Infective Endocarditis Risk with Melody versus Sapien Valves Following Transcatheter Pulmonary Valve Implantation: A Systematic Review and Meta-Analysis of Prospective Cohort Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategies
2.2. Study Selection, Data Extraction, and Quality Assessment
2.3. Study Outcomes
2.4. Statistical Analysis
3. Results
3.1. Search Results
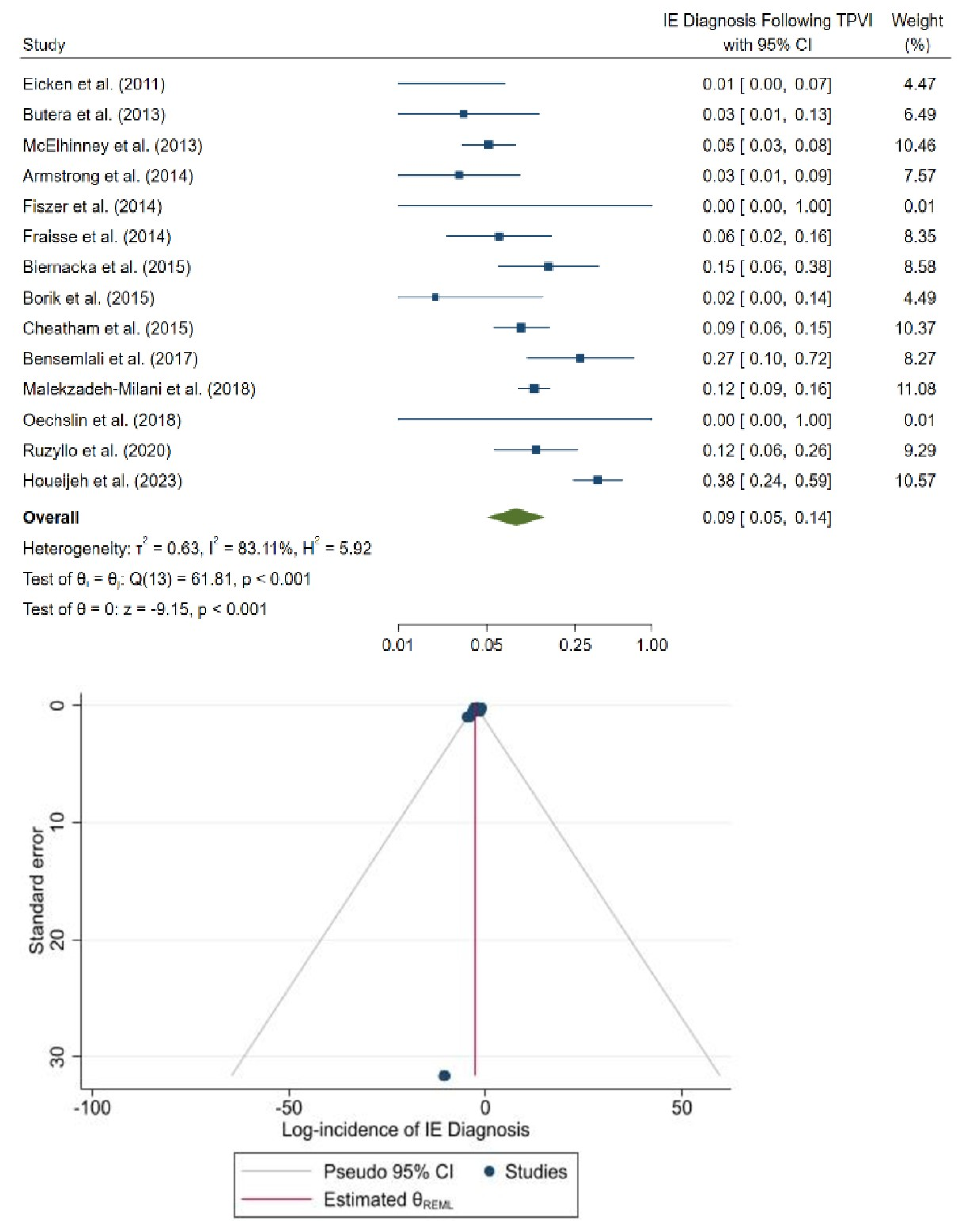
3.2. Incidence of IE Following TPVI
3.3. Sensitivity Analysis
3.4. IE Treatment Characteristics
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CI | confidence intervals |
| CHD | congenital heart disease |
| IE | infective endocarditis |
| NOS | Newcastle–Ottawa Scale |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PPVI | percutaneous pulmonary valve implantation |
| RR | relative risk |
| RVOT | right ventricular outflow tract |
| TPVI | transcatheter pulmonary valve implantation |
References
- Bonhoeffer, P.; Boudjemline, Y.; Saliba, Z.; Merckx, J.; Aggoun, Y.; Bonnet, D.; Acar, P.; Le Bidois, J.; Sidi, D.; Kachaner, J. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet 2000, 356, 1403–1405. [Google Scholar] [CrossRef] [PubMed]
- Boudjemline, Y.; Agnoletti, G.; Bonnet, D.; Sidi, D.; Bonhoeffer, P. Percutaneous pulmonary valve replacement in a large right ventricular outflow tract: An experimental study. J. Am. Coll. Cardiol. 2004, 43, 1082–1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malekzadeh-Milani, S.; Ladouceur, M.; Cohen, S.; Iserin, L.; Boudjemline, Y. Results of transcatheter pulmonary valvulation in native or patched right ventricular outflow tracts. Arch. Cardiovasc. Dis. 2014, 107, 592–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hascoet, S.; Martins, J.D.; Baho, H.; Kadirova, S.; Pinto, F.; Paoli, F.; Bitar, F.; abu Haweleh, A.; Uebing, A.; Acar, P.; et al. Percutaneous pulmonary valve implantation in small conduits: A multicenter experience. Int. J. Cardiol. 2018, 254, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Medtronic. Melody Transcatheter Pulmonary Valve. Media Kits. 2016. Available online: https://www.medtronic.com/uk-en/about/news/media-resources/media-kits/melody-transcatheter-pulmonary-valve.html (accessed on 11 January 2023).
