Differences in the Time Course of Recovery from Brain and Liver Dysfunction in Conventional Long-Term Treatment of Wilson Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Recruitment
2.2. Scoring of the Neurological Findings
2.3. Analysis of the Biochemical Parameters
2.4. Statistics
3. Results
3.1. Demographic and Treatment-Related Data and Spectrum of Initial Clinical Symptoms
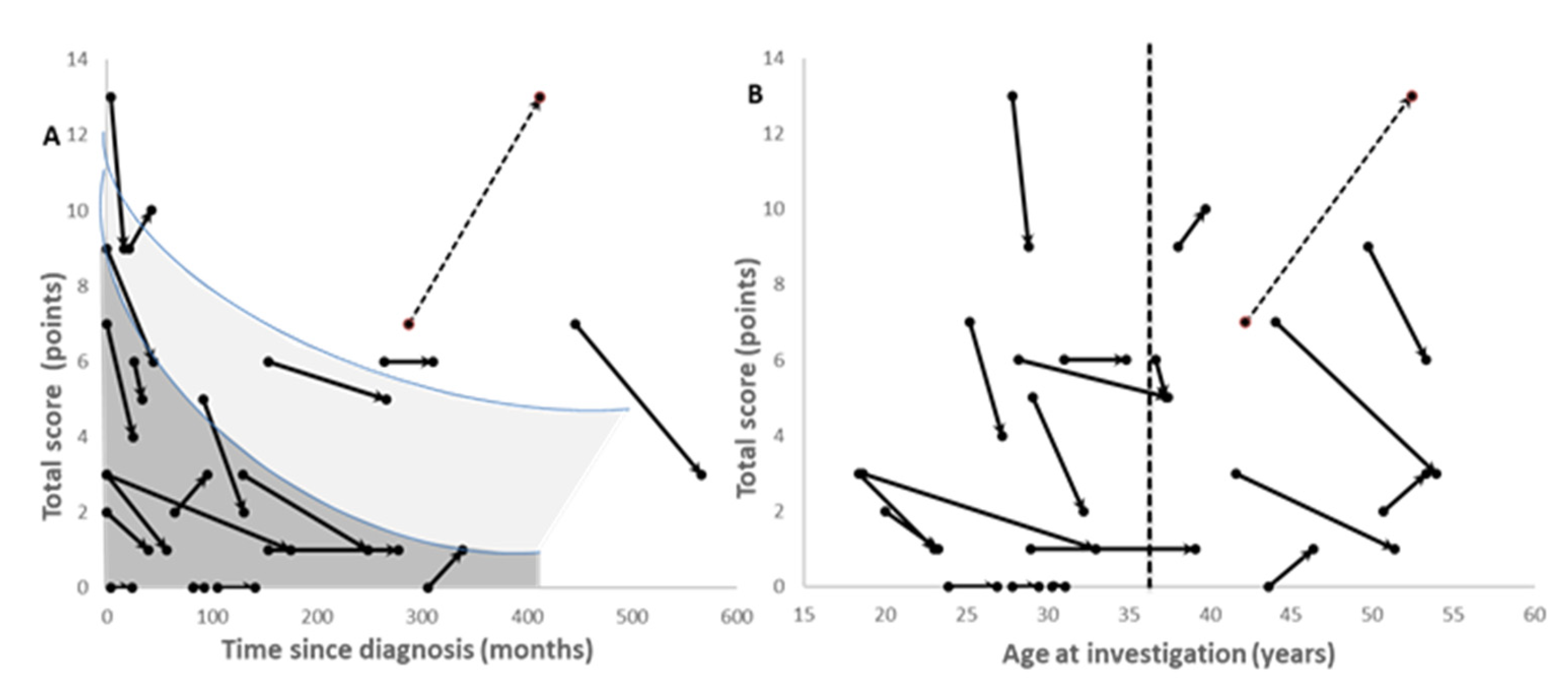
3.2. Course of Treatment and Improvement in Neurological Symptoms
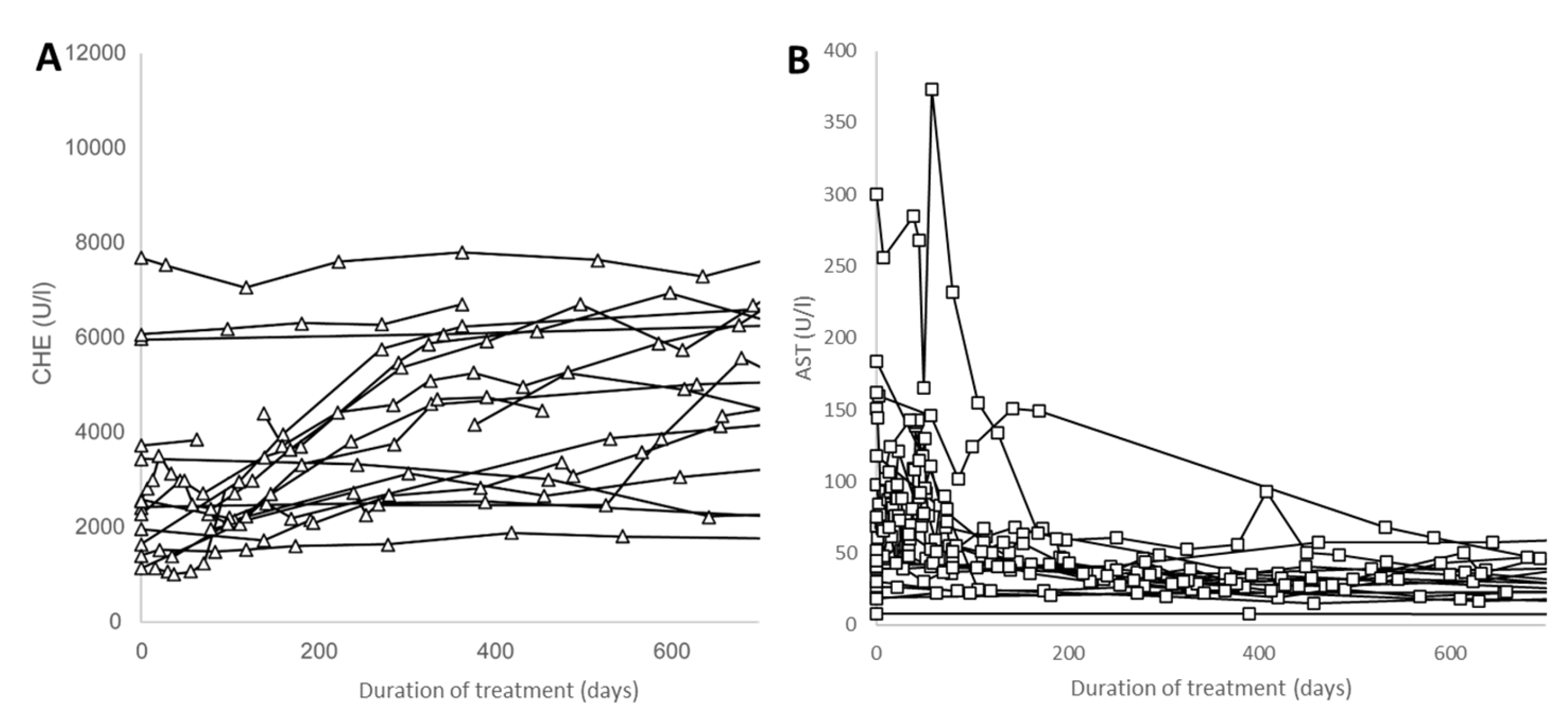
3.3. Improvement in the MELD Score and Biochemical Parameters
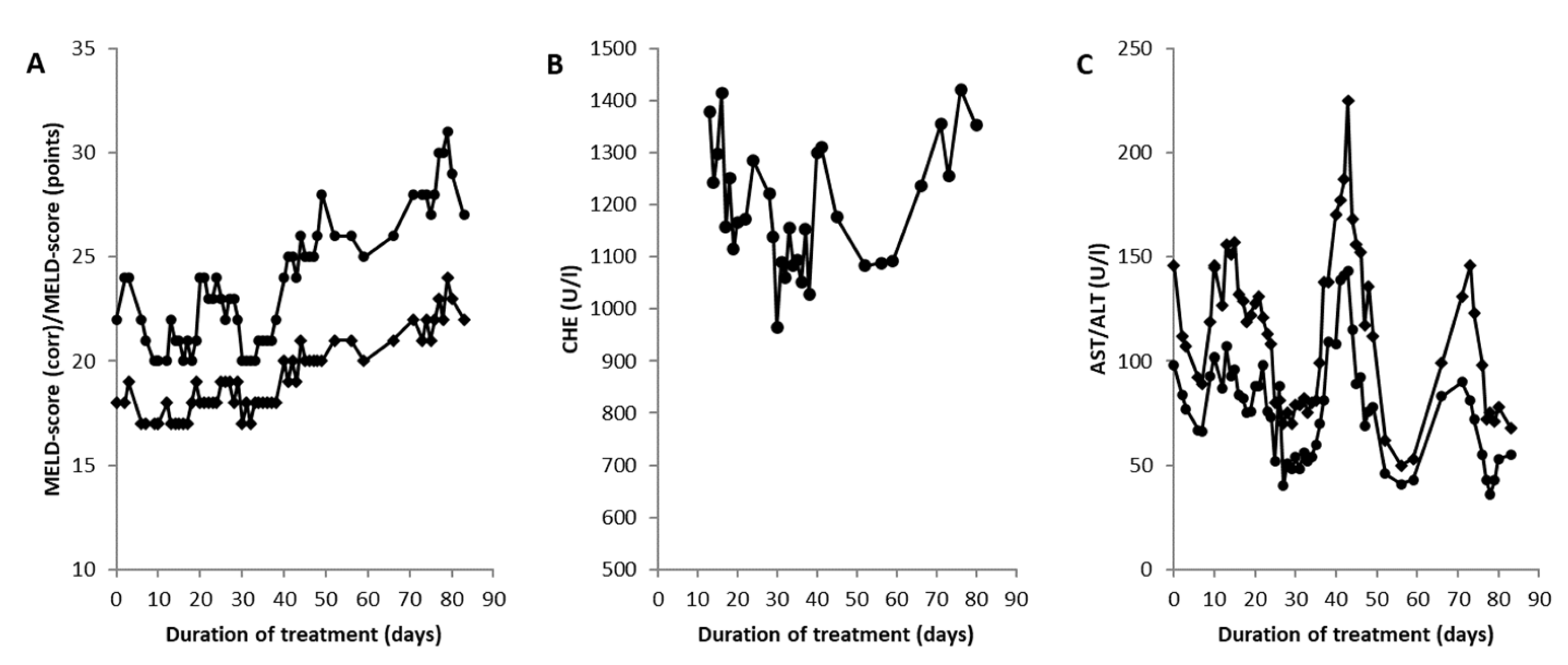
3.4. Liver Transplantation in a Wilson Disease Patient with Acute Liver Failure
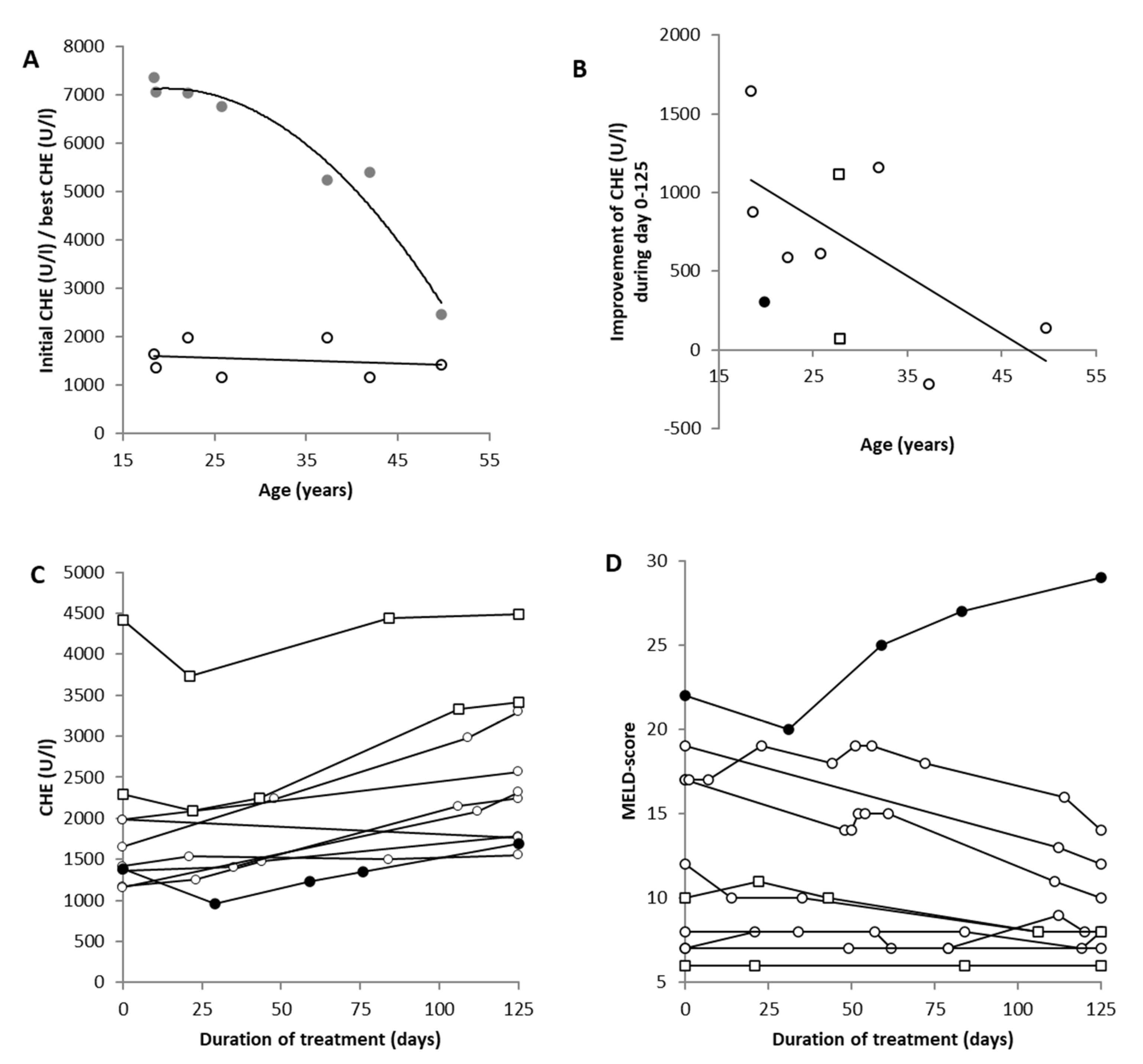
3.5. Recovery of Pseudocholinesterase in 10 Selected Patients
4. Discussion
