Diagnosis, Treatment and Prognosis of Mesonephric Adenocarcinoma of the Vagina: A Literature Review and a Case Report
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Search Strategy
2.2. Inclusion Criteria
2.3. Study Selection and Data Extraction
2.4. Data Synthesis
3. Results
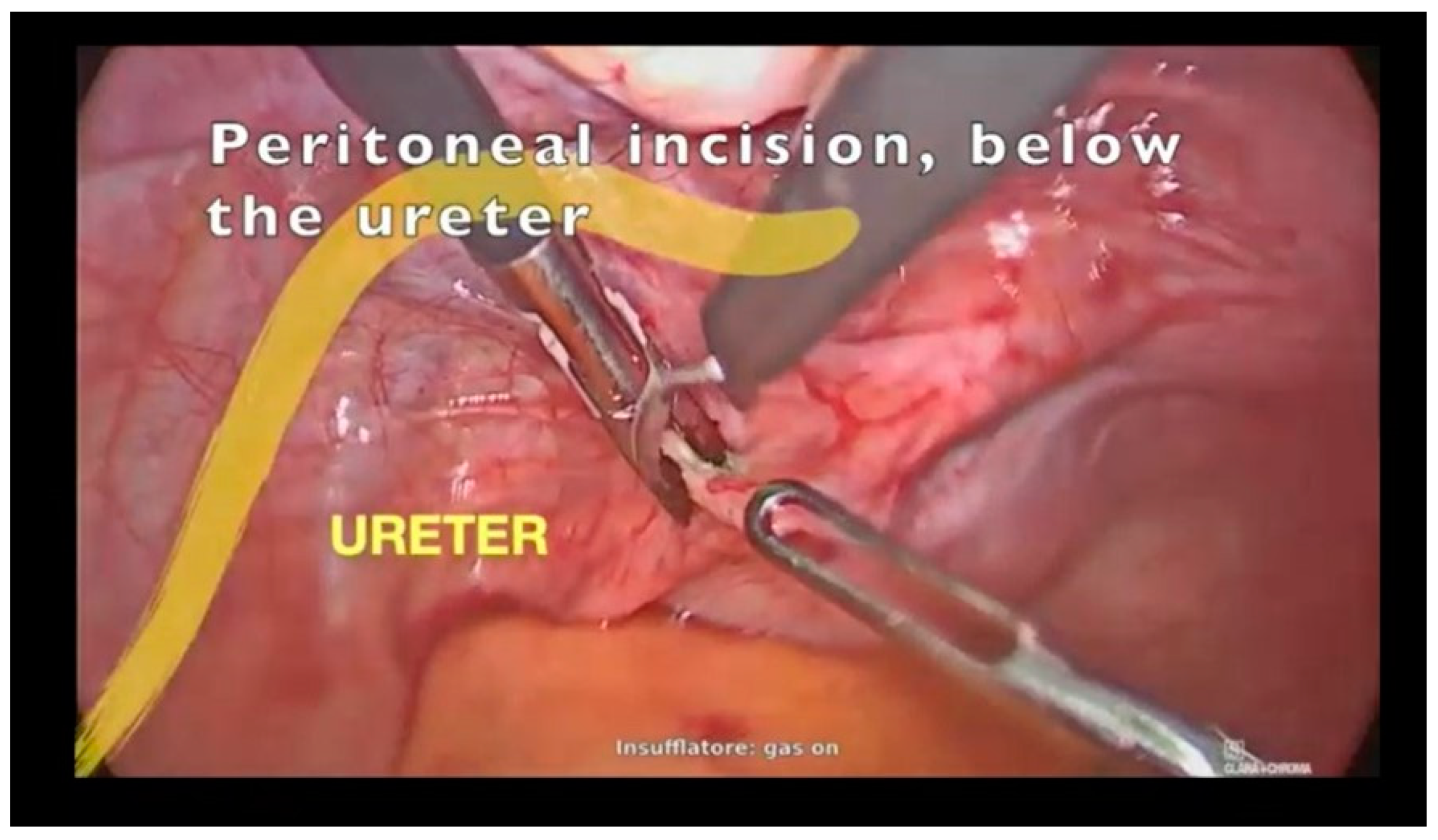
3.1. Case Report
3.2. Systematic Review of the Literature
3.2.1. Historical Findings
3.2.2. Characteristics of Patients, Clinical Manifestations and Diagnostical Features
3.2.3. Therapeutical Management and Prognosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Howitt, B.E.; Nucci, M.R. Mesonephric proliferations of the female genital tract. Pathology 2018, 50, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Saklani, B.; Kapil, R.; Sen, R. Post hysterectomy mesonephric carcinoma: A case report and literature review. J. Cancer Res. Ther. 2022, 18, 277–279. [Google Scholar] [CrossRef]
- Soleymani Majd, H.; Ferrari, F.; Gubbala, K.; Campanile, R.G.; Tozzi, R. Latest developments and techniques in gynaecological oncology surgery. Curr. Opin. Obstet. Gynecol. 2015, 27, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Adams, T.S.; Rogers, L.J.; Cuello, M.A. Cancer of the vagina: 2021 update. Int. J. Gynaecol. Obstet. 2021, 155 (Suppl. S1), 19–27. [Google Scholar] [CrossRef] [PubMed]
- Wahlen, T.; Gynning, I. Carcinoma of Gärtner’s duct; a vaginal case. Acta Obstet. Gynecol. Scand. 1955, 34, 120–130. [Google Scholar] [CrossRef]
- Grünberger, W.; Pantucek, F. Case report of a Gartner’s duct carcinoma of the vagina (author’s transl). Wien. Klin. Wochenschr. 1977, 89, 313–315. [Google Scholar]
- Shevchuk, M.M.; Fenoglio, C.M.; Lattes, R.; Frick, H.C.; Richart, R.M. Malignant mixed tumor of the vagina probably arising in mesonephric rests. Cancer 1978, 42, 214–223. [Google Scholar] [CrossRef]
- Nemoto, S.; Yazaki, T.; Nemoto, R.; Kanoh, S.; Kitagawa, R. A case of mesonephric adenocarcinoma arising from the Gartner’s duct cyst. Nihon Hinyokika Gakkai Zasshi 1983, 74, 2148–2153. [Google Scholar] [CrossRef]
- Tanigawa, T.; Ueda, S.; Nomura, Y.; Ogata, J. A case of malignant Gartner’s duct tumor presenting urinary symptoms. Nihon Hinyokika Gakkai Zasshi 1985, 76, 104–109. [Google Scholar] [CrossRef] [Green Version]