- Edwards Lifesciences. Transcatheter Heart Valves. 2018. Available online: https://www.edwards.com/devices/heart-valves/transcatheter-sapien-xt-valve-pulmonic (accessed on 11 January 2023).
- Baumgartner, H.; De Backer, J.; Babu-Narayan, S.V.; Budts, W.; Chessa, M.; Diller, G.-P.; Lung, B.; Kluin, J.; Lang, I.M.; Meijboom, F.; et al. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur. Heart J. 2021, 42, 563–645. [Google Scholar] [CrossRef] [PubMed]
- Mongeon, F.-P.; Ben Ali, W.; Khairy, P.; Bouhout, I.; Therrien, J.; Wald, R.M.; Dallaire, F.; Bernier, P.-L.; Poirier, N.; Dore, A.; et al. Pulmonary Valve Replacement for Pulmonary Regurgitation in Adults with Tetralogy of Fallot: A Meta-analysis—A Report for the Writing Committee of the 2019 Update of the Canadian Cardiovascular Society Guidelines for the Management of Adults with Congenital Heart Disease. Can. J. Cardiol. 2019, 35, 1772–1783. [Google Scholar] [CrossRef] [PubMed]
- Stout, K.K.; Daniels, C.J.; Aboulhosn, J.A.; Bozkurt, B.; Broberg, C.S.; Colman, J.M.; Crumb, S.R.; Dearani, J.A.; Fuller, S.; Gurvitz, M.; et al. 2018 AHA/ACC Guideline for the Management of Adults with Congenital Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, 1494–1563, Correction in J. Am. Coll. Cardiol. 2019, 73, 2361. [Google Scholar] [CrossRef]
- Knirsch, W.; Mackenzie, C.R.; Schäfers, H.J.; Baumgartner, H.; Kramer, H.H. Infective endocarditis in childhood and adolescence. In The German Society of Paediatric Cardiology (DGPK) Guidelines for the Management of Congenital Heart Diseases in Childhood and Adolescence; Weil, J., Ed.; Cambridge University Press: Cambridge, UK, 2017; pp. 81–84. [Google Scholar]
- McElhinney, D.B.; Zhang, Y.; Aboulhosn, J.A.; Morray, B.H.; Biernacka, E.K.; Qureshi, A.M.; Torres, A.J.; Shahanavaz, S.; Goldstein, B.H.; Cabalka, A.K.; et al. Multicenter Study of Endocarditis After Transcatheter Pulmonary Valve Replacement. J. Am. Coll. Cardiol. 2021, 78, 575–589. [Google Scholar] [CrossRef]
- Sadeghi, S.; Wadia, S.; Lluri, G.; Tarabay, J.; Fernando, A.; Salem, M.; Sinha, S.; Levi, D.S.; Aboulhosn, J. Risk factors for infective endocarditis following transcatheter pulmonary valve replacement in patients with congenital heart disease. Catheter. Cardiovasc. Interv. 2019, 94, 625–635. [Google Scholar] [CrossRef]
- Haas, N.A.; Bach, S.; Vcasna, R.; Laser, K.T.; Sandica, E.; Blanz, U.; Jakob, A.; Dietl, M.; Fischer, M.; Kanaan, M.; et al. The risk of bacterial endocarditis after percutaneous and surgical biological pulmonary valve implantation. Int. J. Cardiol. 2018, 268, 55–60. [Google Scholar] [CrossRef]
- Bos, D.; De Wolf, D.; Cools, B.; Eyskens, B.; Hubrechts, J.; Boshoff, D.; Louw, J.; Frerich, S.; Ditkowski, B.; Rega, F.; et al. Infective endocarditis in patients after percutaneous pulmonary valve implantation with the stent-mounted bovine jugular vein valve: Clinical experience and evaluation of the modified Duke criteria. Int. J. Cardiol. 2021, 323, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Van Dijck, I.; Budts, W.; Cools, B.; Eyskens, B.; Boshoff, D.E.; Heying, R.; Frerich, S.; Vanagt, W.Y.; Troost, E.; Gewillig, M. Infective endocarditis of a transcatheter pulmonary valve in comparison with surgical implants. Heart 2015, 101, 788–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hascoet, S.; Mauri, L.; Claude, C.; Fournier, E.; Lourtet, J.; Riou, J.-Y.; Brenot, P.; Petit, J. Infective Endocarditis Risk after Percutaneous Pulmonary Valve Implantation with the Melody and Sapien Valves. JACC Cardiovasc. Interv. 