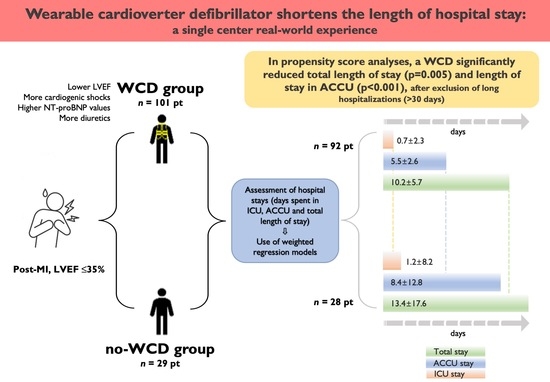
Wearable Cardioverter Defibrillator Shortens the Lengths of Stay in Patients with Left Ventricular Dysfunction after Myocardial Infarction: A Single-Centre Real-World Experience
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Hospitalization Times
3.2. Follow-Up Period
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACCU | Acute cardiac care unit |
| ACS | Acute coronary syndrome |
| AMI | Acute myocardial infarction |
| CRT-D | Cardiac resynchronization therapy defibrillator |
| ECG | Electrocardiogram |
| ECMO | Extra-corporeal membrane oxygenation |
| HTX | Heart transplantation |
| ICD | Implantable cardioverter-defibrillator |
| ICU | Intensive care unit |
| IQR | Interquartile ranges |
| LVEF | Left ventricular ejection fraction |
| OMT | Optimal medical therapy |
| SCD | Sudden cardiac death |
| SD | Standard deviation |
| WCD | Wearable cardioverter defibrillator |
References
- Solomon, S.D.; Zelenkofske, S.; McMurray, J.J.; Finn, P.V.; Velazquez, E.; Ertl, G.; Harsanyi, A.; Rouleau, J.L.; Maggioni, A.; Kober, L.; et al. Sudden Death in Patients with Myocardial Infarction and Left Ventricular Dysfunction, Heart Failure, or Both. N. Engl. J. Med. 2005, 352, 2581–2588. [Google Scholar] [CrossRef] [PubMed]
- Buxton, A.E.; Lee, K.L.; Fisher, J.D.; Josephson, M.E.; Prystowsky, E.N.; Hafley, G. A Randomized Study of the Prevention of Sudden Death in Patients with Coronary Artery Disease. N. Engl. J. Med. 1999, 341, 1882–1890. [Google Scholar] [CrossRef] [PubMed]
- Elayi, C.S.; Charnigo, R.J.; Heron, P.M.; Lee, B.K.; Olgin, J.E. Primary Prevention of Sudden Cardiac Death Early Post-Myocardial Infarction. Circ. Arrhythmia Electrophysiol. 2017, 10, 194. [Google Scholar] [CrossRef] [PubMed]
- Moss, A.J.; Zareba, W.; Hall, W.J.; Klein, H.; Wilber, D.J.; Cannom, D.S.; Daubert, J.P.; Higgins, S.L.; Brown, M.W.; Andrews, M.L. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N. Engl. J. Med. 2002, 346, 877–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinbeck, G.; Andresen, D.; Seidl, K.; Brachmann, J.; Hoffmann, E.; Wojciechowski, D.; Kornacewicz-Jach, Z.; Sredniawa, B.; Lupkovics, G.; Hofgärtner, F.; et al. Defibrillator Implantation Early after Myocardial Infarction. N. Engl. J. Med. 2009, 361, 1427–1436. [Google Scholar] [CrossRef] [Green Version]
- Hohnloser, S.H.; Kuck, K.H.; Dorian, P.; Roberts, R.S.; Hampton, J.R.; Hatala, R.; Fain, E.; Gent, M.; Connolly, S.J. Prophylactic Use of an Implantable Cardioverter–Defibrillator after Acute Myocardial Infarction. N. Engl. J. Med. 2004, 351, 2481–2488. [Google Scholar] [CrossRef] [Green Version]
- Solomon, S.D.; Glynn, R.J.; Greaves, S.; Ajani, U.; Rouleau, J.-L.; Menapace, F.; Arnold, J.M.O.; Hennekens, C.; Pfeffer, M.A. Recovery of ventricular function after myocardial infarction in the reperfusion era: The healing and early afterload reducing therapy study. Ann. Intern. Med. 2001, 134, 451–458. [Google Scholar] [CrossRef]