4.1. The Broad Spectrum of Neurological Symptoms in Wilson´s Disease before and after Therapy
4.2. Improvement in Biochemical Parameters during the Course of Therapy in Wilson´s Disease
4.3. Difference in Time Course of the Improvement in Neurological and Hepatic Symptoms
4.4. Is There a Time Window for Conservative Therapy in Patients with Acute Liver Failure?
5. Conclusions
6. Strengths and Limits of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Wilson, S.A.K. Progressive lenticular degeneration: A familial nervous disease associated with cirrhosis of the liver. Brain 1912, 34, 295–507. [Google Scholar] [CrossRef]
- Bandmann, O.; Weiss, K.H.; Kaler, S.G. Wilson’s disease and other neurological copper disorders. Lancet Neurol. 2015, 15, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Członkowska, A.; Litwin, T.; Dusek, P.; Ferenci, P.; Lutsenko, S.; Medici, V.; Rybakowski, J.K.; Weiss, K.H.; Schilsky, M.L. Wilson disease. Nat. Rev. Dis. Prim. 2018, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Cumings, J.N. The copper and iron content of brain and liver in the normal and in hepato-lenticular degeneration. Brain 1948, 71, 410–415. [Google Scholar] [CrossRef]
- Madsen, E.; Gitlin, J.D. Copper and iron disorders of the brain. Annu. Rev. Neurosci. 2007, 30, 317–337. [Google Scholar] [CrossRef]
- Nose, Y.; Kim, B.E.; Thiele, D.J. Ctr1 drives intestinal copper absorption and is essential for growth, iron metabolism, and neonatal cardiac function. Cell Metab. 2006, 4, 235–244. [Google Scholar] [CrossRef]
- Hellman, N.E.; Gitlin, J.D. Ceruloplasmin metabolism and function. Ann. Rev. Nut 2002, 22, 439–458. [Google Scholar] [CrossRef]
- Mukhopadhyay, C.K.; Attieh, Z.K.; Fox, P.L. Role of ceruloplasmin in cellular iron uptake. Science 1998, 279, 714. [Google Scholar] [CrossRef]
- Stremmel, W.; Merle, U.; Weiskirchen, R. Clinical features of Wilson disease. Ann. Transl. Med. 2019, 7 (Suppl. S2), S61. [Google Scholar] [CrossRef]
- Ferenci, P. Wilson’s disease. Clin. Gastroenterol. Hepatol. 2005, 3, 726–733. [Google Scholar] [CrossRef]
- Bull, P.C.; Thomas, G.R.; Rommens, J.M.; Forbes, J.R.; Cox, D.W. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat. Genet. 1993, 5, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.N.; Ball, E.V.; Stenson, P.D.; Phillips, A.D.; Evans, K.; Heywood, S.; Hayden, M.J.; Chapman, M.M.; Mort, M.E.; Azevedo, L.; et al. The Human Gene Mutation Database. QIAGEN. 2018. Available online: http://www.hgmd.cf.ac.uk/ac/index.php (accessed on 2 May 2023).