2017, 10, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Lehner, A.; Haas, N.A.; Dietl, M.; Jakob, A.; Schulze-Neick, I.; Pozza, R.D.; Rodriguez, S.F.; Fischer, M. The risk of infective endocarditis following interventional pulmonary valve implantation: A meta-analysis. J. Cardiol. 2019, 74, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Abdelghani, M.; Nassif, M.; Blom, N.A.; Van Mourik, M.S.; Straver, B.; Koolbergen, D.R.; Kluin, J.; Tijssen, J.G.; Mulder, B.J.M.; Bouma, B.J.; et al. Infective Endocarditis After Melody Valve Implantation in the Pulmonary Position: A Systematic Review. J. Am. Heart Assoc. 2018, 7, e008163. [Google Scholar] [CrossRef] [Green Version]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.A.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Zhou, X.; Zorzela, L.; Ju, K.; Furuya-Kanamori, L.; Lin, L.; Lu, C.; Musa, O.A.H.; Vohra, S. Utilization of the evidence from studies with no events in meta-analyses of adverse events: An empirical investigation. BMC Med. 2021, 19, 141. [Google Scholar] [CrossRef]
- Hascoet, S.; Pozza, R.D.; Bentham, J.; Carere, R.G.; Kanaan, M.; Ewert, P.; Biernacka, E.K.; Kretschmar, O.; Deutsch, C.; Lecerf, F.; et al. Early outcomes of percutaneous pulmonary valve implantation using the Edwards SAPIEN 3 transcatheter heart valve system. Eurointervention 2019, 14, 1378–1385. [Google Scholar] [CrossRef]
- Haas, N.A.; Moysich, A.; Neudorf, U.; Mortezaeian, H.; Abdel-Wahab, M.; Schneider, H.; De Wolf, D.; Petit, J.; Narayanswami, S.; Laser, K.T.; et al. Percutaneous implantation of the Edwards SAPIEN™ pulmonic valve: Initial results in the first 22 patients. Clin. Res. Cardiol. 2013, 102, 119–128. [Google Scholar] [CrossRef]
- Pilati, M.; Gagliardi, M.G.; Guccione, P.; Pongiglione, G. Infective Endocarditis Risk after Percutaneous Pulmonary Valve Implantation with the Melody and Sapien Valves. Transcatheter Pulmonary Valve Implantation Using the Edwards Sapien Transcatheter Heart Valve: Experience from a Single Centre. Giornale Italiano di Cardiologia. In Proceedings of the Conference: 41st Congresso Nazionale della Societa Italiana di Cardiologia Pediatrica Congiunto con la Sezione Pediatrica e delle Cardiopatie Congenite della Societa Italiana di Chirurgia Cardiaca. 2011; Volume 12, p. 10S. Available online: https://www.giornaledicardiologia.it/#current (accessed on 19 July 2023).
- Odemis, E.; Guzeltas, A.; Saygi, M.; Ozyilmaz, I.; Momenah, T.; Bakir, I. Percutaneous pulmonary valve implantation using Edwards SAPIEN transcatheter heart valve in different types of conduits: Initial results of a single center experience. Congenit. Heart Dis. 2013, 8, 411–417. [Google Scholar] [CrossRef]
- Demkow, M.; Rużyłło, W.; Biernacka, E.K.; Kalińczuk, Ł.; Śpiewak, M.; Kowalski, M.; Sitkowska, E.; Kuśmierczyk, M.; Różanski, J.; Banaś, S.; et al. Percutaneous Edwards SAPIEN™ valve implantation for significant pulmonary regurgitation after previous surgical repair with a right ventricular outflow patch. Catheter. Cardiovasc. Interv. 2013, 83, 474–481. [Google Scholar] [CrossRef]
- Biernacka, E.K.; Rużyłło, W.; Demkow, M.; Kowalski, M.; Śpiewak, M.; Piotrowski, W.; Kuśmierczyk, M.; Banaś, S.; Różanski, J.; Hoffman, P. Transcatheter pulmonary valve implantation in patients with right ventricular outflow tract dysfunction: Early and mid-term results. J. Invasive Cardiol. 2015, 27, E82–E89. [Google Scholar]
- Kenny, D.; Rhodes, J.F.; Fleming, G.A.; Kar, S.; Zahn, E.M.; Vincent, J.; Shirali, G.S.; Gorelick, J.; Fogel, M.A.; Fahey, J.T.; et al. 3-Year Outcomes of the Edwards SAPIEN Transcatheter Heart Valve for Conduit Failure in the Pulmonary Position From the COMPASSION Multicenter Clinical Trial. JACC Cardiovasc. Interv. 2018, 11, 1920–1929. [Google Scholar] [CrossRef]