- Chieng, D.; Paul, V.; Denman, R. Current Device Therapies for Sudden Cardiac Death Prevention—The ICD, Subcutaneous ICD and Wearable ICD. Heart Lung Circ. 2018, 28, 65–75. [Google Scholar] [CrossRef]
- Piccini, J.P.; Allen, L.A.; Kudenchuk, P.J.; Page, R.L.; Patel, M.R.; Turakhia, M.P. Wearable Cardioverter-Defibrillator Therapy for the Prevention of Sudden Cardiac Death. Circulation 2016, 133, 1715–1727. [Google Scholar] [CrossRef] [Green Version]
- Feldman, A.M.; Klein, H.; Tchou, P.; Murali, S.; Hall, W.J.; Mancini, D.; Boehmer, J.; Harvey, M.; Heilman, M.S.; Szymkiewicz, S.J.; et al. Use of a Wearable Defibrillator in Terminating Tachyarrhythmias in Patients at High Risk for Sudden Death: Results of WEARIT/BIROAD. Pacing Clin. Electrophysiol. 2004, 27, 4–9. [Google Scholar] [CrossRef]
- Olgin, J.E.; Pletcher, M.J.; Vittinghoff, E.; Wranicz, J.; Malik, R.; Morin, D.P.; Zweibel, S.; Buxton, A.E.; Elayi, C.S.; Chung, E.H.; et al. Wearable Cardioverter–Defibrillator after Myocardial Infarction. N. Engl. J. Med. 2018, 379, 1205–1215. [Google Scholar] [CrossRef]
- Olgin, J.E.; Lee, B.K.; Vittinghoff, E.; Morin, D.P.; Zweibel, S.; Rashba, E.; Chung, E.H.; Borggrefe, M.; Hulley, S.; Lin, F.; et al. Impact of wearable cardioverter-defibrillator compliance on outcomes in the VEST trial: As-treated and per-protocol analyses. J. Cardiovasc. Electrophysiol. 2020, 31, 1009–1018. [Google Scholar] [CrossRef]
- Healy, C.A.; Carrillo, R.G. Wearable cardioverter-defibrillator for prevention of sudden cardiac death after infected implantable cardioverter-defibrillator removal: A cost-effectiveness evaluation. Heart Rhythm. 2015, 12, 1565–1573. [Google Scholar] [CrossRef]
- Sanders, G.D.; Owens, D.K.; Hlatky, M.A. Potential Cost-effectiveness of Wearable Cardioverter-Defibrillator Early Post Myocardial Infarction. J. Innov. Card. Rhythm. Manag. 2015, 6, 1929–1940. [Google Scholar] [CrossRef]
- Botto, G.L.; Mantovani, L.G.; Cortesi, P.A.; De Ponti, R.; D’Onofrio, A.; Biffi, M.; Capucci, A.; Casu, G.; Notarstefano, P.; Scaglione, M.; et al. The value of wearable cardioverter defibrillator in adult patients with recent myocardial infarction: Economic and clinical implications from a health technology assessment perspective. Int. J. Cardiol. 2022, 356, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.A.; Szymkiewicz, S.; Volosin, K.; Kabulski, G.M.; Northup, A.; Wiggins, B.S. Mortality and Costs Associated with Wearable Cardioverter-defibrillators after Acute Myocardial Infarction: A Retrospective Cohort Analysis of Medicare Claims Data. J. Innov. Card. Rhythm Manag. 2019, 10, 3866–3873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boriani, G.; Mantovani, L.G.; Cortesi, P.A.; De Ponti, R.; D’Onofrio, A.; Arena, G.; Curnis, A.; Forleo, G.; Guerra, F.; Porcu, M.; et al. Cost-minimization analysis of a wearable cardioverter defibrillator in adult patients undergoing ICD explant procedures: Clinical and economic implications. Clin. Cardiol. 2021, 44, 1497–1505. [Google Scholar] [CrossRef] [PubMed]
- Gage, A.; Higgins, A.; Lee, R. Cardiac Critical Care: The Evolution of a Novel Subspecialty. Methodist DeBakey Cardiovasc. J. 2022, 18, 24–29. [Google Scholar] [CrossRef]