- Stenson, P.D.; Mort, M.; Ball, E.V.; Evans, K.; Hayden, M.; Heywood, S.; Hussain, M.; Phillips, A.D.; Cooper, D.N. The Human Gene Mutation Database: Towards a comprehensive repository of inherited mutation data for medical research, genetic diagnosis, and next-generation sequencing studies. Hum. Genet. 2017, 136, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Ferenci, P.; Stremmel, W.; Czlonkowska, A.; Szalay, F.; Viveiros, A.; Stättermayer, A.F.; Bruha, R.; Houwen, R.; Pop, T.L.; Stauber, R.; et al. Age and sex but not ATP7B genotype effectively influence the clinical phenotype of Wilson disease. Hepatology 2018, 69, 1464–1476. [Google Scholar] [CrossRef]
- Samadzadeh, S.; Kruschel, T.; Novak, M.; Kallenbach, M.; Hefter, H. Different Response Behavior to Therapeutic Approaches in Homozygotic Wilson’s Disease Twins with Clinical Phenotypic Variability: Case Report and Literature Review. Genes 2022, 13, 1217. [Google Scholar] [CrossRef]
- Castillo-Fernandez, J.E.; Spector, T.D.; Bell, J.T. Epigenetics of discordant monozygotic twins: Implications for disease. Genome Med. 2014, 6, 60. [Google Scholar] [CrossRef]
- Członkowska, A.; Gromadzka, G.; Chabik, G. Monozygotic female twins discordant for phenotype of Wilson’s disease. Mov. Disord. 2009, 24, 1066–1069. [Google Scholar] [CrossRef]
- Eisenbach, C.; Sieg, O.; Stremmel, W.; Encke, J.; Merle, U. Diagnostic criteria for acute liver failure due to Wilson disease. World J. Gastroenterol. 2007, 13, 1711–1714. [Google Scholar] [CrossRef]
- Reyes, C.V. Hepatocellular carcinoma in Wilson disease-related liver cirrhosis. Gastroenterol. Hepatol. 2008, 4, 435–437. [Google Scholar]
- Hartmann, C.J.; Hefter, H. Manifestation of Wilson disease despite ongoing zinc-monotherapy and improvement after adding trientine: A case report. Basal Ganglia 2014, 4, 81–83. [Google Scholar] [CrossRef]
- Ghika, J.; Vingerhoets, F.; Maeder, P.; Borruat, F.-X.; Bogousslavsky, J. Maladie de Wilson. EMC Neurol. 2004, 1, 481–511. [Google Scholar] [CrossRef]
- Rajan, A.; Kalita, J.; Kumar, V.; Misra, U.K. MRI and oxidative stress markers in neurological worsening of Wilson disease following penicillamine. Neurotoxicology 2015, 49, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Borchard, S.; Raschke, S.; Zak, K.M.; Eberhagen, C.; Einer, C.; Weber, E.; Müller, S.M.; Michalke, B.; Lichtmannegger, J.; Wieser, A.; et al. Bis-choline tetrathiomolybdate prevents copper-induced blood-brain barrier damage. Life Sci. Alliance 2021, 4, e202101164. [Google Scholar] [CrossRef]
- Burke, J.F.; Dayalu, P.; Nan, B.; Askari, F.; Brewer, G.J.; Lorincz, M.T. Prognostic significance of neurologic examination findings in Wilson disease. Park. Relat. Disord. 2011, 17, 551–556. [Google Scholar] [CrossRef]
- Senzolo, M.; Loreno, M.; Fagiuoli, S.; Zanus, G.; Canova, D.; Masier, A.; Russo, F.P.; Sturniolo, G.C.; Burra, P. Different neurological outcome of liver transplantation for Wilson’s disease in two homozygotic twins. Clin. Neurol. Neurosurg. 2007, 109, 71–75. [Google Scholar] [CrossRef]
- Broniek-Kowalik, K.; Dziezyc, K.; Litwin, T.; Członkowska, A.; Szaflik, J.P. Anterior segment optical coherence tomography (AS-OCT) as a new method of detecting copper deposits forming the Kayser-Fleischer ring in patients with Wilson disease. Acta Ophthalmol. 2019, 97, e757–e760. [Google Scholar] [CrossRef]
