Prevalence of Geographic Atrophy in Advanced Age-Related Macular Degeneration (AMD) in Daily Practice
Abstract
1. Introduction
- early AMD: medium drusen > 63 μm and ≤125 μm without RPE abnormalities.
- Intermediate AMD: large drusen >125 μm and/or any AMD RPE abnormalities
- Advanced AMD: characterised by the development of either macular neovascularisation (MNV) or geographic atrophy (GA), or both
2. Methods
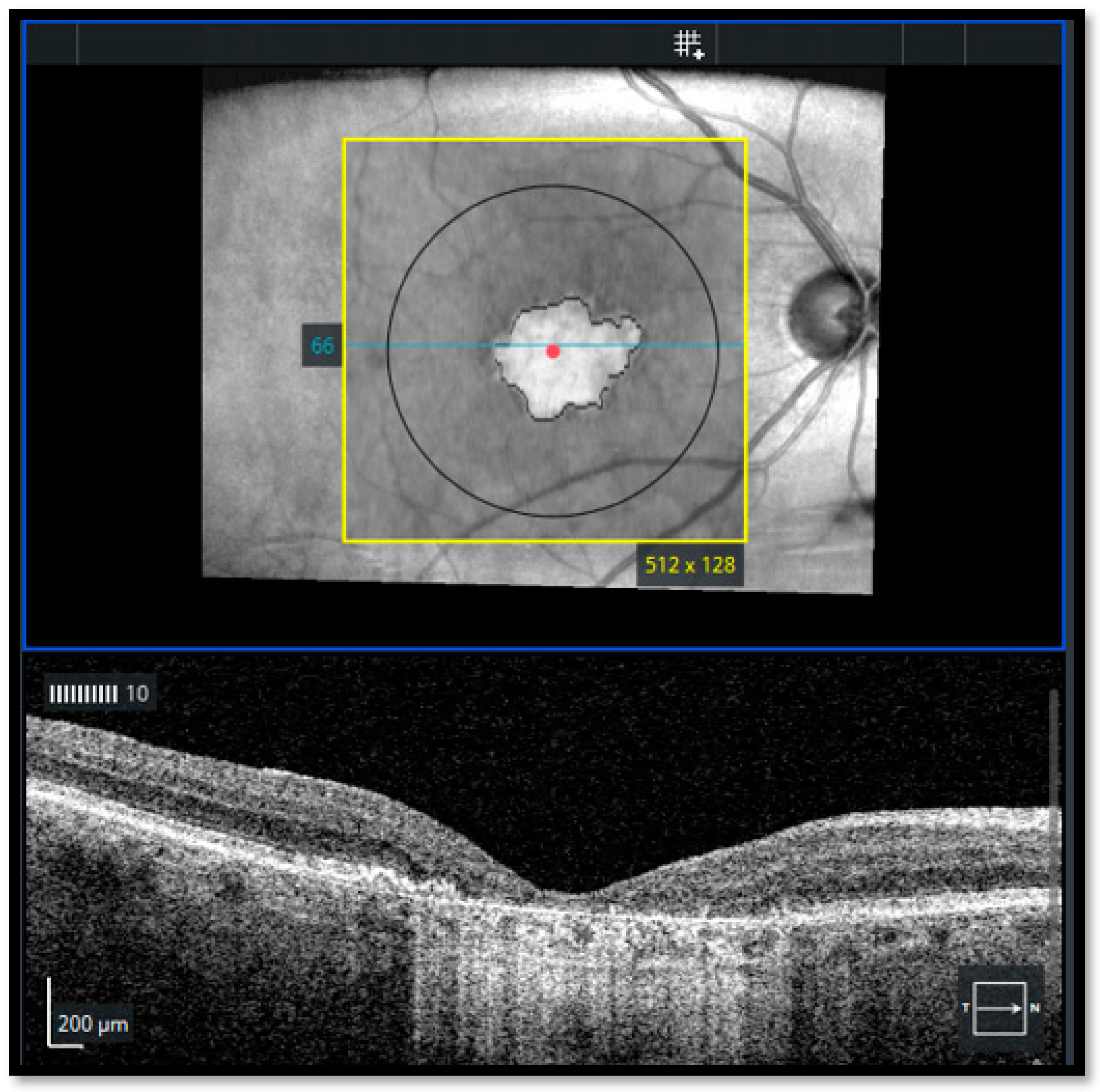
- In FAF, well-defined areas of decreased autofluorescence were manually outlined with the polygon selection tool and considered as atrophic areas and transferred for matching with en face OCT images
- In OCT-A, areas lacking choriocapillaris were classified as atrophic areas using the automatic segmentation of the AngioPlex software with minor manual adjustments
- Group 1: eyes with foveal GA (FGA)
- Group 2: eyes with non-foveal GA (NFGA) detected outside the central subfield of the Early Treatment Diabetic Retinopathy Study (EDTRS)
- Patient history and concomitant diseases
- Previous findings
- Examination of both eyes with multimodality imaging
- Observation of all OCT- B scans
- Evaluation of FAF and OCTA
- ≥55 years
- GA lesions due to AMD (no other dystrophies)
- BCVA ≥ 1.3 in logarithm of the minimum angle of resolution (logMAR)
- GA lesions with or without subfoveal involvement
- GA size: ≥2.5 and ≤17.5 mm2; if multifocal: at least one lesion with a size of ≥1.25 mm2
- No MNV in the study eye (active or history of)
- No high myopia (>26 mm/>−6 dpt)
- MNV in the fellow eye was not exclusionary