- Chen, R.; Strait, K.M.; Dharmarajan, K.; Li, S.-X.; Ranasinghe, I.; Martin, J.; Fazel, R.; Masoudi, F.A.; Cooke, C.R.; Nallamothu, B.K.; et al. Hospital variation in admission to intensive care units for patients with acute myocardial infarction. Am. Heart J. 2015, 170, 1161–1169. [Google Scholar] [CrossRef] [Green Version]
- Reek, S.; Burri, H.; Roberts, P.R.; Perings, C.; Epstein, A.E.; Klein, H.U.; Lip, G.; Gorenek, B.; Sticherling, C.; Fauchier, L.; et al. The wearable cardioverter-defibrillator: Current technology and evolving indications. Europace 2016, 19, 335–345. [Google Scholar] [CrossRef] [Green Version]
- Klein, H.U.; Goldenberg, I.; Moss, A.J. Risk stratification for implantable cardioverter defibrillator therapy: The role of the wearable cardioverter-defibrillator. Eur. Heart J. 2013, 34, 2230–2242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, E.; Weeda, E.; Kohn, C.; D’Souza, B.; Russo, A.; Noreika, S.; Coleman, C. Wearable Cardioverter-defibrillators for the Prevention of Sudden Cardiac Death: A Meta-analysis. J. Innov. Card. Rhythm. Manag. 2018, 9, 3151–3162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wäßnig, N.K.; Günther, M.; Quick, S.; Pfluecke, C.; Rottstädt, F.; Szymkiewicz, S.J.; Ringquist, S.; Strasser, R.H.; Speiser, U. Experience with the Wearable Cardioverter-Defibrillator in Patients at High Risk for Sudden Cardiac Death. Circulation 2016, 134, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, A.N.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef]
- Bodin, A.; Bisson, A.; Fauchier, L. When is a wearable defibrillator indicated? Expert Rev. Med Devices 2021, 18, 51–56. [Google Scholar] [CrossRef]
- Cho, K.H.; Han, X.; Ahn, J.H.; Hyun, D.Y.; Kim, M.C.; Sim, D.S.; Hong, Y.J.; Kim, J.H.; Ahn, Y.; Hwang, J.Y.; et al. Long-Term Outcomes of Patients with Late Presentation of ST-Segment Elevation Myocardial Infarction. J. Am. Coll. Cardiol. 2021, 77, 1859–1870. [Google Scholar] [CrossRef]
- Sud, M.; Yu, B.; Wijeysundera, H.C.; Austin, P.C.; Ko, D.T.; Braga, J.; Cram, P.; Spertus, J.A.; Domanski, M.; Lee, D.S. Associations Between Short or Long Length of Stay and 30-Day Readmission and Mortality in Hospitalized Patients With Heart Failure. JACC Heart Fail. 2017, 5, 578–588. [Google Scholar] [CrossRef]
- Hoogervorst-Schilp, J.; Langelaan, M.; Spreeuwenberg, P.; De Bruijne, M.C.; Wagner, C. Excess length of stay and economic consequences of adverse events in Dutch hospital patients. BMC Health Serv. Res. 2015, 15, 531. [Google Scholar] [CrossRef] [Green Version]
- Toušek, P.; Bauer, D.; Neuberg, M.; Nováčková, M.; Mašek, P.; Tu˚ma, P.; Kočka, V.; Moťovská, Z.; Widimský, P. Patient characteristics, treatment strategy, outcomes, and hospital costs of acute coronary syndrome: 3 years of data from a large high-volume centre in Central Europe. Eur. Heart J. Suppl. 2022, 24, B3–B9. [Google Scholar] [CrossRef]