- Yap, W.W.; Kirke, R.; Yoshida, E.M.; Owen, D.; Harris, A.C. Non-invsive assessment of liver fibrosis using ARFI with pathological correlation, a prospective study. Ann. Hepatol. 2013, 12, 440–447. [Google Scholar] [CrossRef]
- Li, Y.; Ma, J.; Li, B.; Zhu, X.; Wang, J. Cirrhosis of Wilson’s disease: High and low cutoff using acoustic radiation force pulse (ARFI)-comparison and combination of serum fibrosis index. Clin. Hemorheol. Microcirc. 2021, 79, 575–585. [Google Scholar] [CrossRef]
- Hefter, H.; Tezayak, O.; Rosenthal, D. Long-term outcome of neurological Wilson’s disease. Park. Relat. Disord. 2018, 49, 48–53. [Google Scholar] [CrossRef]
- Medici, V.; Mirante, V.G.; Fassati, L.R.; Pompili, M.; Forti, D.; Del Gaudio, M.; Trevisan, C.P.; Cillo, U.; Sturniolo, G.C.; Fagiuoli, S. Liver transplantation for Wilson´s disease: The burden of neurological and psychiatric disorders. Liver Transpl. 2005, 11, 1056–1063. [Google Scholar] [CrossRef]
- Shribman, S.; Warner, T.T.; Dooley, J.S. Clinical presentations of Wilson disease. Ann. Transl. Med. 2019, 7 (Suppl. S2), S60. [Google Scholar] [CrossRef]
- Londono, M.-C.; Cardenas, A.; Guevara, M.; Quintó, L.; Heras, D.D.L.; Navasa, M.; Rimola, A.; Garcia-Valdecasas, J.-C.; Arroyo, V.; Ginès, P. MELD score and serum sodium in the prediction of survival of patients with cirrhosis awaiting liver transplantation. Gut 2007, 56, 1283–1290. [Google Scholar] [CrossRef]
- Samadzadeh, S. Long-Term Follow-Up of 115 Patients with Wilson’s Disease. Ph.D. Thesis, University of Düsseldorf, Düsseldorf, Germany, 2022. Available online: https://docserv.uni-duesseldorf.de/servlets/DocumentServlet?id=59305 (accessed on 3 May 2023).
- Horoupian, D.S.; Sternlieb, I.; Scheinberg, I.H. Neuropathological findings in penicillamine-treated patients with Wilson’s disease. Clin. Neuropathol. 1988, 7, 62–67. [Google Scholar]
- Stravitz, R.T.; Ellerbe, C.; Durkalski, V.; Schilsky, M.; Fontana, R.J.; Peterseim, C.; Lee, W.M.; The Acute Liver Failure Study Group. Bleeding complications in acute liver failure. Hepatology 2018, 67, 1931–1942. [Google Scholar] [CrossRef]
- Walshe, J.M.; Gibbs, K.R. Brain copper in Wilson’s disease. Lancet 1987, 2, 1030. [Google Scholar] [CrossRef]
- Hefter, H.; Arendt, G.; Kuwert, T.; Herzog, H.; Feinendegen, L.E.; Stremmel, W. Relationship between striatal glucose consumption and copper excretion in patients with Wilson’s disease treated with D-penicillamine. J. Neurol. 1993, 241, 49–53. [Google Scholar] [CrossRef]
- Ijomone, O.M.; Ifenatuoha, C.W.; Aluko, O.M.; Ijomone, O.K.; Aschner, M. The aging brain: Impact of heavy metal neurotoxicity. Crit. Rev. Toxicol. 2020, 50, 801–814. [Google Scholar] [CrossRef]
- Stuerenburg, H.J. CSF copper concentrations, blood barrier function, and coeruloplasmin synthesis during treatment of Wilson’s disease. J. Neural Transm. 2000, 107, 321–329. [Google Scholar] [CrossRef]
- Polson, R.J.; Rolles, K.; Calne, R.Y.; Williams, R.; Marsden, D. Reversal of severe neurological manifestations of Wilson’s disease following liver transplantation. Q. J. Med. 1987, 64, 685–691. [Google Scholar]
- Bax, R.T.; Hässler, A.; Luck, W.; Hefter, H.; Krägeloh-Mann, I.; Neuhaus, P.; Emmrich, P. Cerebral manifestation of Wilson´s disease successfully treated with liver transplantation. Neurology 1998, 51, 863–865. [Google Scholar] [CrossRef]
