capon, G.; Yacoub, M.; Alezra, E.; Bernhard, J.C.; Bladou, F.; Robert, G. Incidental prostate cancer after holmium laser enucleation of the prostate: Incidence and predictive factors for clinical progression. Int. J. Clin. Oncol. 2022, 27, 1077–1083. [Google Scholar] [CrossRef]
- Pellegrino, F.; Stabile, A.; Mazzone, E.; Sorce, G.; Barletta, F.; De Angelis, M.; Brembilla, G.; Gandaglia, G.; De Cobelli, F.; Montorsi, F.; et al. Does previous prostate surgery affect multiparametric magnetic resonance imaging accuracy in detecting clinically significant prostate cancer? Results from a single institution series. Prostate 2022, 82, 1170–1175. [Google Scholar] [CrossRef]
- Weinreb, J.C.; Barentsz, J.O.; Choyke, P.L.; Cornud, F.; Haider, M.A.; Macura, K.J.; Margolis, D.; Schnall, M.D.; Shtern, F.; Tempany, C.M.; et al. PI-RADS Prostate Imaging—Reporting and Data System: 2015, Version 2. Eur. Urol. 2016, 69, 16–40. [Google Scholar] [CrossRef]
| Variable | p |
|---|---|
| No. of patients, n (%) | 62 (100) |
| Age, years | |
| Median (IQR) | 72.5 (66.5–78.0) |
| Body mass index, kg/m2 | |
| Median (IQR) | 24.6 (22.8–26.3) |
| Total PSA, ng/mL | |
| Median (IQR) | 3.49 (1.82–5.03) |
| Prostate volume, cm3 | |
| Median (IQR) | 49.6 (38.5–85.4) |
| PSA density, ng/mL/cm3 | |
| Median (IQR) | 0.06 (0.03–0.09) |
| PI-RADS v2 score, n (%) | |
| 1–2 | 32 (51.6) |
| 3 | 14 (22.6) |
| 4 | 11 (17.7) |
| 5 | 5 (8.1) |
| T stage, n (%) | |
| T1a | 40 (64.5) |
| T1b | 22 (35.5) |
| IPSS total | |
| Mean (SD) | 20.6 (5.2) |
| Variable | Value |
|---|---|
| Gleason grade, n (%) | 62 (100) |
| GG1 | 42 (67.7) |
| GG2 | 13 (21.0) |
| GG3 | 2 (3.2) |
| GG4 | 1 (1.6) |
| GG5 | 4 (6.5) |
| Tumor volume, % | |
| Median (IQR) | 2.0 (1.0–5.0) |
| Incidental Prostate Cancer after HoLEP | |||
|---|---|---|---|
| Gleason Grade (n, %) | mpMRI Results (n, %) | Clinical T Stage (n, %) | Treatment (n, %) |
| Gleason grade 1 (42, 67.7%) | PI-RADS 2 (27, 64.3%) | cT1 (27, 100.0%) | Active surveillance (24, 88.9%) |
| Radical prostatectomy (2, 7.4%) | |||
| Radiotherapy (1, 3.7%) | |||
| PI-RADS 3 (9, 21.4%) | cT1 (2, 22.2%) | Active surveillance (1, 50.0%) | |
| Radical prostatectomy (1, 50.0%) | |||
| cT2a (6, 66.7%) | Active surveillance (5, 83.3%) | ||
| Radiotherapy (1, 16.7%) | |||
| cT2c (1, 11.1%) | Radical prostatectomy (1, 100.0%) | ||
| PI-RADS 4 (6, 14.3%) | cT2a (2, 33.3%) | Active surveillance (2, 100.0%) | |
| cT2b (1, 16.7%) | Active surveillance (1, 100.0%) | ||
| cT2c (2, 33.3%) | Active surveillance (1, 50.0%) | ||
| Radical prostatectomy (1, 50.0%) | |||
| cT3a (1, 16.7%) | Hormone therapy (1, 100.0%) | ||
| Gleason grade 2 (13, 21.0%) | PI-RADS 2 (5, 38.5%) | cT1 (4, 80.0%) | Active surveillance (4, 100.0%) |
| cT2a (1, 20.0%) | Radical prostatectomy (1, 100.0%) | ||
| PI-RADS 3 (5, 38.5%) | cT2a (4, 80.0%) | Active surveillance (2, 50.0%) | |
| Radical prostatectomy (2, 50.0%) | |||
| cT3a (1, 20.0%) | Radiotherapy (1, 100.0%) | ||
| PI-RADS 4 (3, 23.0%) | cT2a (1, 33.3%) | Radical prostatectomy (1, 100.0%) | |
| cT2c (2, 66.7%) | Radical prostatectomy (1, 50.0%) | ||
| Active surveillance (1, 50.0%) | |||
| Gleason grade 3 (2, 3.2%) | PI-RADS 4 (1, 50.0%) | cT3a (1, 100.0%) | Radiotherapy (1, 100.0%) |
| PI-RADS 5 (1, 50.0%) | cT3a (1, 100.0%) | Hormone therapy (1, 100.0%) | |
| Gleason grade 4–5 (5, 8.1%) | PI-RADS 4 (1, 20.0%) | cT3b (1, 100.0%) | Radiotherapy + hormone therapy (1, 100.0%) |
| PI-RADS 5 (4, 80.0%) | cT3b (4, 100.0%) | Radiotherapy + hormone therapy (4, 100.0%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, K.-J.; Choi, S.; Song, W. The Impact of Multiparametric Magnetic Resonance Imaging on Treatment Strategies for Incidental Prostate Cancer after Holmium Laser Enucleation of the Prostate. J. Clin. Med. 2023, 12, 4826. https://doi.org/10.3390/jcm12144826
Ko K-J, Choi S, Song W. The Impact of Multiparametric Magnetic Resonance Imaging on Treatment Strategies for Incidental Prostate Cancer after Holmium Laser Enucleation of the Prostate. Journal of Clinical Medicine. 2023; 12(14):4826. https://doi.org/10.3390/jcm12144826
Chicago/Turabian StyleKo, Kwang-Jin, Seongik Choi, and Wan Song. 2023. "The Impact of Multiparametric Magnetic Resonance Imaging on Treatment Strategies for Incidental Prostate Cancer after Holmium Laser Enucleation of the Prostate" Journal of Clinical Medicine 12, no. 14: 4826. https://doi.org/10.3390/jcm12144826
APA StyleKo, K.-J., Choi, S., & Song, W. (2023). The Impact of Multiparametric Magnetic Resonance Imaging on Treatment Strategies for Incidental Prostate Cancer after Holmium Laser Enucleation of the Prostate. Journal of Clinical Medicine, 12(14), 4826. https://doi.org/10.3390/jcm12144826