2.1. Ethical Approval
2.2. Statistical Analysis
3. Results
3.1. Main Characteristics for All Eyes with AMD
3.2. Prevalence of GA
4. Discussion
- The retrospective cross-sectional design
- Eyes with other ocular comorbidities were not excluded
- GA was not assessed in relation to the number of injections and the type of MNV or retinal fluids
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.Q.; Welchowski, T.; Schmid, M.; Mauschitz, M.M.; Holz, F.G.; Finger, R.P. Prevalence and incidence of age-related macular degeneration in Europe: A systematic review and meta-analysis. Br. J. Ophthalmol. 2020, 104, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Colijn, J.M.; Buitendijk, G.H.S.; Prokofyeva, E.; Alves, D.; Cachulo, M.L.; Khawaja, A.P.; Cougnard-Gregoire, A.; Merle, B.M.J.; Korb, C.; Erke, M.G.; et al. Prevalence of age-related macular degeneration in Europe: The past and the future. Ophthalmology 2017, 124, 1753–1763. [Google Scholar] [CrossRef] [PubMed]
- Finger, R.P.; Bertram, B.; Wolfram, C.; Holz, F.G. Blindness and visual impairment in Germany. Dtsch. Ärzteblatt Int. 2012, 109, 484–489. [Google Scholar] [CrossRef]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Health (NIH). National Eye Institute (NEI): Age-Related Macular Degeneration (AMD) Data and Statistics. Available online: https://www.nei.nih.gov/learn-about-eye-health/outreach-campaigns-andresources/eye-health-data-and-statistics/agerelated-macular-degeneration-amd-data-and-statistics/age-related-macular-degeneration-amdtables (accessed on 12 April 2023).