- Oechslin, L.; Corti, R.; Greutmann, M.; Kretschmar, O.; Gaemperli, O. Percutaneous pulmonary valve implantation in grown-up congenital heart disease patients: Insights from the Zurich experience. J. Interv. Cardiol. 2018, 31, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Plessis, J.; Hascoet, S.; Baruteau, A.-E.; Godart, F.; Le Gloan, L.; Fresse, K.W.; Tahhan, N.; Riou, J.-Y.; Guyomarch, B.; Petit, J.; et al. Edwards SAPIEN Transcatheter Pulmonary Valve Implantation: Results From a French Registry. JACC Cardiovasc. Interv. 2018, 11, 1909–1916. [Google Scholar] [CrossRef] [PubMed]
- Rużyłło, W.; Biernacka, E.K.; Woźniak, O.; Kowalski, M.; Śpiewak, M.; Cicha-Mikołajczyk, A.; Szczęsny, A.; Kuśmierczyk, M.; Hoffman, P.; Demkow, M. Transcatheter pulmonary valve implantation in 100 patients: A 10-year single-center experience. Postępy Kardiol. Interwencyjnej 2020, 16, 235–243. [Google Scholar] [CrossRef]
- Houeijeh, A.; Batteux, C.; Karsenty, C.; Ramdane, N.; Lecerf, F.; Valdeolmillos, E.; Lourtet-Hascoet, J.; Cohen, S.; Belli, E.; Petit, J.; et al. Long-term outcomes of transcatheter pulmonary valve implantation with melody and SAPIEN valves. Int. J. Cardiol. 2023, 370, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Eicken, A.; Ewert, P.; Hager, A.; Peters, B.; Fratz, S.; Kuehne, T.; Busch, R.; Hess, J.; Berger, F. Percutaneous pulmonary valve implantation: Two-centre experience with more than 100 patients. Eur. Heart J. 2011, 32, 1260–1265. [Google Scholar] [CrossRef] [Green Version]
- Butera, G.; Milanesi, O.; Spadoni, I.; Piazza, L.; Donti, A.; Ricci, C.; Agnoletti, G.; Pangrazi, A.; Chessa, M.; Carminati, M. Melody transcatheter pulmonary valve implantation. Results from the registry of the Italian society of pediatric cardiology. Catheter. Cardiovasc. Interv. 2012, 81, 310–316. [Google Scholar] [CrossRef]
- Armstrong, A.K.; Balzer, D.T.; Cabalka, A.K.; Gray, R.G.; Javois, A.J.; Moore, J.W.; Rome, J.J.; Turner, D.R.; Zellers, T.M.; Kreutzer, J. One-year follow-up of the Melody transcatheter pulmonary valve multicenter post-approval study. JACC Cardiovasc. Interv. 2014, 7, 1254–1262. [Google Scholar] [CrossRef] [Green Version]
- Fiszer, R.; Dryżek, P.; Szkutnik, M.; Góreczny, S.; Krawczuk, A.; Moll, J.; Moszura, T.; Pawlak, S.; Białkowski, J. Immediate and long-term outcomes of percutaneous transcatheter pulmonary valve implantation. Cardiol. J. 2017, 24, 604–611. [Google Scholar] [CrossRef] [Green Version]
- Fraisse, A.; Aldebert, P.; Malekzadeh-Milani, S.; Thambo, J.-B.; Piéchaud, J.-F.; Aucoururier, P.; Chatelier, G.; Bonnet, D.; Iserin, L.; Bonello, B.; et al. Melody® transcatheter pulmonary valve implantation: Results from a French registry. Arch. Cardiovasc. Dis. 2014, 107, 607–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borik, S.; Crean, A.; Horlick, E.; Osten, M.; Lee, K.-J.; Chaturvedi, R.; Friedberg, M.K.; McCrindle, B.W.; Manlhiot, C.; Benson, L. Percutaneous pulmonary valve implantation: 5 years of follow-up: Does age influence outcomes? Circ. Cardiovasc. Interv. 2015, 8, e001745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheatham, J.P.; Hellenbrand, W.E.; Zahn, E.M.; Jones, T.K.; Berman, D.P.; Vincent, J.A.; McElhinney, D.B. Clinical and hemodynamic outcomes up to 7 years after transcatheter pulmonary valve replacement in the US melody valve investigational device exemption trial. Circulation 2015, 131, 1960–1970. [Google Scholar] [CrossRef] [Green Version]