- Chung, M.K.; Szymkiewicz, S.J.; Shao, M.; Zishiri, E.; Niebauer, M.J.; Lindsay, B.D.; Tchou, P.J. Aggregate National Experience with the Wearable Cardioverter-Defibrillator: Event Rates, Compliance, and Survival. J. Am. Coll. Cardiol. 2010, 56, 194–203. [Google Scholar] [CrossRef] [Green Version]

| Total n = 130 | Life Vest n = 101 | Control n = 29 | p Value | |
|---|---|---|---|---|
| Age (years)—mean (±SD) | 60.6 ± 10.9 | 60.3 ± 10.4 | 61.7 ± 12.8 | 0.532 |
| Male sex—n. (%) | 106 (81.5) | 81 (80.2) | 25 (86.2) | 0.462 |
| Past Medical history—no. (%) | ||||
| Hypertension | 53 (40.8) | 40 (39.6) | 13 (44.8) | 0.614 |
| Dyslipidemia | 35 (26.9) | 26 (25.7) | 9 (31.0) | 0.571 |
| Type 2 diabetes | 23 (17.7) | 16 (15.8) | 7 (24.1) | 0.302 |
| Smoking history | 87 (66.9) | 65 (64.4) | 22 (75.9) | 0.246 |
| Stroke/TIA | 9 (6.9) | 9 (8.9) | 0 (0.0) | 0.096 |
| COPD | 9 (6.9) | 8 (7.9) | 1 (3.4) | 0.403 |
| Chronic renal failure | 7 (5.4) | 7 (6.9) | 0 (0.0) | 0.145 |
| Previous CAD | 18 (13.8) | 13 (12.9) | 5 (17.2) | 0.548 |
| Previous PCI | 11 (8.5) | 7 (6.9) | 4 (13.8) | 0.241 |
| Previous CABG | 2 (1.5) | 2 (2.0) | 0 (0.0) | 0.445 |
| Peripheral vascular disease | 4 (3.1) | 3 (3.0) | 1 (3.4) | 0.896 |
| Atrial fibrillation | 9 (6.9) | 7 (6.9) | 2 (6.9) | 0.995 |
| Index event—n. (%) | ||||
| ACS type | ||||
| STE-ACS early (≤12 h) | 55 (42.3) | 41 (40.6) | 14 (48.3) | 0.239 |
| STE-ACS late (>12 h) | 52 (40.0) | 40 (39.6) | 12 (41.4) | 0.863 |
| NSTE-ACS | 23 (17.7) | 19 (19.8) | 3 (10.3) | 0.402 |
| Multivessel disease | 90 (69.2) | 72 (71.3) | 18 (62.1) | 0.343 |
| PCI | 122 (93.8) | 94 (93.1) | 28 (96.6) | 0.492 |
| Multivessel PCI | 47 (36.2) | 37 (36.3) | 10 (34.5) | 0.832 |
| In-hospital echocardiographic evaluation | ||||
| LVEF %—mean (±SD) | 29.0 ± 4.7 | 28.1 ± 4.7 | 32.1 ± 3.6 | <0.001 |
| LVEDD (mm)—mean (±SD) | 55.8 ± 7.4 | 56.1 ± 7.4 | 54.8 ± 7.7 | 0.560 |
| LVEDV (ml)—mean (±SD) | 165.3 ± 57.4 | 172.5 ± 60.6 | 138.3 ± 32.7 | 0.013 |
| Right ventricular dysfunction—n. (%) | 10 (7.9) | 8 (8.2) | 2 (7.1) | 0.860 |
| Severe valvulopathy—n (%) | 7 (5.4) | 7 (7.0) | 0 (0.0) | 0.143 |
| Aneurysm—n. (%) | 12 (9.4) | 10 (10.2) | 2 (6.9) | 0.593 |
| Left ventricular thrombosis—n. (%) | 25 (19.2) | 23 (22.8) | 2 (6.9) | 0.056 |
| In-hospital electrocardiographic evaluation at presentation | ||||
| Sinus rhythm—n. (%) | 122 (93.8) | 95 (94.1) | 27 (93.1) | 0.850 |
| Duration of the QRS interval (msec)—mean (±SD) | 100.5 ± 20.0 | 102.4 ± 20.4 | 94.0 ± 17.5 | 0.055 |
| QTc duration (msec)—mean (±SD) | 429.2 ± 36.0 | 432.2 ± 33.9 | 418.3 ± 41.7 | 0.076 |
| Bundle branch block—n. (%) | 22 (17.5) | 19 (19.2) | 3 (11.1) | 0.327 |
| In-hospital laboratory evaluation at presentation | ||||
| Anemia (Hb < 11 g/dL)—n. (%) | 7 (5.4) | 7 (6.9) | 0 (0.0) | 0.348 |
| White blood cells count (109/L), mean (±SD) | 13.6 ± 4.9 | 13.4 ± 4.8 | 14.3 ± 5.3 | 0.338 |
| Platelet (109/L)—mean (±SD) | 260.9 ± 96.4 | 258.7 ± 89.7 | 268.8 ± 118.2 | 0.673 |
| Creatinine (µmol/L)—mean (±SD) | 91.0 ± 35.0 | 92.0 ± 37.5 | 87.9 ± 23.9 | 0.485 |
| Laboratory evaluation during hospitalization | ||||
| Creatinine peak (µmol/L)—mean (±SD) | 117.7 ± 55.1 | 121.5 ± 60.5 | 104.1 ± 24.4 | 0.025 |