- Poujois, A.; Sobesky, R.; Meissner, W.G.; Brunet, A.-S.; Broussolle, E.; Laurencin, C.; Lion-François, L.; Guillaud, O.; Lachaux, A.; Maillot, F.; et al. Liver transplantation as a rscue therapy for severe forms of Wilson disease. Neurology 2020, 94, e2189–e2202. [Google Scholar] [CrossRef]
- Hefter, H.; Arslan, M.; Kruschel, T.; Novak, M.; Rosenthal, D.; Meuth, S.G.; Albrecht, P.; Hartmann, C.J.; Samadzadeh, S. Pseudocholinesterase as a biomarker for untreated Wilson´s disease. Biomolecules 2022, 12, 1791. [Google Scholar] [CrossRef]
- Gheorge, L.; Popescu, I.; Iacob, R.; Gheorghe, C. Predictors of death on the waiting list for liver transplantation characterized by a long waiting time. Transpl. Intern. 2005, 18, 572–576. [Google Scholar] [CrossRef]
- Ahmad, J.; Bryce, C.L.; Cacciarelli, T.; Roberts, M.S. Differences in access to liver transplantation: Disease severity, waiting time, and transplantation center volume. Ann. Intern. Med. 2007, 146, 735–741. [Google Scholar] [CrossRef]
- Kido, J.; Matsumoto, S.; Sakamoto, R.; Mitsubuchi, H.; Inomata, Y.; Nakamura, K. Recovery of severe acute liver failure without transplantation in patients with Wilson disease. Pediatr. Transplant. 2018, 22, e13292. [Google Scholar] [CrossRef]
- Summer, K.H.; Lichtmannegger, J.; Bandow, N.; Choi, D.W.; Di Spirito, A.A.; Michalke, B. The biogenic methanobactin is an effective chelator for copper in a rat model for Wilson disease. J. Trace Elem. Med. Biol. 2011, 25, 36–41. [Google Scholar] [CrossRef]
- Dispirito, A.A.; Choi, D.W.; Semrau, J.D.; Keeney, D. Use of Methanobactin. U.S. Patent US 8,735,538 B1, 27 May 2014. [Google Scholar]
- Lichtmannegger, J.; Leitzinger, C.; Wimmer, R.; Schmitt, S.; Schulz, S.; Kabiri, Y.; Eberhagen, C.; Rieder, T.; Janik, D.; Neff, F.; et al. Methanobactin reverses acute liver failure in a rat model of Wilson disease. J. Clin. Investig. 2016, 126, 2721–2735. [Google Scholar] [CrossRef]
- Müller, J.C.; Lichtmannegger, J.; Zischka, H.; Sperling, M.; Karst, U. High spatial resolution LA-ICP-MS demonstrates massive liver copper depletion in Wilson disease rats upon Methanobactin treatment. J. Trace Elem. Med. Biol. 2018, 49, 119–127. [Google Scholar] [CrossRef]


| No. of Patients | Type | General Clinical Classification |
|---|---|---|
| 1 | Asymptomatic | No symptoms at all |
| 3 | Hepatic | 1 patient with acute liver failure 2 patients with reduced daily activities and fatigue |
| 1 | Neuropsychiatric | Intellectual decline and moderate depression |
| 15 | Neurologic | 7 patients with tremor of the extremities, head, and/or trunk 5 patients with Parkinsonian symptoms 3 patients with other movement disorders (cerebellar ataxia, chorea, generalized dystonia) |
| No. of Patients | Symptom | Initial Neurological Investigation |
| Motor Symptoms | ||
| 14 | Bradykinesia | Slowness in fast alternating movements of the fingers or tongue |
| 9 | Tremor | Clinically manifest tremors of the extremities, head, and/or trunk |
| 8 | Dysarthria | Dysarthria and/or dysphagia |
| 7 | Gait disorder | Spastic, cerebellar, or Parkinsonian gait disorder |
| 7 | Dystonia | Focal and/or generalized dystonia |
| 6 | Cerebellar | Ataxia of extremities |
| 5 | Oculomotor deficits | Oculomotor impairment or oculogyric crisis |
| Non-Motor Symptoms | ||