- Bagué, S.; Rodríguez, I.M.; Prat, J. Malignant mesonephric tumors of the female genital tract: A clinicopathologic study of 9 cases. Am. J. Surg. Pathol. 2004, 28, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Erşahin, C.; Huang, M.; Potkul, R.K.; Hammadeh, R.; Salhadar, A. Mesonephric adenocarcinoma of the vagina with a 3-year follow-up. Gynecol. Oncol. 2005, 99, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Bifulco, G.; Mandato, V.D.; Mignogna, C.; Giampaolino, P.; Di Spiezio Sardo, A.; De Cecio, R.; De Rosa, G.; Piccoli, R.; Radice, L.; Nappi, C. A case of mesonephric adenocarcinoma of the vagina with a 1-year follow-up. Int. J. Gynecol. Cancer 2008, 18, 1127–1131. [Google Scholar] [CrossRef]
- Mueller, I.; Kametriser, G.; Jacobs, V.R.; Bogner, G.; Staudach, A.; Koch, H.; Wolfrum-Ristau, P.; Schausberger, C.; Fischer, T.; Sedlmayer, F. Mesonephric adenocarcinoma of the vagina: Diagnosis and multimodal treatment of a rare tumor and analysis of worldwide experience. Strahlenther. Onkol. 2016, 192, 668–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roma, A.A. Mesonephric carcinosarcoma involving uterine cervix and vagina: Report of 2 cases with immunohistochemical positivity For PAX2, PAX8, and GATA-3. Int. J. Gynecol. Pathol. 2014, 33, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Amal, B.; Hind, E.F.; Hanane, S.; Hayat, R.; Kaoutar, M.; Abdelaziz, B.; Taoufik, H.; Affaf, A. A tumor of the vagina not to overlook, the mesonephric adenocarcinoma: About a case report and review of literature. Pan Afr. Med. J. 2015, 21, 126. [Google Scholar] [CrossRef]
- Plesinac-Karapandzic, V.; Stojanovic Rundic, S.; Jankovic, R.; Nadrljanski, M.; Milovanovic, Z.; Tomasevic, A.; Perisie Jeremic, N. Non-diethylstilbestrol exposed vaginal adenocarcinoma in young patients associated with unilateral renal agenesis: Two case reports and literature review. Eur. J. Gynaecol. Oncol. 2017, 38, 157–161. [Google Scholar] [PubMed]
- Shoeir, S.; Balachandran, A.A.; Wang, J.; Sultan, A.H. Mesonephric adenocarcinoma of the vagina masquerading as a suburethral cyst. BMJ Case Rep. 2018, 2018, bcr-2018-224758. [Google Scholar] [CrossRef]
- Xie, C.; Chen, Q.; Shen, Y. Mesonephric adenocarcinomas in female genital tract: A case series. Medicine 2021, 100, e27174. [Google Scholar] [CrossRef]
- Lee, H.; Kim, H.; Kim, H.-S. Mesonephric Adenocarcinoma of the Vagina Harboring TP53 Mutation. Diagnostics 2022, 12, 119. [Google Scholar] [CrossRef]