- Bensemlali, M.; Malekzadeh-Milani, S.; Mostefa-Kara, M.; Bonnet, D.; Boudjemline, Y. Percutaneous pulmonary Melody® valve implantation in small conduits. Arch. Cardiovasc. Dis. 2017, 110, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Malekzadeh-Milani, S.; Houeijeh, A.; Jalal, Z.; Hascoet, S.; Bakloul, M.; Aldebert, P.; Piéchaud, J.-F.; Heitz, F.; Bouvaist, H.; Dauphin, C.; et al. French national survey on infective endocarditis and the Melody™ valve in percutaneous pulmonary valve implantation. Arch. Cardiovasc. Dis. 2018, 111, 497–506. [Google Scholar] [CrossRef]
- Efthimiou, O. Practical guide to the meta-analysis of rare events. Evid. Based Ment. Health 2018, 21, 72–76. [Google Scholar] [CrossRef]
- Kuss, O. Statistical methods for meta-analyses including information from studies without any events-add nothing to nothing and succeed nevertheless. Stat. Med. 2015, 34, 1097–1116. [Google Scholar] [CrossRef]
- Xu, C.; Lin, L. The impact of studies with no events in both arms on meta-analysis of rare events: A simulation study using generalized linear mixed model. medRxiv 2021, 2021, 21262461. [Google Scholar] [CrossRef]
- Xu, C.; Li, L.; Lin, L.; Chu, H.; Thabane, L.; Zou, K.; Sun, X. Exclusion of studies with no events in both arms in meta-analysis impacted the conclusions. J. Clin. Epidemiol. 2020, 123, 91–99. [Google Scholar] [CrossRef]
- Wei, J.-J.; Lin, E.-X.; Shi, J.-D.; Yang, K.; Hu, Z.-L.; Zeng, X.-T.; Tong, T.-J. Meta-analysis with zero-event studies: A comparative study with application to COVID-19 data. Mil. Med. Res. 2021, 8, 1–11. [Google Scholar] [CrossRef]
- Lin, L.; Chu, H. Meta-analysis of Proportions Using Generalized Linear Mixed Models. Epidemiology 2020, 31, 713–717. [Google Scholar] [CrossRef]
- Spittal, M.J.; Pirkis, J.; Gurrin, L.C. Meta-analysis of incidence rate data in the presence of zero events. BMC Med. Res. Methodol. 2015, 15, 42. [Google Scholar] [CrossRef] [Green Version]
- Böhning, D.; Sangnawakij, P. The identity of two meta-analytic likelihoods and the ignorability of double-zero studies. Biostatistics 2021, 22, 890–896. [Google Scholar] [CrossRef]
- Wilson, W.M.; Benson, L.N.; Osten, M.D.; Shah, A.; Horlick, E.M. Horlick EM. Transcatheter Pulmonary Valve Replacement with the Edwards Sapien System: The Toronto Experience. JACC Cardiovasc. Interv. 2015, 8, 1819–1827. [Google Scholar] [CrossRef] [PubMed]
- Villafañe, J.; Baker, G.H.; Austin, E.H., 3rd; Miller, S.; Peng, L.; Beekman, R. Melody®pulmonary valve bacterial endocarditis: Experience in four pediatric patients and a review of the literature. Catheter. Cardiovasc. Interv. 2014, 84, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.; Vogt, M.; Boekenkamp, R.; Hoerer, J.; Eicken, A.; Foth, R.; Kriebel, T.; Paul, T.; Sigler, M. Melody transcatheter valve: Histopathology and clinical implications of nine explanted devices. Int. J. Cardiol. 2015, 189, 124–131. [Google Scholar] [CrossRef]
- Mery, C.M.; Guzmán-Pruneda, F.A.; De León, L.E.; Zhang, W.; Terwelp, M.D.; Bocchini, C.E.; Adachi, I.; Heinle, J.S.; McKenzie, E.D.; Fraser, C.D. Risk factors for development of endocarditis and reintervention in patients undergoing right ventricle to pulmonary artery valved conduit placement. J. Thorac. Cardiovasc. Surg. 2016, 151, 432–441.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jalal, Z.; Galmiche, L.; Lebeaux, D.; Villemain, O.; Brugada, G.; Patel, M.; Ghigo, J.-M.; Beloin, C.; Boudjemline, Y. Selective propensity of bovine jugular vein material to bacterial adhesions: An in-vitro study. Int. J. Cardiol. 2015, 198, 201–205. [Google Scholar] [CrossRef]
- Veloso, T.R.; Claes, J.; Van Kerckhoven, S.; Ditkowski, B.; Hurtado-Aguilar, L.G.; Jockenhoevel, S.; Mela, P.; Jashari, R.; Gewillig, M.; Hoylaerts, M.F.; et al. Bacterial adherence to graft tissues in static and flow conditions. J. Thorac. Cardiovasc. Surg. 2018, 155, 325–332.e4. [Google Scholar] [CrossRef] [Green Version]