| 0 | Sensory abnormalities | Sensory deficits of the legs, arms, hands, or fingers |
| 3 | Reflexes abnormalities | Reflex abnormalities (either enhanced reflexes with positive pyramidal tract signs or reduced or missing reflexes) |
| 4 | Neuropsychiatric symptoms | Neuropsychiatric symptoms; only 1 patient needed specific treatment |
| Parameter | Units | Initial Values | Final Values | Significance | ||
|---|---|---|---|---|---|---|
| Initial MV | Initial SD | Final MV | Final SD | p< | ||
| 24 h-PROT/L | mg/L | 107.9 | 107.9 | 142.0 | 329.4 | 0.00049 |
| 24 h-PROT/d | mg/d | 173.5 | 152.2 | 195.5 | 354.5 | 0.00604 |
| CHE | U/L | 3028 | 1886 | 5220 | 1379 | 0.00740 |
| AP | U/L | 131.0 | 71.6 | 87.3 | 25.7 | 0.01010 |
| ALB (%) | % | 54.5 | 6.6 | 60.2 | 2.7 | 0.02095 |
| AST | U/L | 78.8 | 76.2 | 31.6 | 17.3 | 0.04024 |
| ALB (g/dL) | g/dL | 6.1 | 7.6 | 4.4 | 1.0 | 0.04813 |
| MELD≤ 10 | Initial | MELD > 10 | Significance | |||
| Parameter | Units | MV: Initial MELD≤ 10 | SD: Initial MELD≤ 10 | MV: Initial MELD > 10 | SD: Initial MELD > 10 | p< |
| Quick | % | 91.1 | 5.93 | 54.5 | 16.07 | 0.00004 |
| HB | g/L | 14.73 | 0.69 | 12.66 | 1.14 | 0.00069 |
| CHE | U/L | 4502 | 1939 | 1849 | 567 | 0.00077 |
| PTT | Sec | 29.0 | 2.65 | 41.28 | 7.50 | 0.00106 |
| ERY | 106/µL | 4.97 | 0.31 | 4.14 | 0.54 | 0.00252 |
| 24 h-UCU/L | mg/L | 0.16 | 0.20 | 1.31 | 0.98 | 0.00740 |
| ALB (%) | % | 58.9 | 3.56 | 50.5 | 6.2 | 0.00763 |
| 24 h-UCU/d | mg/d | 0.26 | 0.36 | 2.41 | 1.89 | 0.00846 |
| INR | 1.04 | 0.05 | 1.55 | 0.44 | 0.00865 | |
| MELD≤ 10 | Final | MELD > 10 | Significance | |||
| Parameter | Units | MV: Final MELD≤ 10 | SD: Final MELD≤ 10 | MV: Final MELD > 10 | SD: Final MELD > 10 | p< |
| PTT | s | 25.67 | 2.00 | 29.86 | 4.59 | 0.02172 |
| CER | mg/dL | 7.22 | 3.31 | 12.40 | 5.64 | 0.02910 |
| TRANS | mg/dL | 254.6 | 31.8 | 285.7 | 24.5 | 0.03343 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hefter, H.; Kruschel, T.S.; Novak, M.; Rosenthal, D.; Luedde, T.; Meuth, S.G.; Albrecht, P.; Hartmann, C.J.; Samadzadeh, S. Differences in the Time Course of Recovery from Brain and Liver Dysfunction in Conventional Long-Term Treatment of Wilson Disease. J. Clin. Med. 2023, 12, 4861. https://doi.org/10.3390/jcm12144861
Hefter H, Kruschel TS, Novak M, Rosenthal D, Luedde T, Meuth SG, Albrecht P, Hartmann CJ, Samadzadeh S. Differences in the Time Course of Recovery from Brain and Liver Dysfunction in Conventional Long-Term Treatment of Wilson Disease. Journal of Clinical Medicine. 2023; 12(14):4861. https://doi.org/10.3390/jcm12144861
Chicago/Turabian StyleHefter, Harald, Theodor S. Kruschel, Max Novak, Dietmar Rosenthal, Tom Luedde, Sven G. Meuth, Philipp Albrecht, Christian J. Hartmann, and Sara Samadzadeh. 2023. "Differences in the Time Course of Recovery from Brain and Liver Dysfunction in Conventional Long-Term Treatment of Wilson Disease" Journal of Clinical Medicine 12, no. 14: 4861. https://doi.org/10.3390/jcm12144861
APA StyleHefter, H., Kruschel, T. S., Novak, M., Rosenthal, D., Luedde, T., Meuth, S. G., Albrecht, P., Hartmann, C. J., & Samadzadeh, S. (2023). Differences in the Time Course of Recovery from Brain and Liver Dysfunction in Conventional Long-Term Treatment of Wilson Disease. Journal of Clinical Medicine, 12(14), 4861. https://doi.org/10.3390/jcm12144861