- Ferris, F.L., 3rd; Wilkinson, C.P.; Bird, A.; Chakravarthy, U.; Chew, E.; Csaky, K.; Sadda, S.R. Beckman Initiative for Macular Research Classification Committee. Clinical classification of age-related macular degeneration. Ophthalmology 2013, 120, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Schatz, H.; McDonald, H.R. Atrophic macular degeneration. Rate of spread of geographic atrophy and visual loss. Ophthalmology 1989, 96, 1541–1551. [Google Scholar] [CrossRef] [PubMed]
- Maguire, P.; Vine, A.K. Geographic atrophy of the retinal pigment epithelium. Am. J. Ophthalmol. 1986, 102, 621–625. [Google Scholar] [CrossRef]
- Fleckenstein, M.; Mitchell, P.; Freund, K.B.; Sadda, S.; Holz, F.G.; Brittain, C.; Henry, E.C.; Ferrara, D. The progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmology 2018, 125, 369–390. [Google Scholar] [CrossRef]
- Klein, R.; Klein, B.E.; Linton, K.L. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology 1992, 99, 933–943. [Google Scholar] [CrossRef]
- Klein, R.; Klein, B.E.; Knudtson, M.D.; Meuer, S.M.; Swift, M.; Gangnon, R.E. Fifteen-year cumulative incidence of age-related macular degeneration: The Beaver Dam Eye Study. Ophthalmology 2007, 114, 253–262. [Google Scholar] [CrossRef]
- Sunness, J.S.; Rubin, G.S.; Zuckerbrod, A.; Applegate, C.A. Foveal-sparing scotomas in advanced dry age-related macular degeneration. J. Vis. Impair. Blind. 2008, 102, 600–610. [Google Scholar] [CrossRef]
- Halpern, M.T.; Schmier, J.K.; Covert, D.; Venkataraman, K. Resource utilization and costs of age-related macular degeneration. Health Care Financ. Rev. 2006, 27, 37–47. [Google Scholar]
- Schmier, J.K.; Covert, D.W.; Lau, E.C. Patterns and costs associated with progression of age-related macular degeneration. Am. J. Ophthalmol. 2012, 154, 675–681.e1. [Google Scholar] [CrossRef]
- Cabral de Guimaraes, T.A.; Daich Varela, M.; Georgiou, M.; Michaelides, M. Treatments for dry age-related macular degeneration: Therapeutic avenues, clinical trials and future directions. Br. J. Ophthalmol. 2022, 106, 297–304. [Google Scholar] [CrossRef]
- Goldberg, R.; Heier, J.S.; Wykoff, C.C.; Staurenghi, G.; Singh, R.P.; Steinle, N.; David, S.B.; Mones, J.; Holz, F.G.; Bliss, C.; et al. Efficacy of intravitreal pegcetacoplan in patients with geographic atrophy (GA): 12-month results from the phase 3 OAKS and DERBY studies. Investig. Ophthalmol. Vis. Sci. 2022, 63, 1500. [Google Scholar]
- Rosenfeld, P.J.; Brown, D.M.; Heier, J.S.; Boyer, D.S.; Kaiser, P.K.; Chung, C.Y.; Kim, R.Y.; MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1419–1431. [Google Scholar] [CrossRef]
- Abdin, A.D.; Aljundi, W.; Jawhari, K.E.; Suffo, S.; Weinstein, I.; Seitz, B. First year real life experience with intravitreal brolucizumab for treatment of refractory neovascular age-related macular degeneration. Front. Pharmacol. 2022, 13, 860784. [Google Scholar] [CrossRef]
- Abdin, A.D.; Mohamed, A.; Munteanu, C.; Weinstein, I.; Langenbucher, A.; Seitz, B.; Suffo, S. Intravitreal aflibercept following treat and extend protocol versus fixed protocol for treatment of neovascular age-related macular degeneration. Int. J. Retin. Vitr. 2021, 7, 74. [Google Scholar] [CrossRef]