- Esmaeili, A.; Bollmann, S.; Khalil, M.; De Rosa, R.; Fichtlscherer, S.; Akintuerk, H.; Schranz, D. Percutaneous pulmonary valve implantation for reconstruction of a patch-repaired right ventricular outflow tract. J. Interv. Cardiol. 2017, 31, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Fuente, M.; Haas, N.A.; Del Cerro, M.J. Edwards valve-in-valve implantation in tricuspid position. Cardiol. Young 2017, 27, 1633–1636. [Google Scholar] [CrossRef]
- Boone, R.H.; Webb, J.G.; Horlick, E.; Benson, L.; Cao, Q.-L.; Nadeem, N.; Kiess, M.; Hijazi, Z.M. Transcatheter pulmonary valve implantation using the Edwards SAPIEN™ transcatheter heart valve. Catheter. Cardiovasc. Interv. 2010, 75, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Giugno, L.; Faccini, A.; Carminati, M. Percutaneous Pulmonary Valve Implantation. Korean Circ. J. 2020, 50, 302–316. [Google Scholar] [CrossRef] [Green Version]
- Patel, N.D.; Levi, D.S.; Cheatham, J.P.; Qureshi, S.A.; Shahanavaz, S.; Zahn, E.M. Transcatheter Pulmonary Valve Replacement: A Review of Current Valve Technologies. J. Soc. Cardiovasc. Angiogr. Interv. 2022, 1, 100452. [Google Scholar] [CrossRef]
- Kirklin, J.K.; Kirklin, J.W.; Blackstone, E.H.; Milano, A.; Pacifico, A.D. Effect of transannular patching on outcome after repair of tetralogy of Fallot. Ann. Thorac. Surg. 1989, 48, 783–791. [Google Scholar] [CrossRef]
- Stammnitz, C.; Huscher, D.; Bauer, U.M.M.; Urban, A.; Nordmeyer, J.; Schubert, S.; Photiadis, J.; Berger, F.; Klaassen, S. The German Competence Network for Congenital Heart Defects Investigators. Nationwide Registry-Based Analysis of Infective Endocarditis Risk After Pulmonary Valve Replacement. J. Am. Heart Assoc. 2022, 11, 22231. [Google Scholar] [CrossRef]
- Nordmeyer, J.; Ewert, P.; Gewillig, M.; Aljufan, M.; Carminati, M.; Kretschmar, O.; Uebing, A.; Dähnert, I.; Röhle, R.; Schneider, H.; et al. Acute and midterm outcomes of the post-approval MELODY Registry: A multicentre registry of transcatheter pulmonary valve implantation. Eur. Heart J. 2019, 40, 2255–2264. [Google Scholar] [CrossRef]
- Balakrishna, A.M.; Ismayl, M.; Thandra, A.; Walters, R.; Ganesan, V.; Anugula, D.; Shah, D.J.; Aboeata, A. Diagnostic Value of Cardiac Magnetic Resonance Imaging and Intracoronary Optical Coherence Tomography in Patients with a Working Diagnosis of Myocardial Infarction with Non-obstructive Coronary Arteries—A Systematic Review and Meta-analysis. Curr. Probl. Cardiol. 2023, 48, 101126. [Google Scholar] [CrossRef]
- Machanahalli Balakrishna, A.; Ismayl, M.; Palicherla, A.; Aboeata, A.; Goldsweig, A.M.; Zhao, D.X.; Vallabhajosyula, S. Impact of prior coronary artery bypass grafting on periprocedural and short-term outcomes of patients undergoing transcatheter aortic valve replacement: A systematic review and meta-analysis. Coron Artery Dis. 2023, 34, 42–51. [Google Scholar] [CrossRef]
- Machanahalli Balakrishna, A.; Ismayl, M.; Srinivasamurthy, R.; Gowda, R.M.; Aboeata, A. Early Outcomes of Percutaneous Coronary Intervention in Patients with Cancer: A Systematic Review and Meta-analysis. Curr. Probl. Cardiol. 2022, 47, 101305. [Google Scholar] [CrossRef] [PubMed]
- Ismayl, M.; Machanahalli Balakrishna, A.; Abusnina, W.; Thandra, A.; Walters, R.W.; Alugubelli, N.R.; Yackley, S.; Betts, L.; Smer, A.; Goldsweig, A.M.; et al. Surgical Aortic Valve Replacement Versus Conservative Treatment in Asymptomatic Severe Aortic Stenosis: An Updated Systematic Review and Meta-Analysis. Cardiovasc. Revasc. Med. 2022, 42, 36–44. [Google Scholar] [CrossRef] [PubMed]