- Lin, D.I.; Shah, N.; Tse, J.Y.; Killian, J.K.; Hemmerich, A.; Edgerly, C.; Haberberger, J.; Severson, E.A.; Huang, R.S.P.; Ramkissoon, S.H.; et al. Molecular profiling of mesonephric and mesonephric-like carcinomas of cervical, endometrial and ovarian origin. Gynecol. Oncol. Rep. 2020, 34, 100652. [Google Scholar] [CrossRef]
- Tozzi, R.; Soleymani Majd, H.; Campanile, R.G.; Ferrari, F. Feasibility of laparoscopic diaphragmatic peritonectomy during Visceral-Peritoneal Debulking (VPD) in patients with stage IIIC-IV ovarian cancer. J. Gynecol. Oncol. 2020, 31, e71. [Google Scholar] [CrossRef]
- Addley, S.; Vinti, D.; Soleymani Majd, H. Laparoscopic Upper Colpectomy for VAIN III after Previous Total Hysterectomy. J. Minim. Invasive Gynecol. 2021, 28, 1137. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Hur, S.Y.; Park, J.S.; Lee, K.H. Laparoscopic upper vaginectomy for post-hysterectomy high risk vaginal intraepithelial neoplasia and superficially invasive vaginal carcinoma. World J. Surg. Oncol. 2013, 11, 126. [Google Scholar] [CrossRef] [Green Version]
- Bats, A.S.; Metzger, U.; Le Frere-Belda, M.A.; Brisa, M.; Lecuru, F. Malignant transformation of Gartner cyst. Int. J. Gynecol. Cancer 2009, 19, 1655–1657. [Google Scholar] [CrossRef] [PubMed]
- Besnard, C.; Lemanski, C.; Vendrely, V. Toxicité sexuelle induite par la radiothérapie [Radiation-induced sexual toxicity]. Cancer Radiother. 2021, 25, 816–821. [Google Scholar] [CrossRef]




| Author, Year | Baguè, 2004 | Baguè, 2004 | Ersahin, 2005 | Bifulco, 2008 | Mueller, 2016 | Roma, 2014 | Amal, 2015 | Plesinac, 2017 | Shoeir, 2018 | Xie, 2021 | Lee, 2022 | Kumar, 2022 | Case Report |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Previous Surgery | - | - | VH | TAH, BSO | - | SCH | - | - | 2 CS | - | - | TAH | - |
| Cystoscopy | - | - | - | - | yes | - | - | - | yes | - | - | - | - |
| Presurgical Biopsy | MH | - | ADC | - | Hyperplasia | Mullerian tumor | ADC | MA | - | - | ADC | - | IOA: ADC |
| CA 125 | - | - | - | pos | - | - | - | - | - | neg | neg | - | pos |
| Imaging | - | - | - | US, CT | MRI | US, CT | MRI | CT | US, MRI | - | MRI | - | US, MRI |
| Size (cm) | 4 | - | 1 | 14 × 7 × 6 | 2.5 × 1.8 | 5 × 2.5 × 0.5 | 4 | - | 3.1 × 2.7 × 2.9 | - | 2.5 | 2 × 1.5 × 1 | 5.1 × 4.2 × 4.9 |
| Symptoms | Leiomyomas | Dyspareunia | Polyp, + PAP smear | Pelvic pain, Pruritus vulvae | Post-coital vaginal bleeding | Vaginal bleeding | Vaginal bleeding | - | Vaginal swelling, urinary urgency | Vaginal discomfort | Vaginal bleeding | Vaginal bleeding | Ovaric cyst |