- Avery, R.L.; Pieramici, D.J.; Rabena, M.D.; Castellarin, A.A.; Nasir, M.A.; Giust, M.J. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology 2006, 113, 363–372.e5. [Google Scholar] [CrossRef]
- Abdin, A.D.; Suffo, S.; Asi, F.; Langenbucher, A.; Seitz, B. Intravitreal ranibizumab versus aflibercept following treat and extend protocol for neovascular age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 1671–1677. [Google Scholar] [CrossRef]
- Rosenfeld, P.J.; Shapiro, H.; Tuomi, L.; Webster, M.; Elledge, J.; Blodi, B.; MARINA and ANCHOR Study Groups. Characteristics of patients losing vision after 2 years of monthly dosing in the phase III ranibizumab clinical trials. Ophthalmology 2011, 118, 523–530. [Google Scholar] [CrossRef]
- Morris, B.; Imrie, F.; Armbrecht, A.M.; Dhillon, B. Age-related macular degeneration and recent developments: New hope for old eyes? Postgrad. Med. J. 2007, 83, 301–307. [Google Scholar] [CrossRef][Green Version]
- Corbelli, E.; Sacconi, R.; Rabiolo, A.; Mercuri, S.; Carnevali, A.; Querques, L.; Bandello, F.; Querques, G. Optical coherence tomography angiography in the evaluation of geographic atrophy area extension. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5201–5208. [Google Scholar] [CrossRef]
- Fogel-Levin, M.; Sadda, S.R.; Rosenfeld, P.J.; Waheed, N.; Querques, G.; Freund, B.K.; Sarraf, D. Advanced retinal imaging and applications for clinical practice: A consensus review. Surv. Ophthalmol. 2022, 67, 1373–1390. [Google Scholar] [CrossRef]
- Spaide, R.F.; Jaffe, G.J.; Sarraf, D.; Freund, K.B.; Sadda, S.R.; Staurenghi, G.; Waheed, N.K.; Chakravarthy, U.; Rosenfeld, P.J.; Holz, F.G.; et al. Consensus Nomenclature for Reporting Neovascular Age-Related Macular Degeneration Data: Consensus on Neovascular Age-Related Macular Degeneration Nomenclature Study Group. Ophthalmology 2020, 127, 616–636. [Google Scholar] [CrossRef]
- Fouad, Y.A.; Santina, A.; Bousquet, E.; Sadda, S.R.; Sarraf, D. Pathways of Fluid Leakage in Age-Related Macular Degeneration. Retina 2023, 43, 873–881. [Google Scholar] [CrossRef]
- Rasmussen, A.; Fuchs, J.; Hansen, L.H.; Larsen, M.; Sander, B.; Lund-Andersen, H. Neovascular age-related macular degeneration: Is it worthwhile treating an eye with poor visual acuity, if the visual acuity of the fellow eye is good? Eye 2017, 31, 978–980. [Google Scholar] [CrossRef]
- Nakata, I.; Yamashiro, K.; Nakanishi, H.; Akagi-Kurashige, Y.; Miyake, M.; Tsujikawa, A.; Matsuda, F.; Yoshimura, N.; Nagahama Cohort Research Group. Prevalence and characteristics of age-related macular degeneration in the Japanese population: The Nagahama study. Am. J. Ophthalmol. 2013, 156, 1002–1009.e2. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Rochtchina, E.; Lee, A.J.; Chia, E.M.; Smith, W.; Cumming, R.G.; Mitchell, P. Ten-year incidence and progression of age-related maculopathy: The blue Mountains Eye Study. Ophthalmology 2007, 114, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Sadda, S.R.; Guymer, R.; Holz, F.G.; Schmitz-Valckenberg, S.; Curcio, C.A.; Bird, A.C.; Blodi, B.A.; Bottoni, F.; Chakravarthy, U.; Chew, E.Y.; et al. Consensus definition for atrophy associated with age-related macular degeneration on OCT: Classification of atrophy report 3. Ophthalmology 2018, 125, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Waheed, N.K.; Moult, E.M.; Fujimoto, J.G.; Rosenfeld, P.J. Optical Coherence Tomography Angiography of Dry Age-Related Macular Degeneration. Dev. Ophthalmol. 2016, 56, 91–100. [Google Scholar] [CrossRef]