| TnT hs peak (ng/L)—mean (±SD) | 8516.2 ± 7503.6 | 8517.5 ± 7881.3 | 8511.6 ± 6128.8 | 0.997 |
| NT-proBNP peak (ng/L)—median (IQR) | 3920.0 (1808.5–7073.5) | 3999.5 (1968.0–7285.3) | 1537.0 (3224.0–5149.5) | 0.169 |
| Total n = 130 | Life Vest n = 101 | Control n = 29 | p Value | |
|---|---|---|---|---|
| CABG | 3 (2.3) | 3 (3.0) | 0 (0.0) | 0.348 |
| Impella | 8 (6.2) | 7 (6.9) | 1 (3.4) | 0.492 |
| ECMO | 11 (8.5) | 9 (8.9) | 2 (6.9) | 0.731 |
| Heart transplant | 3 (2.3) | 2 (2.0) | 1 (3.4) | 0.643 |
| Dialysis | 2 (1.5) | 2 (2.0) | 0 (0.0) | 0.445 |
| Non-invasive ventilation (C-PAP or BiPAP) | 7 (5.4) | 7 (6.9) | 0 (0.0) | 0.145 |
| Mechanical ventilation | 15 (11.5) | 13 (12.9) | 2 (6.9) | 0.374 |
| Temporary pacemaker | 3 (2.3) | 3 (3.0) | 0 (0.0) | 0.347 |
| RBC transfusion | 11 (8.5) | 10 (9.9) | 1 (3.4) | 0.271 |
| Inotropic drugs | 19 (14.6) | 17 (16.8) | 2 (6.9) | 0.182 |
| Vasopressor drugs | 21 (16.2) | 18 (17.8) | 3 (10.3) | 0.335 |
| Complications during hospitalization—n. (%) | ||||
| Stroke | 3 (2.3) | 3 (3.0) | 0 (0.0) | 0.348 |
| Cardiogenic shock | 26 (20.0) | 24 (23.8) | 2 (6.9) | 0.045 |
| Pulmonary embolism | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| Sepsis | 12 (9.2) | 10 (9.9) | 2 (6.9) | 0.622 |
| Acute kidney failure a | 62 (47.7) | 52 (51.5) | 10 (34.5) | 0.106 |
| Bradyarrhythmias | 2 (1.5) | 2 (2.0) | 0 (0.0) | 0.445 |
| Ventricular tachyarrhythmias b | 68 (52.3) | 55 (54.5) | 13 (44.8) | 0.360 |
| Therapy at discharge—n. (%) | ||||
| Angiotensin-converting enzyme inhibitors (ACEi) or angiotensin II receptor blockers (ARB) | 114 (87.7) | 89 (88.1) | 25 (86.2) | 0.659 |
| Angiotensin Receptor-Neprilysin Inhibitor | 2 (1.6) | 2 (2.0) | 0 (0.0) | 0.453 |
| Beta-blockers | 125 (96.9) | 99 (98.0) | 26 (92.9) | 0.163 |
| Mineralocorticoid Receptor Antagonists | 106 (82.2) | 87 (86.1) | 19 (67.9) | 0.025 |
| Sodium-glucose cotransporter 2 inhibitors (SGLT2i) | 26 (20.2) | 24 (23.8) | 2 (7.1) | 0.052 |
| Ivabradine | 9 (7.0) | 7 (6.9) | 2 (7.1) | 0.969 |
| Diuretics | 65 (50.4) | 56 (55.4) | 9 (32.1) | 0.029 |
| Statin | 118 (91.5) | 92 (91.1) | 26 (92.9) | 0.767 |
| Acetylsalicylic acid | 120 (93.0) | 93 (92.1) | 27 (96.4) | 0.424 |
| P2Y12 receptor blockers | 119 (92.2) | 94 (93.1) | 25 (89.3) | 0.508 |
| Novel Oral Anticoagulants/Warfarin | 40 (31.0) | 33 (32.7) | 7 (25.0) | 0.437 |
| Amiodarone | 13 (10.1) | 11 (10.9) | 2 (7.1) | 0.559 |
| (a) | |||||
| Base Model | Total n = 130 mean (±SD) | Life Vest n = 101 mean (±SD) | Control n = 29 mean (±SD) | Estimate Effect [95%-CI] | p Value |
| Total hospital length (days) | 15.7 ± 21.4 | 13.6 ± 14.6 | 17.2 ± 36.3 | −3.5 [−8.4; 1.2] | 0.143 |
| Days in ICU (days) | 2.4 ± 8.8 | 1.7 ± 5.6 | 2.9 ± 15.4 | −1.1 [−3.1; 0.8] | 0.251 |
| Days in ACCU (days) | 7.6 ± 7.5 | 5.8 ± 3.9 | 8.9 ± 13.5 | −3.1 [−4.7; −1.4] | <0.001 |
| Weighted number of days adjusted for extra-corporeal membrane oxygenation (ECMO) use and heart transplantation (HTX). SD, standard deviation; ICU, intensive care unit; ACCU, acute cardiac care unit. | |||||