| Age | 54 | 38 | 55 | 58 | 54 | 58 | 50 | 22 | 63 | 31 | 52 | 40 | 39 |
| Authors, Year | Baguè, 2004 | Baguè, 2004 | Ersahin, 2005 | Bifulco, 2008 | Mueller, 2016 | Roma, 2014 | Amal, 2015 | Plesinac, 2017 | Shoeir, 2018 | Xie, 2021 | Lee, 2022 | Kumar, 2022 | Case Report |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Follow-up | 8y7m—PFS | - | 3y—PFS | 1y—PFS | 4y—PFS | 1m—PFS | - | 11y—PFS | 2m—PFS | 7y6m—PFS | 10m—PFS | - | 1y3m—PFS |
| Adjuvant Treatment | - | - | BT, EBRT, CHT | - | BT, EBRT, CHT | - | BT (NeoAdj.) | EBRT, CHT | BT | CHT | BT, EBRT, CHT | BT | - |
| N | - | - | pos | neg | neg | neg | - | - | neg | neg | neg | - | Neg |
| Local Invasion | pos | - | pos | neg | pos | pos | pos | - | neg | neg | pos | - | Neg |
| FIGO Stage | II | - | III | I | II | II | III | III | I | I | II | I | I |
| Washing | - | - | pos | - | - | - | - | - | - | - | - | - | neg |
| Surgery | TAH, BSO, CPT | ResTu | BSO, CPT, PLND | ResTu, PLND | ResTu (R1) | Pelvis exenteration, Ileal conduit | unknown | - | ResTu (+ spilling) | TAH, BSO | ResTu, BSO, PLND | ResTu | ResTu, BS |
| Surgical Approach | LPT | VS | LPT | LPT | VS | LPT | unknown | - | VS | LPT | LPT | VS | LPS |
| Age | 54 | 38 | 55 | 58 | 54 | 58 | 50 | 22 | 63 | 31 | 52 | 40 | 39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrari, F.; Omodei, A.S.; Ferrari, F.A.; Soleymani Majd, H.; Ardighieri, L.; Vitale, S.G.; Laganà, A.S.; Angioni, S.; Ciravolo, G.; Odicino, F. Diagnosis, Treatment and Prognosis of Mesonephric Adenocarcinoma of the Vagina: A Literature Review and a Case Report. J. Clin. Med. 2023, 12, 4846. https://doi.org/10.3390/jcm12144846
Ferrari F, Omodei AS, Ferrari FA, Soleymani Majd H, Ardighieri L, Vitale SG, Laganà AS, Angioni S, Ciravolo G, Odicino F. Diagnosis, Treatment and Prognosis of Mesonephric Adenocarcinoma of the Vagina: A Literature Review and a Case Report. Journal of Clinical Medicine. 2023; 12(14):4846. https://doi.org/10.3390/jcm12144846
Chicago/Turabian StyleFerrari, Federico, Andrea Salvatore Omodei, Filippo Alberto Ferrari, Hooman Soleymani Majd, Laura Ardighieri, Salvatore Giovanni Vitale, Antonio Simone Laganà, Stefano Angioni, Giuseppe Ciravolo, and Franco Odicino. 2023. "Diagnosis, Treatment and Prognosis of Mesonephric Adenocarcinoma of the Vagina: A Literature Review and a Case Report" Journal of Clinical Medicine 12, no. 14: 4846. https://doi.org/10.3390/jcm12144846