- Thulliez, M.; Zhang, Q.; Shi, Y.; Zhou, H.; Chu, Z.; de Sisternes, L.; Durbin, M.K.; Feuer, W.; Gregori, G.; Wang, R.K.; et al. Correlations between Choriocapillaris Flow Deficits around Geographic Atrophy and Enlargement Rates Based on Swept-Source OCT Imaging. Ophthalmol. Retin. 2019, 3, 478–488. [Google Scholar] [CrossRef]
- Sacconi, R.; Corbelli, E.; Carnevali, A.; Querques, L.; Bandello, F.; Querques, G. Optical coherence tomography angiography in geographic atrophy. Retina 2018, 38, 2350–2355. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.S.; Huang, D. Artifacts in Optical Coherence Tomography Angiography. In Optical Coherence Tomography Angiography of the Eye; Huang, D., Lumbroso, B., Jia, Y., Waheed, N., Eds.; SLACK Publishing: Thorofare, NJ, USA, 2017; pp. 33–40. [Google Scholar]
- Camino, A.; Guo, Y.; You, Q.; Wang, J.; Huang, D.; Bailey, S.T.; Jia, Y. Detecting and measuring areas of choriocapillaris low perfusion in intermediate, non-neovascular age-related macular degeneration. Neurophotonics 2019, 6, 041108. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Erfurth, U.; Waldstein, S.M. A paradigm shift in imaging biomarkers in neovascular age-related macular degeneration. Prog. Retin. Eye Res. 2016, 50, 1–24. [Google Scholar] [CrossRef]
- Hilely, A.; Au, A.; Freund, K.B.; Loewenstein, A.; Souied, E.H.; Zur, D.; Sacconi, R.; Borrelli, E.; Peiretti, E.; Iovino, C.; et al. Non-neovascular age-related macular degeneration with subretinal fluid. Br. J. Ophthalmol. 2021, 105, 1415–1420. [Google Scholar] [CrossRef]
- Samanta, A.; Jhingan, M.; Arora, S.; Singh, S.; Tucci, D.; Cagini, C.; Lupidi, M.; Chhablani, J. Intraretinal, sub-retinal, and sub-retinal pigmented epithelium fluid in non-exudative age-related macular degeneration: Follow-up with OCT imaging. Eur. J. Ophthalmol. 2022, 32, 2419–2426. [Google Scholar] [CrossRef] [PubMed]
- Bacci, T.; Essilfie, J.O.; Leong, B.C.S.; Freund, K.B. Exudative non-neovascular age-related macular degeneration. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 1123–1134. [Google Scholar] [CrossRef]
- Framme, C.; Junker, B.; Feltgen, N.; Hoerauf, H.; Striebe, N.-A.; Wachtlin, J.; Volkmann, I. Avoiding mistakes in anti-VEGF intravitreal injection therapy. Ophthalmologe 2022, 119, 309–326. [Google Scholar] [CrossRef]
- Hoy, S.M. Pegcetacoplan: First Approval. Drugs 2021, 81, 1423–1430. [Google Scholar] [CrossRef]
- Liao, D.S.; Grossi, F.V.; El Mehdi, D.; Gerber, M.R.; Brown, D.M.; Heier, J.S.; Wykoff, C.C.; Singerman, L.J.; Abraham, P.; Grassmann, F.; et al. Complement C3 inhibitor pegcetacoplan for geographic atrophy secondary to age-related macular degeneration: A randomized phase 2 trial. Ophthalmology 2020, 127, 186–195. [Google Scholar] [CrossRef]