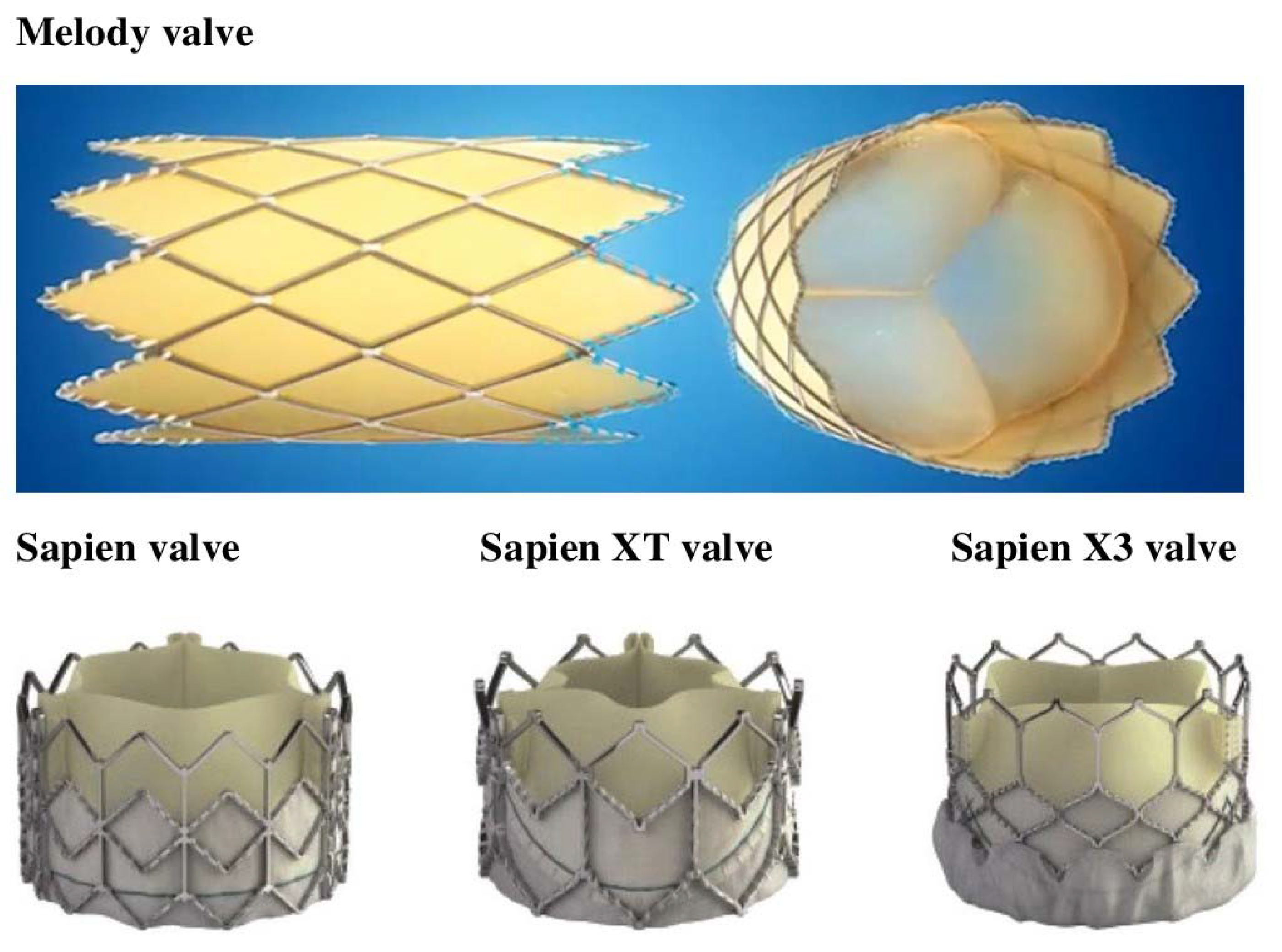


| Study | Year | Region/ Country | Study Design | Enrollment Period | Number of Patients | Types of Valve Used | Events | Time to IE (Months) | Management and In-Hospital Outcome | Microorganism | Follow-Up Duration |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Eicken et al. [32] | 2011 | Germany | Prospective, observational study, single-arm | 2006 to 2010 | 102 | Melody | 1 | 6 | SX = 1 | Staphylococcus aureus = 1 | 12 months |
| Pilati et al. [23] | 2011 | Italy | Prospective, observational study, single-arm | 2010 to 2011 | 7 | Sapien | 0 | - | - | - | 3 months |
| Butera et al. [33] | 2013 | Italy | Prospective, observational study, single-arm | 2007 to 2010 | 61 | Melody | 2 | 5 | SX = 2 | Staphylococcus aureus = 2 | 30 months |
| Haas et al. [22] | 2013 | Germany | Prospective, observational study, single-arm | 2007 to 2010 | 22 | Sapien | 0 | - | - | - | 6 months |
| McElhinney et al. [11] | 2013 | USA | Prospective, observational study, single-arm | 2007 to 2009 | 311 | Melody | 16 | 16 | ABX = 8, TI = 2, SX = 4, D = 2 | Staphylococcus aureus = 5, other Staphylococcus = 5, Streptococcus = 6, other gram-negative bacteria = 1 | 24 months |
| Odemis et al. [24] | 2013 | Turkey | Prospective, observational study, single-arm | 2007 to 2010 | 14 | Sapien | 0 | - | - | - | 30 months |
| Armstrong et al. [34] | 2014 | USA | Prospective, observational study, single-arm | 2007 to 2010 | 100 | Melody | 3 | 3 | ABX = 2, SX = 1 | Staphylococcus aureus = 2, other gram-negative bacteria = 1 | 12 months |
| Demkow et al. [25] | 2014 | Poland | Prospective, observational study, single-arm | 2011 to 2012 | 10 | Sapien | 0 | - | - | - | 2 months |
| Fiszer et al. [35] | 2014 | Poland | Prospective, observational study, single-arm | 2009 to 2016 | 44 | Melody | 0 | - | - | - | 35 months |
| Fraisse et al. [36] | 2014 | France | Prospective, observational study, single-arm | 2008 to 2010 | 64 | Melody | 4 | 26 | SX = 3, D = 2 | Other Staphylococcus = 2, Streptococcus = 1 | 54 months |
| Biernacka et al. [26] | 2015 | Poland | Prospective, observational study, multi-arm | 2008 to 2012 | 26 | Melody and Sapien | 4 | - | SX = 4, D = 1 | - | 20 months |
| Borik et al. [37] | 2015 | Canada | Prospective, observational study, single-arm | 2005 to 2011 | 51 | Melody | 1 | 60 | SX = 1 | Other gram-positive bacteria = 1 | 54 months |
| Cheatham et al. [38] | 2015 | USA | Prospective, observational study, single-arm | 2007 to 2014 | 150 | Melody | 14 | - | ABX = 6, TI = 8, D = 1 | - | 54 months |
| Bensemlali et al. [39] | 2017 | France | Prospective, observational study, single-arm | 2000 to 2015 | 11 | Melody | 3 | - | ABX = 1, SX = 2 | - | 46 months |
| Haas et al. [13] | 2018 | Germany | Prospective, observational study, single-arm | 2000 to 2015 | 46 | Sapien | 0 | - | - | - | 60 months |
| Kenny et al. [27] | 2018 | USA | Prospective, observational study, single-arm | 2008 to 2014 | 79 | Sapien | 3 | 2 | ABX = 3 | Other Staphylococcus = 2, HACEK = 1 | 36 months |
| Malekzadeh-Milani et al. [40] | 2018 | France | Prospective, observational study, single-arm | 2008 to 2016 | 365 | Melody | 43 | 31 | ABX = 27, TI = 7, SX = 6, D = 3 | Other Staphylococcus = 7, Staphylococcus aureus = 12, Streptococcus = 16, GP = 5, GN = 4 | 43 months |
| Oechslin et al. [28] | 2018 | Switzerland | Prospective, observational study, multi- arm | 2008 to 2016 | 29 | Melody and Sapien | 0 | - | - | - | 43 months |
| Plessis et al. [29] | 2018 | France | Prospective, observational study, single-arm | 2011 to 2017 | 71 | Sapien | 1 | - | SX = 1 | - | 12 months |