| (b) | |||||
| Model without ECMO, HTX | Total n = 119 mean (±SD) | Life Vest n = 92 mean (±SD) | Control n = 27 mean (±SD) | Estimate Effect [95%-CI] | p Value |
| Total hospital length (days) | 11.8 ± 9.7 | 10.4 ± 7.2 | 12.4 ± 15.2 | −2.0 [−4.1; 0.1] | 0.066 |
| Days in ICU (days) | 0.3 ± 1.8 | 0.6 ± 1.7 | 0.2 ± 1.8 | 0.3 [0.0; 0.7] | 0.079 |
| Days in ACCU (days) | 7.9 ± 8.0 | 5.7 ± 3.5 | 9.1 ± 14.6 | −3.4 [−5.1; −1.7] | <0.001 |
| Weighted number of days after exclusion of patients with extra-corporeal membrane oxygenation (ECMO) and heart transplantation (HTX). SD, standard deviation; ICU, intensive care unit; ACCU, acute cardiac care unit. | |||||
| (c) | |||||
| Model without hospital stays >30 days | Total n = 120 mean (±SD) | Life Vest n = 92 mean (±SD) | Control n = 28 mean (±SD) | Estimate Effect [95%-CI] | p Value |
| Total hospital length (days) | 12.2 ± 10.1 | 10.2 ± 5.7 | 13.4 ± 17.6 | −3.2 [−5.4; −1.0] | 0.005 |
| Days in ICU (days) | 1.0 ± 4.4 | 0.7 ± 2.3 | 1.2 ± 8.2 | −0.5 [−1.5; 0.5] | 0.356 |
| Days in ACCU (days) | 7.3 ± 6.9 | 5.5 ± 2.6 | 8.4 ± 12.8 | −3.0 [−4.4; −1.5] | <0.001 |
| Weighted number of days after exclusion of patients with hospital stays > 30 days. SD, standard deviation; ICU, intensive care unit; ACCU, acute cardiac care unit. | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardelli, L.S.; Delbaere, Q.; Massin, F.; Granier, M.; Casella, G.; Barbato, G.; Dupasquier, V.; Macia, J.-C.; Leclercq, F.; Pasquie, J.-L.; et al. Wearable Cardioverter Defibrillator Shortens the Lengths of Stay in Patients with Left Ventricular Dysfunction after Myocardial Infarction: A Single-Centre Real-World Experience. J. Clin. Med. 2023, 12, 4884. https://doi.org/10.3390/jcm12154884
Cardelli LS, Delbaere Q, Massin F, Granier M, Casella G, Barbato G, Dupasquier V, Macia J-C, Leclercq F, Pasquie J-L, et al. Wearable Cardioverter Defibrillator Shortens the Lengths of Stay in Patients with Left Ventricular Dysfunction after Myocardial Infarction: A Single-Centre Real-World Experience. Journal of Clinical Medicine. 2023; 12(15):4884. https://doi.org/10.3390/jcm12154884
Chicago/Turabian StyleCardelli, Laura Sofia, Quentin Delbaere, François Massin, Mathieu Granier, Gianni Casella, Gaetano Barbato, Valentin Dupasquier, Jean-Christophe Macia, Florence Leclercq, Jean-Luc Pasquie, and et al. 2023. "Wearable Cardioverter Defibrillator Shortens the Lengths of Stay in Patients with Left Ventricular Dysfunction after Myocardial Infarction: A Single-Centre Real-World Experience" Journal of Clinical Medicine 12, no. 15: 4884. https://doi.org/10.3390/jcm12154884
APA StyleCardelli, L. S., Delbaere, Q., Massin, F., Granier, M., Casella, G., Barbato, G., Dupasquier, V., Macia, J.-C., Leclercq, F., Pasquie, J.-L., & Roubille, F. (2023). Wearable Cardioverter Defibrillator Shortens the Lengths of Stay in Patients with Left Ventricular Dysfunction after Myocardial Infarction: A Single-Centre Real-World Experience. Journal of Clinical Medicine, 12(15), 4884. https://doi.org/10.3390/jcm12154884