- Annamalai, B.; Parsons, N.; Nicholson, C.; Obert, E.; Jones, B.; Rohrer, B. Subretinal rather than intravitreal adeno-associated virus-mediated delivery of a complement alternative pathway inhibitor is effective in a mouse model of RPE damage. Investig. Ophthalmol. Vis. Sci. 2021, 62, 11. [Google Scholar] [CrossRef]
- Csaky, K.; Curcio, C.A.; Mullins, R.F.; Rosenfeld, P.J.; Fujimoto, J.; Rohrer, B.; Ribero, R.; Malek, G.; Waheed, N.; Guymer, R.; et al. New approaches to the treatment of Age-Related Macular Degeneration (AMD). Exp. Eye Res. 2022, 221, 109–134. [Google Scholar] [CrossRef]
- Sayegh, R.G.; Sacu, S.; Dunavölgyi, R.; Kroh, M.E.; Roberts, P.; Mitsch, C.; Montuoro, A.; Ehrenmüller, M.; Schmidt-Erfurth, U. Geographic atrophy and foveal-sparing changes related to visual acuity in patients with dry age-related macular degeneration over time. Am. J. Ophthalmol. 2017, 179, 118–128. [Google Scholar] [CrossRef]
- Saßmannshausen, M.; Behning, C.; Weinz, J.; Goerdt, L.; Terheyden, J.H.; Chang, P.; Schmid, M.; Poor, S.H.; Zakaria, N.; Finger, R.P.; et al. MACUSTAR consortium members. Characteristics and spatial distribution of structural features in age-related macular degeneration: A MACUSTAR study report. Ophthalmol. Retin. 2023, 7, 420–430. [Google Scholar] [CrossRef]
- Sarks, S.H. Ageing and degeneration in the macular region: A clinico-pathological study. Br. J. Ophthalmol. 1976, 60, 324–341. [Google Scholar] [CrossRef]
- Green, W.R.; Key, S.N., 3rd. Senile macular degeneration: A histopathologic study. Trans. Am. Ophthalmol. Soc. 1977, 75, 180–254. [Google Scholar]
- Corvi, F.; Cozzi, M.; Invernizzi, A.; Pace, L.; Sadda, S.R.; Staurenghi, G. Optical coherence tomography angiography for detection of macular neovascularization associated with atrophy in age-related macular degeneration. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 291–299. [Google Scholar] [CrossRef]
- Sadda, S.R.; Tuomi, L.L.; Ding, B.; Fung, A.E.; Hopkins, J.J. Macular atrophy in the HARBOR study for neovascular age-related macular degeneration. Ophthalmology 2018, 125, 878–886. [Google Scholar] [CrossRef]
- Gune, S.; Abdelfattah, N.S.; Karamat, A.; Balasubramanian, S.; Marion, K.M.; Morgenthien, E.; Sadda, S.R. Spectral-domain OCT-based prevalence and progression of macular atrophy in the HARBOR study for neovascular age-related macular degeneration. Ophthalmology 2020, 127, 523–532. [Google Scholar] [CrossRef]
- Airaldi, M.; Corvi, F.; Cozzi, M.; Nittala, M.G.; Staurenghi, G.; Sadda, S.R. Differences in long-term progression of atrophy between neovascular and nonneovascular age-related macular degeneration. Ophthalmol. Retin. 2022, 6, 914–921. [Google Scholar] [CrossRef]
- Bhutto, I.; Lutty, G. Understanding age-related macular degeneration (AMD): Relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol. Asp. Med. 2012, 33, 295–317. [Google Scholar] [CrossRef]
- McLeod, D.S.; Grebe, R.; Bhutto, I.; Merges, C.; Baba, T.; Lutty, G.A. Relationship between RPE and choriocapillaris in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4982–4991. [Google Scholar] [CrossRef]
- Grob, S.; Luo, J.; Hughes, G.; Lee, C.; Zhou, X.; Lee, J.; Du, H.; Ferreyra, H.; Freeman, W.R.; Kozak, I.; et al. Genetic analysis of simultaneous geographic atrophy and choroidal neovascularization. Eye 2012, 26, 1106–1113. [Google Scholar] [CrossRef]