| Hascoet et al. [21] | 2019 | France | Prospective, observational study, single-arm | 2016 to 2018 | 82 | Sapien | 0 | - | - | - | 17 months |
| Rużyłło et al. [30] | 2020 | Poland | Prospective, observational study, multi-arm | 2008 to 2019 | 49 | Melody and Sapien | 6 | 35 | - | - | 66 months |
| Houeijeh et al. [31] | 2023 | France | Prospective, observational study, multi-arm | 2008 to 2020 | 32 | Melody and Sapien | 12 | - | SX = 17 | - | 2.8 years |
| Method | Sapien | Melody | Incidence in Sapien vs. Melody | |
|---|---|---|---|---|
| Incidence (95% CI) [%] | Incidence (95% CI) [%] | Risk Ratio (95% CI) | p | |
| Primary Analysis | 2.1 (0.9, 5.1) | 8.5 (4.8, 15.2) | 0.21 (0.06, 0.76) | 0.019 |
| Sensitivity Analysis | ||||
| 0.5 Continuity Correction | 2.6 (1.3, 5.2) | 7.7 (4.3, 1.4) | 0.32 (0.12, 0.82) | 0.020 |
| Exclusion of Zero-Event Studies | 2.1 (0.6, 7.7) | 8.5 (4.7, 15.4) | 0.21 (0.05, 0.81) | 0.026 |
| Poisson Regression | 0.4 (0.1, 2.2) | 7.2 (2.8, 18.2) | 0.06 (0.01, 0.30) | 0.011 |
| Binomial Regression | 0.4 (0.1, 2.0) | 7.3 (2.8, 18.8) | 0.05 (0.01, 0.26) | 0.010 |
| Melody | Sapien | |
|---|---|---|
| Number of patients | 1395 | 572 |
| Mean age | 20 ± 7 | 23 ± 6 |
| Mean follow-up | 33 ± 23 months | 22 ± 18 months |
| IE events (cumulative incidence) | 109 (7.8%) | 6 (1%) |
| Time to IE | 21 ± 18 months | 2 ± 1 months |
| Management | n = 105 | n = 4 |
| Only antibiotic treatment | 45% | 75% |
| Explantation by surgery | 33% | 25% |
| Transcatheter pulmonary valve interventions (redoing TPV implantation or TPV explantation) | 14% | – |
| In-hospital outcomes | n = 105 | n = 4 |
| Death | 8% | – |
| Microbiological data | n = 78 | n = 4 |
| Staphylococcus aureus | 26% | 25% |
| Other staphylococci | 19% | 50% |
| Streptococcus | 32% | – |
| Other gram-positive bacteria | 10% | – |
| Other gram-negative bacteria | 6% | – |
| HACEK | 7% | 25% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machanahalli Balakrishna, A.; Dilsaver, D.B.; Aboeata, A.; Gowda, R.M.; Goldsweig, A.M.; Vallabhajosyula, S.; Anderson, J.H.; Simard, T.; Jhand, A. Infective Endocarditis Risk with Melody versus Sapien Valves Following Transcatheter Pulmonary Valve Implantation: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. J. Clin. Med. 2023, 12, 4886. https://doi.org/10.3390/jcm12154886
Machanahalli Balakrishna A, Dilsaver DB, Aboeata A, Gowda RM, Goldsweig AM, Vallabhajosyula S, Anderson JH, Simard T, Jhand A. Infective Endocarditis Risk with Melody versus Sapien Valves Following Transcatheter Pulmonary Valve Implantation: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Journal of Clinical Medicine. 2023; 12(15):4886. https://doi.org/10.3390/jcm12154886
Chicago/Turabian StyleMachanahalli Balakrishna, Akshay, Danielle B. Dilsaver, Ahmed Aboeata, Ramesh M. Gowda, Andrew M. Goldsweig, Saraschandra Vallabhajosyula, Jason H. Anderson, Trevor Simard, and Aravdeep Jhand. 2023. "Infective Endocarditis Risk with Melody versus Sapien Valves Following Transcatheter Pulmonary Valve Implantation: A Systematic Review and Meta-Analysis of Prospective Cohort Studies" Journal of Clinical Medicine 12, no. 15: 4886. https://doi.org/10.3390/jcm12154886
APA StyleMachanahalli Balakrishna, A., Dilsaver, D. B., Aboeata, A., Gowda, R. M., Goldsweig, A. M., Vallabhajosyula, S., Anderson, J. H., Simard, T., & Jhand, A. (2023). Infective Endocarditis Risk with Melody versus Sapien Valves Following Transcatheter Pulmonary Valve Implantation: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Journal of Clinical Medicine, 12(15), 4886. https://doi.org/10.3390/jcm12154886