- Gemenetzi, M.; Lotery, A.J.; Patel, P.J. Risk of geographic atrophy in age-related macular degeneration patients treated with intravitreal anti-VEGF agents. Eye 2017, 31, 1–9. [Google Scholar] [CrossRef]
- Grunwald, J.E.; Daniel, E.; Huang, J.; Ying, G.-S.; Maguire, M.G.; Toth, C.A.; Jaffe, G.J.; Fine, S.L.; Blodi, B.; Klein, M.L.; et al. Risk of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology 2014, 121, 150–161. [Google Scholar] [CrossRef]
- Sadda, S.R.; Abdelfattah, N.S.; Lei, J.; Shi, Y.; Marion, K.M.; Morgenthien, E.; Gune, S.; Balasubramanian, S. Spectral-Domain OCT analysis of risk factors for macular atrophy development in the HARBOR Study for neovascular age-related macular degeneration. Ophthalmology 2020, 127, 1360–1370. [Google Scholar] [CrossRef]


| All AMD Eyes (N = 1913) | Early (N = 296) | Intermediate (N = 368) | Advanced (N = 1249) | |
|---|---|---|---|---|
| Age (years) | 76.4 ± 9.5 | 73.1 ± 10.1 | 76.3 ± 8.1 | 77.6 ± 9.2 |
| Female:Male | 62.9%:37.1% | 58.8%:41.2% | 61.9%:38.1% | 62.8%:37.2% |
| Visual acuity (logMAR) | 0.51 ± 0.7 | 0.15 ± 0.3 | 0.18 ± 0.2 | 0.71 ± 0.8 |
| Spherical equivalent (dpt) | −0.07 ± 2.0 | −0.41 ± 2.9 | 0.20 ± 1.6 | −0.06 ± 1.7 |
| Cataract:Pseudophakic | 28.4%:68.4% | 40.2%:54.1% | 32.6%:64.6% | 24.4%:73.4% |
| Axial length (mm) | 23.6 ± 1.8 | 24.1 ± 2.1 | 23.5 ± 1.1 | 23.5 ± 1.2 |
| Foveal (N = 932) | Non Foveal (N = 272) | p Value | |
|---|---|---|---|
| Age (years) | 78.0 ± 9.3 | 77.8 ± 8.7 | 0.80 |
| Female: Male | 64.3%:35.7% | 63.6%:36.4% | 0.75 |
| Visual acuity (logMAR) | 0.80 ± 0.20 | 0.40 ± 0.16 | 0.01 |
| Spherical equivalent (dpt) | 0.20 ± 1.7 | 0.32 ± 1.7 | 0.80 |
| Atrophy area (mm2) | 4.2 ± 4.5 | 2.2 ± 2.6 | <0.001 |
| Dry:Neovascular | 334:598 (35.8%:64.2%) | 120:152 (44.2%:55.8%) | 0.01 |
| Cataract:Pseudophakic | 214:698 (23%:75%) | 76:189 (27.9%:69.4%) | 0.08 |
| Axial length (mm) | 23.56 ± 1.4 | 23.59 ± 1.1 | 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdin, A.D.; Devenijn, M.; Fulga, R.; Langenbucher, A.; Seitz, B.; Kaymak, H. Prevalence of Geographic Atrophy in Advanced Age-Related Macular Degeneration (AMD) in Daily Practice. J. Clin. Med. 2023, 12, 4862. https://doi.org/10.3390/jcm12144862
Abdin AD, Devenijn M, Fulga R, Langenbucher A, Seitz B, Kaymak H. Prevalence of Geographic Atrophy in Advanced Age-Related Macular Degeneration (AMD) in Daily Practice. Journal of Clinical Medicine. 2023; 12(14):4862. https://doi.org/10.3390/jcm12144862
Chicago/Turabian StyleAbdin, Alaa Din, Machteld Devenijn, Roxana Fulga, Achim Langenbucher, Berthold Seitz, and Hakan Kaymak. 2023. "Prevalence of Geographic Atrophy in Advanced Age-Related Macular Degeneration (AMD) in Daily Practice" Journal of Clinical Medicine 12, no. 14: 4862. https://doi.org/10.3390/jcm12144862
APA StyleAbdin, A. D., Devenijn, M., Fulga, R., Langenbucher, A., Seitz, B., & Kaymak, H. (2023). Prevalence of Geographic Atrophy in Advanced Age-Related Macular Degeneration (AMD) in Daily Practice. Journal of Clinical Medicine, 12(14), 4862. https://doi.org/10.3390/jcm12144862









